Abstract
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) cells typically have low 2-deoxy-2-[18F] fluoro-D-glucose (FDG) avidity and CLL patients have an increased risk of developing FDG-avid aggressive lymphomas, second malignancies, and infections. We hypothesized that FDG positron emission tomography-computed tomography of the trunk (PET-CT) is a sensitive method of detecting these complications in patients with CLL.
Of the of 2299 CLL patients seen in the Division of Hematology at Mayo Clinic Rochester between January 1, 2006 and December 31, 2011, 272 (11.8%) had 526 PET-CT scans and 472 (89.7%) of these were reported as abnormal. Among the 293 (55.7%) PET-CT scans used for routine evaluation of CLL, the PET component was of clinical value in only one instance. In contrast, in 83 (30.5%) patients, PET-CT scans used to evaluate new clinical complications localized high FDG avidity lesions for biopsies. This resulted in clinically relevant new diagnoses in 32 patients including more aggressive lymphoma (n=16), non-hematological malignancies (n=8), and opportunistic infections (n=3). Twenty-seven patients had high FDG avidity CLL, which was associated with prominent lymph node proliferation centers, an increased frequency of poor prognostic factors (17p13 deletion, unmutated IGHV, expression of ZAP-70 and CD38), and a shorter overall survival.
We conclude that FDG PET scans should not be used for routine surveillance of patients with CLL. However PET-CT scans are sensitive, but not specific, for detection of aggressive lymphomas, other cancers, and systemic infections in patients with CLL.
Keywords: Chronic lymphocytic leukemia, small lymphocytic lymphoma, PET, CT, imaging
Introduction
Positron emission tomography (PET) is a functional imaging technique that can measure cellular uptake of the glucose analogue 2-deoxy-2-[18F] fluoro-D-glucose (FDG).[1] The anatomical localization of FDG uptake is determined by concomitant low-resolution non-contrast computerized tomography (CT) in a PET-CT scan. Appreciable physiological FDG uptake at rest occurs in highly metabolic areas such as the brain and heart and FDG is also concentrated in urine resulting in physiological positron emission from the kidneys, ureters, and urinary bladder.[1] Pathological increased uptake of glucose and its FDG analogue can occur in neoplastic, infected, or inflamed tissue and this characteristic can be used for diagnosis and monitoring of disease.[1]
PET-CT scans are now widely utilized in the diagnosis and management of many lymphoid malignancies.[2] However, FDG uptake is usually not appreciably increased in the malignant lymphocytes of some indolent lymphoid malignancies, including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL).[3] In these diseases the value of the routine use of PET scans has not been established. However, patients with CLL are at an appreciably higher risk of developing second malignancies (including transformation)[4,5] and serious infections[6], so PET-CT scans could be useful in the diagnosis and management of these complications.
This study was designed to test the hypothesis that PET-CT scans are sensitive tests for the evaluation of patients with CLL for second malignancies and serious infections. We show that PET-CT scans are sensitive but not specific for the diagnosis of these complications.
Materials and Methods
This observational study was conducted with Institutional Review Board approval according to the principals of the Declaration of Helsinki. We reviewed the prospectively collected clinical data of all 2299 patients in the Mayo Clinic Rochester CLL database seen in the Division of Hematology at least once during the six-year period between January 1, 2006 and December 31, 2011. These data were used to search the Radiology procedure database to identify the 526 FDG PET-CT scans of the trunk done on 272 (11.8%) of these patients during this study period.
Each patient’s electronic clinical record was reviewed to determine the indications for PET-CT, the results of the imaging, and the management decisions based on these results. CLL and lymphoma diagnoses were made using standard criteria.[7–10] PET-CT scans were performed and reported at Mayo Clinic Rochester according to standard clinical protocols. PET scans were assessed as positive (activity greater than that of the surrounding tissue) or negative (activity at or below the surrounding background) according to the reports. FDG avidity was recorded as high (standardized uptake value (SUV) ≥5) or low (SUV<5) for all lesions.[11,12]
All diagnostic biopsies done at other institutions were routinely reviewed at Mayo Clinic. All available and suitable biopsy specimens from patients with high FDG avidity CLL (SUV≥5) were reviewed by a consultant hematopathologist (D.S.V.). Lymph nodes were considered to have prominent proliferation centers if they exceeded 40% of the CLL cells within the node, an assessment similar to that used in previously published studies[13,14](Figure 1). Genetic analysis was performed on peripheral blood cells by interphase fluorescent in situ hybridization (FISH) in the clinical laboratory as previously described.[15] TP53 exon 4–9 analysis was done by conventional sequencing of exons 4–9[16], somatic hypermutation of IGHV was analyzed by conventional sequencing[17] and ZAP-70, CD38 and CD49d were measured by flow cytometric methods in the clinical laboratory using standard methods.[18–20]
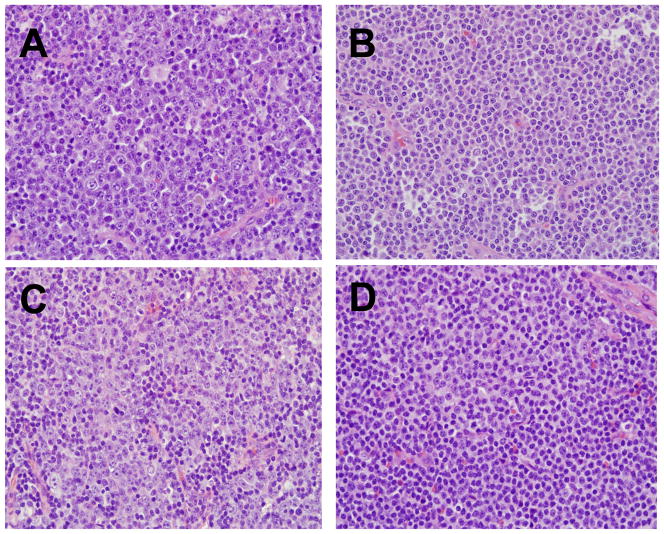
Figure 1. Lymph node Histology in CLL.
Proliferation centers characterized by paraimmunoblasts (prolymphocytes) are a characteristic feature of lymph nodes involved by CLL. Prominent proliferation centers with >40% paraimmunoblasts are shown in sections A to C. In section B there is a more subtle increase in paraimmunoblasts interspersed with small CLL lymphocytes. D: Example of a more typical appearance of CLL with a predominance of small lymphocytes and foci of proliferation center formation. (H&E stain, Nikon objective 20x lens with 200x magnification, Olympus DP controller and Nikon Eclipse 80i camera)
The indication for each PET-CT scan was categorized based on the clinical record as follows: 1. Evaluation of lymphadenopathy or other pathological lesions in patients without an established diagnosis of CLL; 2. Initial staging of newly diagnosed CLL; 3. Evaluation of response to treatment of CLL or complications of CLL; or 4. Evaluation of new or pre-existing non-CLL medical conditions in patients with an established diagnosis of CLL. Patient records were examined to determine the role of the PET component of the PET-CT scan in deciding subsequent patient management and the effect of the PET scan on management outcome. Management was categorized as observation, further investigation (consultation, biopsy and additional evaluation), or treatment. Outcome was defined as: 1. Highly deleterious if a false positive or negative PET scan contributed to an adverse event; 2. Moderately deleterious if a false positive or negative PET scan did not contribute to an adverse event; 3. Neutral if the PET scan result did not influence outcome; 4. Moderately beneficial if the PET scan facilitated the selection of a biopsy site, diagnosis, or treatment; 5. Highly beneficial if the PET scan was critical in making a diagnosis that directly affected outcome.
Statistical Analysis
Descriptive statistics were computed and summarized. Qualitative variables were compared using chi-square or Fisher’s exact tests. Differences in survival were computed using a log-rank test from Kaplan-Meier methodology. All statistical analyses were performed using SAS 9.3 (SAS Institute; Cary, NC).
Results
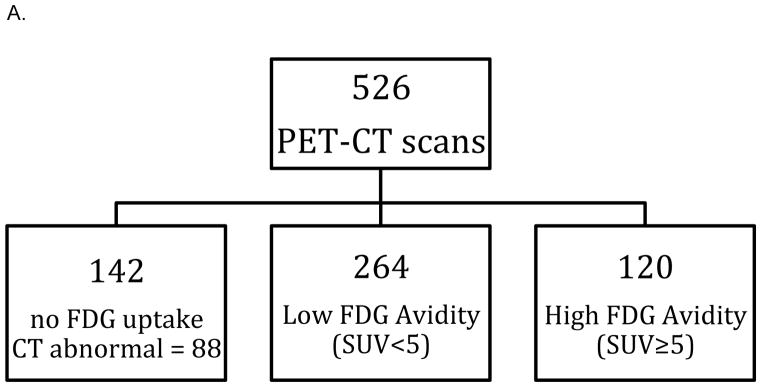
The characteristics of the 272 CLL patients (11.8% of population) who had at least one PET-CT scan are detailed in Table 1. These patients underwent a total of 526 scans as detailed in Table 2. Fifty-four (10.3%) PET-CT scans were reported as showing no abnormalities and an additional 88 (16.7%) had no increased FDG uptake (PET negative) with abnormalities seen only on the CT component of the scan (Figure 2A). There were 384 (73.0%) positive PET scans. One hundred and twenty (31.2%) scans had areas of high FDG avidity (SUV≥5) while 264 (68.8%) had only areas of low FDG avidity (SUV<5).
Table 1.
Patient Characteristics (n=272)
| Variable | N (%) |
|---|---|
|
| |
| Age at diagnosis (years) | |
| Median (range) | 61.5 (21–91) |
| Sex | |
| Female | 75 (28) |
| Male | 197 (72) |
| Stage | |
| Low risk (Rai 0) | 87 (32) |
| Intermediate risk (Rai I - II) | 155 (57) |
| High risk (Rai III – IV) | 29 (11) |
| Missing | 1 |
| CD38 | |
| Negative | 115 (53) |
| Positive | 103 (47) |
| Missing | 54 |
| CD49d | |
| Negative | 71 (49) |
| Positive | 75 (51) |
| Missing | 126 |
| ZAP-70 | |
| Negative | 83 (44) |
| Positive | 108 (56) |
| Missing | 81 |
| IGHV somatic hypermutation | |
| Mutated | 43 (32) |
| Unmutated | 92 (68) |
| Missing | 137 |
| FISH | |
| 13q- | 28 (25) |
| Negative | 28 (25) |
| Trisomy 12 | 35 (31) |
| 11q- | 13 (12) |
| 17p- | 9 (8) |
| Missing | 159 |
| Number of PET scans/patient | |
| Mean (SD) | 1.9 (1.9) |
| Median (range) | 1 (1–11) |
Table 2.
Description of 526 PET-CT Scans in 272 CLL Patients
| N (%) | |
|---|---|
|
| |
| CLL status prior to PET-CT scan | |
| Pre-CLL diagnosis | 20 (3.8) |
| CLL Untreated | 143 (27.2) |
| CLL Remission | 163 (31.0) |
| CLL Relapsed/Progressive | 183 (34.8) |
| Unknown | 17 (3.2) |
| Secondary Diagnosis prior to PET-CT scan | |
| None | 247 (47.0) |
| DLBCL | 115 (21.9) |
| Skin cancer | 57 (10.8) |
| Non-hematological malignancy | 53 (10.1) |
| Other lymphoma | 31 (5.9) |
| Hodgkin’s lymphoma | 19 (3.6) |
| Infection | 3 (0.6) |
| Lung transplant | 1 (0.2) |
| Indication for PET-CT scan | |
| Restaging CLL | 220 (41.8) |
| Restaging other malignancy | 93 (17.7) |
| Restaging DLBCL | 81 (15.4) |
| Staging CLL – initial evaluation | 73 (13.9) |
| Staging DLBCL | 11 (2.1) |
| Evaluation of a new lesion | 27 (5.1) |
| Staging other malignancy | 9 (1.7) |
| Restaging Hodgkin’s lymphoma | 8 (1.5) |
| Other | 4 (0.8) |
| PET-CT scan Result | |
| No abnormalities | 54 (10.3) |
| No FDG avid lesions | 88 (16.7) |
| SUV<5 | 264 (50.2) |
| SUV≥5 | 120 (22.8) |
| Consequences of PET-CT | |
| Additional imaging | 1 (0.2) |
| Biopsy | 126 (24.0) |
| Additional testing NOS | 22 (4.2) |
| Consult | 16 (3.0) |
| Observation | 176 (33.5) |
| Treatment | 185 (35.0) |
DLBCL diffuse large B cell lymphoma, NOS not otherwise specified
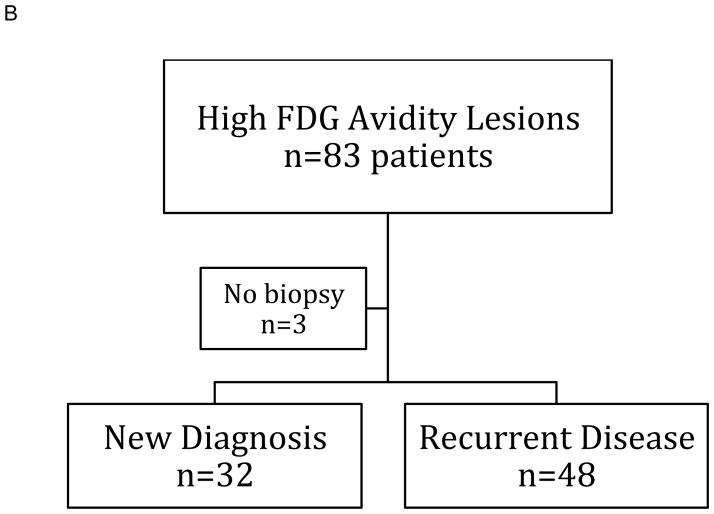
Figure 2. Classification of PET-CT Scans.
A: One hundred and twenty of 526 PET scans performed in 272 patients showed high FDG avidity lesions in 83 patients.
B: Eighty patients underwent biopsies of their high FGD avidity lesions resulting in a new diagnosis in 32 patients.
The 120 scans with high FDG avidity were performed on 83 patients and 80 (96%) of these patients underwent 86 biopsies (Figure 2B and Table 3). Fifty-seven (66%) of these biopsies resulted in a new diagnosis or showed highly FDG-avid CLL for the first time (Table 3). Three patients were either treated without a definitive tissue diagnosis or had palliative care. Thirty-four PET-CT directed biopsies in 32 patients were diagnostic of a new disease process (diffuse large B cell lymphoma (DLBCL) n=13, non-hematological malignancies n=8, CLL n=4, Hodgkin lymphoma n=2, colon adenoma n=1, herpes simplex infection n=1, non caseating granulomas n=1, Pneumocystis pneumonia n=1, EBV-positive lymphoproliferative disorder n=1, cellulitis n=1, benign thyroid nodule n=1). Fifty-two biopsies in 48 patients were compatible with a previous diagnosis including recurrence of non-CLL disease (26 non-CLL, 26 CLL). The 26 previously known non-CLL diagnoses were non-hematological malignancies (n=17), DLBCL (n=6), EBV-positive lymphoproliferative disorder (n=2), and Hodgkin lymphoma (n=1).
Table 3.
Histological Diagnosis of High FDG Avidity Tissue (SUV≥5)
| Diagnosis | Biopsies (%) | New diagnoses* |
|---|---|---|
| Hematological Malignancy | ||
| CLL | 30 (35) | 28** |
| Diffuse large B cell lymphoma | 19 (22) | 13 |
| Hodgkin’s lymphoma | 3 (4) | 3 |
| EBV+ lymphoproliferative disorder | 3 (4) | 1 |
| Non-Hematological Malignancy | ||
| Carcinoma | 19 (22) | 6 |
| Malignant Melanoma | 6 (7) | 0 |
| Infection | ||
| Pneumocystis pneumonia | 1 (1) | 1 |
| Herpes simplex adenitis | 1 (1) | 1 |
| Cellulitis | 1 (1) | 1 |
| Inflammatory | ||
| Non caseating granuloma | 1 (1) | 1 |
| Other | ||
| Adenoma (colon) | 1 (1) | 1 |
| Benign thyroid nodule | 1 (1) | 1 |
EBV+ Epstein Barr Virus positive
Number of biopsies
First detection of highly FDG avid CLL including initial diagnosis of CLL
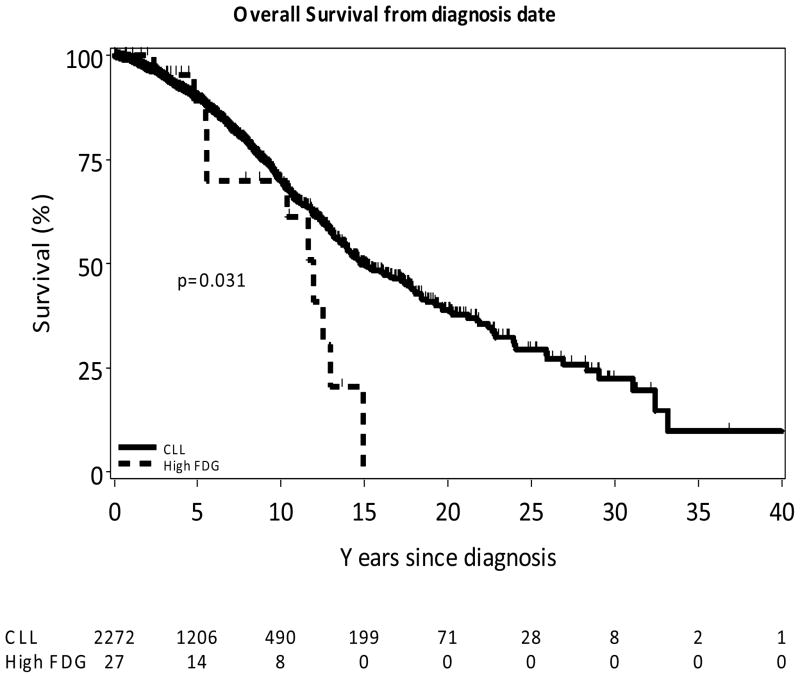
Of the 80 patients with high FDG avidity scans, 27 (34%) had 30 biopsies of the high avidity sites (27 lymph node biopsies and three biopsies of extranodal tissue) that showed CLL. In four of these patients, this was the biopsy that first diagnosed their CLL. An additional two 2 patients had high FDG avidity CLL diagnosed on biopsy of a lesion detected on a PET scan done for re-evaluation of a non-CLL second malignancy. In two patients high FDG avidity CLL was detected in enlarged lymph nodes that previously had low FDG avidity, suggesting a change in the metabolic activity of the CLL cells in that tissue. Of the 27 lymph node biopsies in 25 patients, four specimens were no longer available for review and six (including three fine needle aspiration biopsies) were not adequate for evaluation for proliferation centers. In one patient the initial fine needle aspiration biopsy was not diagnostic, but a subsequent excisional biopsy showed CLL. Among the 16 (64%) patients with suitable lymph node biopsies for evaluation, 13 (81%) exhibited prominent proliferation centers. The extranodal sites with high FDG avidity CLL masses were the nasopharynx (with destruction of the adjacent maxillary bone), lung, and cecum. Among the 24 patients with FISH analysis data, there was a significantly higher frequency with 17p13 deletion (n=5, 20.8%) compared to the other CLL patients with FISH data (49/1074, 4.6%; p=0.005). In contrast, 11q22 deletion detected in an additional 5 (20.8%) patients did not occur at a statistically significantly higher frequency than in the rest of the CLL population with FISH data (97/1074, 9.0%; p=0.06). Only two patients had suitable material for TP53 exons 4–9 analysis by conventional sequencing. One of these patients, who also had 17p13 deletion, had a TP53 mutation in the remaining allele that was predicted to be dysfunctional. Among patients with additional biological prognostic marker data, those with high FDG avidity CLL had significantly higher rates of poor prognosis markers compared to other CLL patients: Unmutated (<2%) IGHV (8/8, 100% vs. 607/1323, 45.9%; p<0.01); ZAP-70 expression (≥20%) (14/18, 77.8% vs. 661/1679, 39.4%, p=0.0009); and CD38 expression (≥30%) (16/22, 72.7% vs. 607/1939, 31.3%, p<0.0001). Ten patients with high FDG avidity CLL tissue, including two of the three patients with high FDG avidity lymph nodes without prominent proliferation centers, had B symptoms. The median time from the diagnosis of CLL to the first PET scan showing highly FDG avid CLL was 1.4 years (range 0–14.4) compared to the median time from diagnosis of CLL to PET scans that showed only CLL tissue with lower FDG avidity of 4.8 years (range 0–17)(p=0.053). The median survival from the first demonstration of high FDG avidity CLL was 4.6 years. The 27 patients with high FDG avidity CLL had a shorter median overall survival (OS) from the time of diagnosis of CLL compared to the rest of the CLL patient population (n=2272) studied (11.9 vs.14.8 years p=0.03)(Figure 3). Detection of high FDG avidity CLL is thus associated with more aggressive disease and poorer prognosis.
Figure 3. Patients with High FDG Avidity CLL Have Decreased Survival.
The 27 patients with biopsy proven high FDG avidity CLL tissue had a significantly shorter median overall survival from the time of diagnosis of their CLL compared to the rest of the CLL population (n=2272) (11.9 years vs. 14.8 years p=0.03).
Twenty-two patients with CLL and clinical features suspicious for complications had negative PET-CT scans and did not undergo non-bone marrow tissue biopsies as part of their evaluation. One of these patients was subsequently diagnosed with peripheral T-cell lymphoma restricted to his bone marrow. This finding is compatible with previous reports that FDG PET-CT has high sensitivity for detection of more aggressive lymphoma at extranodal sites except the bone marrow, for which sensitivity is low.[21] Based on this data, a negative PET scan was at least 96% sensitive for highly FDG avid complications of CLL.
CT-PET scans (n=293 scans, 55.7% of all scans) were also used for routine management of the 199 (73.2%) patients who did not have clinical features of complications of their CLL. These scans were used for initial staging of CLL at diagnosis (n = 73), and for evaluation of disease progression and response to treatment (n = 220). The PET component of these scans provided clinically useful information in only one patient who had high FDG avidity conjunctival tissue that was subsequently found to be involved by malignant melanoma. In all the other studies, any clinical value of the scans was restricted to the CT component.
Five hundred and twenty-six PET-CT scans were scored for clinical utility based on both the PET and CT scan components of the study as detailed in the methods section. One hundred and ninety four (36.9%) were judged to be critically helpful and 327 (62.2%) moderately helpful. However, as detailed above, the PET scan component of the study was only useful in 1 of 292 scans done for routine monitoring of their CLL and in 291 scans, only the CT component was of clinical value. Eleven (2.1%) scans were considered to be neutral or not helpful. No PET-CT scan results were considered to have a negative impact on individual patient care but this study did not specifically evaluate the adverse consequences or cost of these tests.
Discussion
We conducted an observational study using prospectively collected data on a large population of patients with CLL at a single institution to evaluate the clinical utility of PET-CT scans in the diagnosis and management of CLL and its complications. Our data show that targeted biopsies of high FDG avidity (SUV≥5) tissue in patients with CLL are highly informative and that patients with lesions that have low or no FDG avidity (SUV<5) have a very low probability of more aggressive lymphoma, non-hematological malignancies or serious tissue infections. Although CLL cells usually have low FDG avidity, 9.9% of patients with an established diagnosis of CLL undergoing a biopsy of lesion with a SUV≥5 were found to have high FDG avidity CLL. These patients had a high frequency of prominent proliferative centers in lymph nodes, symptomatic disease, and adverse molecular prognostic factors, and a poorer overall survival. Routine use of PET scans for staging and disease re-evaluation of patients with CLL without clinical features of complications was rarely (<0.1% of cases) useful. We propose that PET-CT scans are indicated in evaluation of patients with CLL who have clinical findings suggestive of transformed or complicated disease, and that PET scans should not be used for routine management of CLL.
PET scans are very sensitive for the detection of aggressive lymphomas, including those that occur as secondary lymphoid malignancies in patients with CLL, many non-hematological malignancies, as well as infection and inflammation of solid organs. Because of this wide differential diagnosis, the finding of high FDG avidity tissue in a patient with CLL is non-specific and a definitive diagnosis requires further investigation that frequently includes a surgical biopsy. In contrast, our study shows that patients without highly FDG avid tissue on a PET scan have a low probability of these complications. These findings are similar to previously published studies showing that the negative predictive value of a PET-CT scan for Richter’s transformation was 97%[11] and that PET-CT is useful for evaluation of patients with CLL suspected of having transformed lymphoma or other malignancies.[12]
High FDG avidity CLL was diagnosed by biopsy in 27 patients. Most of the evaluable lymph node biopsies had prominent proliferation centers, which has previously been shown to be associated with shorter survival than patients with smaller or absent proliferation centers.[13,14] The frequency of large and often confluent proliferation centers in these lymph nodes could increase the risk of sampling error and misleading diagnoses of DLBCL on tissue obtained by fine needle biopsies. This finding supports the use of excision or wide incision biopsies in these patients. Patients with high FDG avidity CLL have an increased frequency of high-risk prognostic factors and poorer overall survival, which is compatible with a recent report of increased glucose metabolism in CLL cells obtained from high-risk compared to low-risk patients.[22] However, additional studies would be required to determine if PET scans provide independently useful prognostic data in patients with CLL.
In this study we found that most PET-CT scans in CLL patients were used for routine initial evaluation, determination of treatment response, and surveillance despite the absence of any published data to support this practice. The PET components of these scans were rarely clinically useful, are expensive, and can have adverse consequences in some patients. We would thus strongly recommend that PET scans should not be used for routine evaluation of patients with CLL.
This study provides novel data on a large population of patients with CLL seen at a single large center using standard diagnostic criteria and prognostic testing with uniform prospective data collection and analysis. The limitations of the study include the retrospective collection of some of the data, non-uniform clinical criteria for ordering PET-CT scans, the inability to review all the pathological material, and insufficient pathological material to do more detailed analysis of the highly FDG avid CLL biopsies. However, the study does provide data that is relevant to clinical practice and could provide guidance on the appropriate use of an expensive but valuable resource in the clinical care of patients with CLL.
We conclude that PET-CT scanning has an important role in the clinical evaluation of patients with CLL who have clinical indicators of transformation, second malignancies or serious infections. PET scans that are negative for high FDG avidity tissue (SUV<5) are associated with a low probability of these complications, which supports our hypothesis that PET-CT scans are sensitive tests for the evaluation of patients with CLL for second malignancies and serious infections.
Acknowledgments
This study was supported by the Jackie S. Taylor Memorial Fund and the University of Iowa/Mayo Clinic Lymphoma SPORE (CA097274).
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Basu S, Kwee TC, Surti S, Akin EA, Yoo D, Alavi A. Fundamentals of PET and PET/CT imaging. Ann N Y Acad Sci. 2011;1228:1–18. doi: 10.1111/j.1749-6632.2011.06077.x. [DOI] [PubMed] [Google Scholar]
- 2.Ansell SM, Armitage JO. Positron emission tomographic scans in lymphoma: convention and controversy. Mayo Clin Proc. 2012;87:571–580. doi: 10.1016/j.mayocp.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer. 2006;107:175–183. doi: 10.1002/cncr.21967. [DOI] [PubMed] [Google Scholar]
- 4.Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leukemia & lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- 5.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23:145–153. doi: 10.1016/j.beha.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morice WG, Kurtin PJ, Hodnefield JM, et al. Predictive value of blood and bone marrow flow cytometry in B-cell lymphoma classification: comparative analysis of flow cytometry and tissue biopsy in 252 patients. Mayo Clin Proc. 2008;83:776–785. doi: 10.4065/83.7.776. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 11.Bruzzi JF, Macapinlac H, Tsimberidou AM, et al. Detection of Richter’s transformation of chronic lymphocytic leukemia by PET/CT Journal of nuclear medicine: official publication. Society of Nuclear Medicine. 2006;47:1267–1273. [PubMed] [Google Scholar]
- 12.Papajik T, Myslivecek M, Urbanova R, et al. F-FDG PET/CT examination in patients with chronic lymphocytic leukemia may reveal Richter’s transformation. Leukemia & lymphoma. 2013 doi: 10.3109/10428194.2013.802313. [DOI] [PubMed] [Google Scholar]
- 13.Gine E, Martinez A, Villamor N, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. 2010;95:1526–1533. doi: 10.3324/haematol.2010.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccone M, Agostinelli C, Rigolin GM, et al. Proliferation centers in chronic lymphocytic leukemia: correlation with cytogenetic and clinicobiological features in consecutive patients analyzed on tissue microarrays. Leukemia. 2012;26:499–508. doi: 10.1038/leu.2011.247. [DOI] [PubMed] [Google Scholar]
- 15.Dewald GW, Brockman SR, Paternoster SF, et al. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia. Br J Haematol. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 16.Vasmatzis G, Johnson SH, Knudson RA, et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood. 2012;120:2280–2289. doi: 10.1182/blood-2012-03-419937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschumper RC, Geyer SM, Campbell ME, et al. Immunoglobulin diversity gene usage predicts unfavorable outcome in a subset of chronic lymphocytic leukemia patients. J Clin Invest. 2008;118:306–315. doi: 10.1172/JCI32625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115:854–861. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 19.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 20.Shanafelt TD, Geyer SM, Bone ND, et al. CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: a prognostic parameter with therapeutic potential. Br J Haematol. 2008;140:537–546. doi: 10.1111/j.1365-2141.2007.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerusalem G, Beguin Y, Najjar F, et al. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin’s lymphoma (NHL) Ann Oncol. 2001;12:825–830. doi: 10.1023/a:1011169332265. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Weinberg JB, Davis ED, Volkheimer AD, Rathmell J. The metabolic signature of CLL: Enhanced glucose metabolism in a subset of high-risk CLL patients. Blood. 2012;120:Abstract 1785. [Google Scholar]