Abstract
The homotetrameric E. coli single stranded DNA binding (SSB) protein plays a central role in DNA replication, repair and recombination. E. coli SSB can bind to long single-stranded (ss) DNA in multiple binding modes using all four subunits ((SSB)65 mode) or only two subunits ((SSB)35 binding mode), with the binding mode preference regulated by salt concentration and SSB binding density. These binding modes display very different ssDNA binding properties with the (SSB)35 mode displaying highly cooperative binding to ssDNA. SSB tetramers also bind an array of partner proteins, recruiting them to their sites of action. This is achieved through interactions with the last 9 amino acids (acidic tip) of the intrinsically disordered linkers (IDLs) within the four C-terminal tails connected to the ssDNA binding domains. Here we show that the amino acid composition and length of the IDL affects the ssDNA binding mode preferences of SSB protein. Surprisingly the number of IDLs and the lengths of individual IDLs together with the acidic tip contribute to highly cooperative binding in the (SSB)35 binding mode. Hydrodynamic studies and atomistic simulations suggest that the E. coli SSB IDLs show a preference for forming an ensemble of globular conformations, whereas the IDL from Plasmodium falciparum SSB forms an ensemble of more extended random coils. The more globular conformations correlate with cooperative binding.
Keywords: DNA replication, DNA repair, cooperativity, regulation, simulations
Graphical abstract
Introduction
Single stranded DNA binding proteins (SSBs) play essential roles in all aspects of DNA replication, recombination and repair. They bind selectively and with high affinity to single-stranded (ss)DNA, protecting ssDNA intermediates and inhibiting formation of DNA secondary structures [1–4]. E. coli SSB (EcSSB) protein also serves as a central hub to regulate genome maintenance by interacting with at least fourteen metabolic proteins [5] (SSB interacting proteins (SIPs)).
EcSSB protein functions as a homotetramer, with each subunit (177 amino acids) comprised of two domains: an N-terminal DNA binding domain (112 amino acids), containing an oligonucleotide/oligosaccharide binding fold (OB fold), referred to here as the core, and a C-terminal domain composed of a flexible, intrinsically disordered (ID) linker (56 amino acids (Fig. 1a)) and a nine-residue motif (the “tip”) that is the primary site of interaction of SSB with the SIPs [5]. The tetrameric EcSSB binds ssDNA in multiple modes that differ in the number of subunits that contact DNA. Two of the major modes are denoted (SSB)35 and (SSB)65, where the subscripts indicate the average number of ssDNA nucleotides occluded per SSB tetramer [6, 7]. The relative stabilities of these modes depend on salt concentration and type [6, 7] as well as protein to DNA ratio [8–11]. In the (SSB)65 mode, favored at [NaCl]>0.2M or [Mg2+]>10mM, ~65 nucleotides of ssDNA wrap around all four subunits of the tetramer (see Fig. 1b), while displaying only “limited” cooperativity between adjacent tetramers [12–15]. In this mode SSB can diffuse along ssDNA [16, 17], enabling transient destabilization of DNA hairpins and RecA filament formation [16]. These activities are important for DNA repair and recombination.
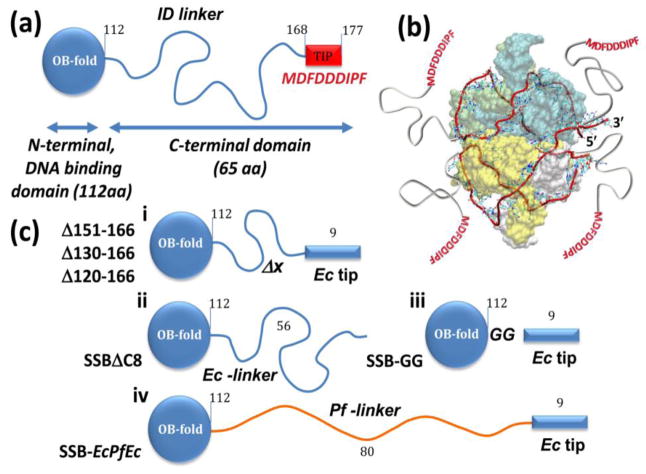
Figure 1. Structural organization of E. coli SSB linker variants.
(a) wt EcSSB subunits (177 aa) are comprised of an N-terminal DNA binding domain (OB-fold) (1-112 residues), and a C-terminal intrinsically disordered (ID) linker (56 aa) and a nine-residue acidic “tip”. (b) Structural model of 65 nucleotides of ssDNA (red ribbon) bound to the EcSSB tetramer [15] with the addition of the C-terminal tails (shown in grey) that are not observed in the crystal structure. (c) Design of SSB-linker variants containing the Ec core (1-112 aa), but varying C-terminal tails: missing part of the linker (i), the whole linker (GG, (ii)), acidic “tip” (SSBΔC8, (iii)) or replacing the Ec linker with the Pf. linker (EcPfEc, (iv)).
In the (SSB)35 mode, favored at [NaCl]<0.02 M or [Mg2+]<1 mM and high SSB to DNA ratios, ssDNA binds to an average of only two subunits of the tetramer, but with high cooperativity such that SSB clusters form on the ssDNA [6, 7, 9, 14]. This property is shared by the phage T4 gene 32 protein [18–20] and is believed to be important for its function during DNA replication [21]. In its (SSB)35 mode, SSB can also undergo direct transfer between separate DNA molecules [22] or intersegment transfer between distant sites within the same DNA molecule [23], an activity that may allow for SSB recycling during replication [22].
The four unstructured C-termini of EcSSB can also influence the relative stability of the SSB-ssDNA binding modes. SSB variants with only one or two C-terminal tails shift the binding mode preference toward the (SSB)35 mode [24]. The highly conserved, negatively charged “tip” of the SSB C-terminus can interact with the DNA binding site within the SSB core at moderate salt concentrations [25, 26] and compete for ssDNA binding. Removal of the C-terminal tip also favors the (SSB)35 binding mode [11, 25]. The (SSB)35 mode seems also to be favored when the C-terminal tip interacts with some SIPs (e.g. PriC [27] and PriA [28]). Interestingly, the similar Plasmodium falciparum (Pf) SSB tetramer, which possesses a C-terminal tail with a higher fraction of charged residues [29], does not form a stable (SSB)35 binding mode [30].
The regions responsible for highly cooperative binding of SSB tetramers to ssDNA have not previously been identified and little is known about the function of the SSB ID linkers. Here, we report the surprising result that highly cooperative binding of EcSSB to ssDNA in its (SSB)35 mode involves the C-terminal tails and is affected by the length, composition and number of IDLs and by the acidic tip. Complete removal of the linker or dramatic changes to its amino acid composition eliminates highly cooperative binding to ssDNA.
Results
SSB tail variants
Biochemical studies suggest that the C terminal tails (residues 113-177, see Fig. 1a) of E. coli SSB lack any substantial structure in its apo form and when bound to ssDNA [31]. The absence of electron density for residues beyond 112 in any of the EcSSB [15, 32, 33] and other SSB [29, 34] crystal structures further suggests that the C-terminal tails are intrinsically disordered. While the 9 amino acid tip of the EcSSB C-terminus is conserved among many bacterial SSBs [5], the IDL (56 amino acids for EcSSB (Fig. 1a)) varies in length from 25 to 135 aa (Fig. S4). In order to examine the effect of linker length and composition on the DNA binding properties of SSB we expressed and purified the SSB variants summarized in Fig. 1c.
We examined SSB variants with 16, 37, 47 and 54 amino acids deleted from the linker region (SSBΔ151-166, SSBΔ130-166, SSBΔ120-166 and SSBΔ115-168, respectively, (Fig. 1c)), while retaining the last 9 amino acids of the tip. The maximum deletion construct SSBΔ115-168 (lacking 54 aa), contains only two glycine residues between the DNA binding core and the tip and is designated hereafter as SSB-GG. SSBΔC8 has a full-length linker but lacks the last 8 amino acids of the tip [25]. SSB-EcPfEc has the Ec-core (1-112 a.a.) and the Ec-tip, connected by the linker from P. falciparum (Pf) SSB that is longer (80 amino acids) and has a higher fraction of charged residues than the E. coli linker [29]. The sequences of the E. coli and P. falciparum C-terminal regions, as well as those of the deletion variants are given in the supplemental material and aligned in Fig. S5.
Hydrodynamic properties of the SSB linker variants
We examined the assembly state of all SSB variants using sedimentation velocity at low and moderate salt concentrations (10 mM and 0.30 M NaCl)), conditions that favor the (SSB)35 or (SSB)65 binding modes, respectively, for wt EcSSB [6, 7]. All variants displayed a single, symmetric peak in a continuous sedimentation (c(s)) analysis consistent with a single species from which we can estimate molecular mass and frictional coefficient ratios [35] (Table 1). All variants form tetramers (±2.5kDa, data not shown). At 0.30 M NaCl the estimated frictional coefficient ratios (f/fo) increase with increasing linker length suggesting that the hydrodynamic radii increase with increasing linker length.
Table 1.
Hydrodynamic properties of SSB linker variants determined by sedimentation velocity (Buffer T, pH 8.1, 25°C).
| SSB variants | 0.3 M NaCl* | 10 mM NaCl* | ||
|---|---|---|---|---|
| s20, W | f/fo | s20, w | f/fo | |
| wtSSB | 4.16 | 1.60 | 4.56 | 1.46 |
| SSBΔC8 | 3.95 | 1.63 | ND | ND |
| SSBΔ151-166 | 4.24 | 1.47 | 4.44 | 1.44 |
| SSBΔ130-166 | 4.04 | 1.42 | ND | ND |
| SSBΔ120-166 | 3.97 | 1.36 | 4.01 | 1.36 |
| SSB-GG | 3.80 | 1.36 | 3.83 | 1.41 |
| SSB-EcPfEc | 4.39 | 1.76 | 4.23 | 1.89 |
The estimated uncertainties for the values of s20,W and f/fo are ±0.09 and ±0.03, respectively, based on the value ±0.10 (1SD) for s25,salt obtained from the global fits of the data.
At lower salt concentrations (10 mM NaCl) s20,w for wt SSB (4.56S) increases by 10% over its value (4.16 S) in 0.30 M NaCl corresponding to a increase in f/fo from 1.46 in 10 mM NaCl to 1.60 in 0.30 M NaCl. This greater compaction of the tails in 10 mM NaCl presumably results from interactions of the 9 aa tips with the DNA binding sites within the tetrameric core that is favored at lower salt concentrations [25, 26]. The fact that the SSB-GG variant that is completely missing the linker has nearly the same f/fo (1.41) as wtSSB in 10 mM NaCl also suggests that the linkers in the wtSSB are more compacted at 10 mM NaCl than at 0.30 M NaCl. However, the opposite effect of [NaCl] is observed for the SSB-EcPfEc construct, which shows a decrease in f/fo from 1.89 at 10 mM NaCl to 1.76 at 0.30 M, suggesting that the Pf-linker is more extended than the Ec linker at both [NaCl].
Atomistic simulations of the intrinsically disordered linkers
We used atomistic simulations based on the ABSINTH implicit solvation model [36] to assess the conformational properties of the linker variants. This approach has yielded accurate assessments of the sequence-encoded conformational properties for a range of intrinsically disordered proteins [37–39]. The central finding is that, as opposed to being non-descript flexible polymers, IDPs fall into distinct conformational classes and these sequence-to-conformation relationships are governed by coarse grain parameters that are dictated primarily by amino acid compositions and secondarily by the specific sequences.
Internal scaling profiles provide an assessment of the most likely value for the mean spatial separation 〈Rij〉 for pairs of residues i and j that are |j–i| apart in the linear sequence. In Fig. 2, EcEc and PfPf refer to sequences that include the C-terminal linker and tip from EcSSB and PfSSB, respectively. The SSB core was not included in the simulations.
Figure 2. Results from simulations summarizing the conformational properties of IDLs.
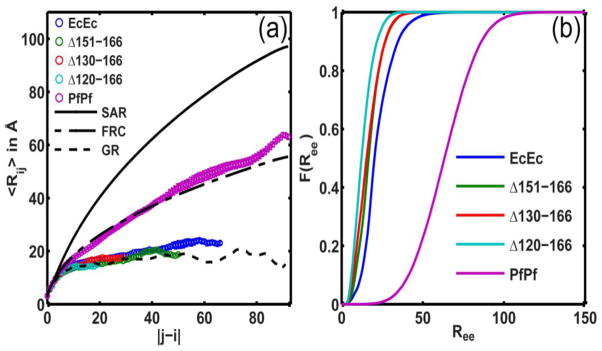
(a) Plot of the internal scaling profiles for the four EcEc linker tail variants and the PfPf linker + tail. The profiles for globular (GR), Flory random coils (FRC), and self-avoiding reference (SAR) ensembles are also shown for calibration. (b) Plot of the cumulative distribution functions (CDFs) of Ree values for each of the linker variants. The mean Ree values are 22.6 ± 0.7Å for EcEc, 17.8 ± 0.8Å for EcEc(Δ151-166), 16.8 ± 1.9Å for EcEc(Δ130-166), 13.7 ± 0.1Å for EcEc(Δ120-166) and 63.6 ± 0.9 Å for PfPf.
The conformational properties of EcEc are clearly distinct from those of PfPf (Fig. 2a). The EcEc sequence adopts compact globular conformations as evidenced by the plateauing behavior of 〈Rij〉 and the similarity of this profile to that of a maximally compact reference globule. The internal scaling profiles for the internal deletion variants, SSBΔ151-166, Δ130-166, and Δ120-166, are congruent with the profile for the wild type EcEc construct. Internal deletions within EcEc do not compromise the intrinsic preferences for compact globular conformations. In contrast, the internal scaling profile for PfPf conforms to that of a Flory random coil implying a preference for expanded and aspherical conformations.
Fig. 2b shows a set of six cumulative distribution functions for the end-to-end distance, Ree. These functions quantify the probabilities associated with realizing a Ree value that is less than or equal to some threshold. The probability of realizing a specific value for Ree shows minimal variation between EcEc and its internal deletion constructs. In contrast, the simulations predict that 〈Ree〉 is approximately four times larger for PfPf than for EcEc. These simulations suggest that the E. coli linkers yield compact, dense globules whereas the linker from P. falciparum forms expanded conformations whose properties are congruent with canonical Flory random coils. The EcEc sequence adopts compact globular conformations (Rg=12.5Å) whereas the conformational properties of the PfPf sequence are congruent with those of Flory random coils (Rg=24.5Å). Since Rg≈0.65Rh for globules and Rg≈Rh for FRCs the hydrodynamic size of the EcEc will be approximately 28% smaller than that of the PfPf. These results are compatible with the inferences drawn from the sedimentation velocity results.
DNA binding properties of the SSB linker variants
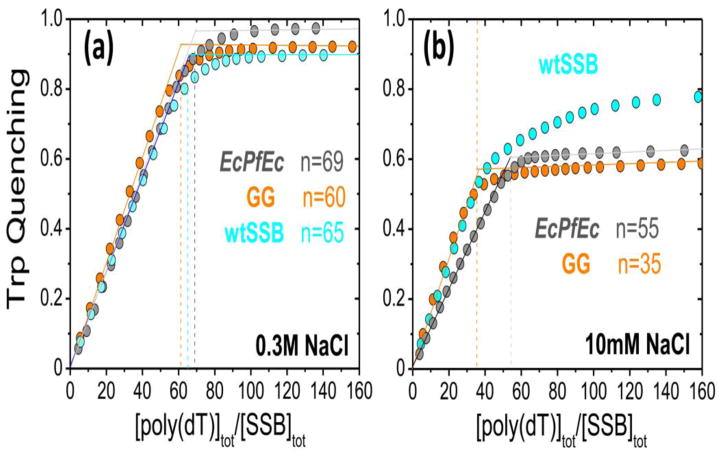
We examined the ssDNA binding mode preferences of the SSB linker variants to determine their occluded site sizes by monitoring the quenching of the SSB Trp fluorescence upon binding poly(dT) [6, 7]. At 0.30 M NaCl (Fig. 3a) the extents of Trp quenching (Qmax~90–95%) and occluded site sizes (nGG=60±2 nt and nEcPfEc =69±2 nt) suggest that the SSB-GG and SSB-EcPfEc variants form fully wrapped (SSB)65 complexes similar to wtSSB (nwt=65±2 nt). Experiments performed with the linker deletion variants and SSBΔC8 show the same behavior (Fig. S1-a).
Figure 3. Occluded site sizes of SSB linker variants on poly(dT).
Results of titrations of 0.30 μM of wtSSB (cyan), SSB-GG (orange) and SSB-EcPfEc (dark grey) with poly(dT) monitoring SSB Trp fluorescence quenching in buffer T, 25°C with (a) 0.30 M NaCl or (b) 10mM NaCl to estimate the occluded site sizes (n) for the SSB variants bound to poly(dT) [54].
At 10 mM NaCl wtSSB binds in its (SSB)35 binding mode with a 35 nt site size [6]. The SSB-GG variant with no linker also shows a 35 nt site size (nGG=35±2 nt; Qmax=0.59±0.01) (Fig. 3b). Interestingly, the SSB-GG variant displays a very stable (SSB)35 binding mode at 10 mM NaCl, in contrast to wtSSB that shows a slow re-equilibration to a higher site size mode upon further addition of poly(dT) due to the metastability of its (SSB)35 mode at low SSB to DNA binding densities [3, 6, 12]. The variants lacking only part of the linker (Δ120-166, Δ130-166 and Δ151-166) demonstrate binding behaviors that are similar to wtSSB (Fig. S1-b). At 10 mM NaCl the SSB-EcPfEc variant shows a larger occluded site size (n=55±2 nt; Fig. 3b). This is the same site size determined for the wt PfSSB under similar conditions [30]. This unexpected result suggests that the Pf linker, and not the Pf DNA binding core, prevents formation of the (SSB)35 binding mode. Therefore, total removal of the Ec linker favors the (SSB)35 mode, whereas replacement of the Ec linker with the Pf-linker favors formation of higher site size complexes.
wtSSB can form a high affinity fully wrapped 1:1 complex with (dT)70 at both low and high salt concentrations [11, 14, 40]. In this complex the (dT)70 interacts with all four subunits, quenching ~90% of the SSB Trp fluorescence. The wtSSB tetramer can also bind two molecules of (dT)35, but with a negative cooperativity that increases as the salt concentration decreases and this is partly responsible for formation of the (SSB)35 binding mode on poly(dT) at low [NaCl] [40, 41]. At 0.30 M NaCl (Fig. S2-a) both SSB-GG and SSB-EcPfEc form fully wrapped 1:1 complexes with (dT)70 as observed for wtSSB. Under conditions (2 M NaBr) that lower the affinity to a range such that the equilibrium constant can be measured accurately, the variants with shorter or no linkers show a 2–5 fold higher affinity than wtSSB for binding to (dT)70 (Fig. S2-c). Hence, even at high salt concentrations, the presence of the IDL lowers ssDNA binding affinity. However, at 10mM NaCl, SSB-GG does not form a 1:1 complex with (dT)70, but rather 2 SSB-GG tetramers bind to (dT)70, even at high DNA to protein ratios (Fig. S2-b), consistent with a very stable (SSB)35 binding mode as seen for binding to poly(dT) (Fig. 3b). Consistent with this observation, SSB-GG shows a much larger negative cooperativity for binding a second molecule of (dT)35 at both 0.3 M and 10 mM NaCl than does wtSSB (Fig. S2-d and e). The SSB-EcPfEc variant shows moderate negative cooperativity for binding of a second molecule of (dT)35 at 0.30 M NaCl (Fig. S2-d).
C-terminal linkers affect cooperative binding of SSB to ssDNA
E. coli SSB is able to bind with high cooperativity to ssDNA, resulting in formation of protein clusters on long ssDNA [12, 42–44], but only at low salt concentrations [12]. Highly cooperative binding requires the (SSB)35 binding mode; the (SSB)65 binding mode displays only a limited cooperativity [12–14]. However, the regions of SSB protein responsible for highly cooperative binding have not previously been identified.
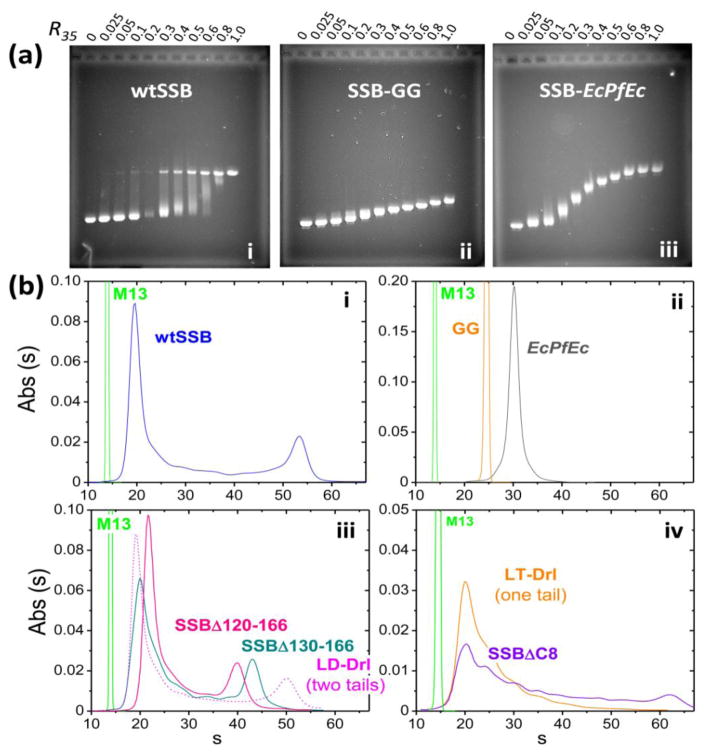
We used two qualitative methods to examine whether the SSB linker modifications affect highly cooperative binding of SSB to M13 mp18 phage ssDNA (~7.25 knt). The first is an agarose gel electrophoretic mobility shift assay (EMSA) [12] and the second is a sedimentation velocity assay. For a non-cooperative or low cooperative binding protein, all of the DNA in the population will have nearly the same amount of protein bound and thus the DNA migrates at the same rate in the gel and only one DNA band is observed for a given protein to DNA ratio. However, highly cooperative binding of wtSSB is evident as a bimodal distribution of ssDNA, resulting from some ssDNA molecules having near saturating amounts of SSB bound while other ssDNA has little bound protein [12].
The behaviors of the SSB variants lacking the linker (GG) or with the E. coli linker replaced by the Pf-linker (EcPfEc) were compared with wtSSB at low and moderate salt (10mM NaCl and 0.30 M NaCl). SSB-DNA complexes were formed at varying SSB to DNA ratios at the indicated NaCl concentration, equilibrated for 1 hour, and loaded onto the gel, followed by electrophoresis (see Materials and Methods). The hallmark of highly cooperative binding of wtSSB to ssM13 DNA at low salt (10 mM NaCl) is a bimodal distribution of ssDNA as shown in Fig. 4a-i, where at intermediate SSB to DNA ratios (e.g., R35 =0.3), free ssDNA exists in the same population as ssDNA that is nearly saturated with SSB [12]. As shown previously [12], this highly cooperative binding is eliminated at higher [NaCl] (0.30 M), where the ssDNA migrates as a single diffuse band at all SSB to DNA ratios (Fig. S3a-i). At 10 mM NaCl neither the SSB-GG (Fig. 4a-ii) nor the SSB-EcPfEc (Fig. 4a-iii) variant show bimodal distributions indicating the absence of highly cooperative binding. At 0.30 M NaCl low cooperativity binding patterns are observed for all of the variants as for wtSSB (Fig. S3-a). Each of the SSB variants, SSBΔ151-166, SSBΔ130-166 and SSBΔ120-166, in which parts of the SSB linker have been deleted, still show bimodal DNA distributions at 10 mM NaCl indicative of highly cooperative binding (Fig. S3-b).
Figure 4. Cooperativity of SSB linker variants bound to M13 ssDNA.
(a) - EMSAs of SSB-M13mp18 ssDNA complexes in buffer T, 10mM NaCl, 22°, formed at different protein/DNA ratios: R35=35[SSB]tot/[M13nts]tot. wtSSB (a-i) shows a bimodal distribution of bound DNA at intermediate ratios (R35=0.05–0.7) indicative of highly cooperative binding, whereas SSB-GG (a-ii) and SSB-EcPfEc (a-iii) show single band at all protein:DNA ratios indicative of low or no cooperativity. (b) - Sedimentation velocity profiles of SSB variants bound to M13 ssDNA at R35=0.3 (buffer T, 10mM NaCl, 25°C). Bimodal distribution of wtSSB-M13 complexes (b-i) indicates highly cooperative binding. SSB-GG and SSB-EcPfEc (b-ii) bind with low cooperativity. Intermediate linker deletion constructs SSBΔ130-166 and SSBΔ120-166, as well as two-tail variant LD-Drl retain high cooperativity (b-iii). SSB missing conserved acidic “tip” (SSBΔC8) and single tail SSB construct, LT-Drl, (b-iv) show decreased cooperativity (see Table S1 for quantification).
Since in the EMSA experiments the electrophoresis running buffer differs from the buffer in which the SSB-M13ssDNA complexes are formed it is possible that the change in salt conditions can affect distribution of the complexes during electrophoresis. For this reason we also examined cooperative binding using sedimentation velocity, analyzed using a c(s) distribution analysis [35]. At low salt concentration (10 mM NaCl) M13 ssDNA sediments as a slow moving (14.0 S), but sharp symmetrical peak in a c(s) distribution analysis (Fig. 4b, green).
We first examined M13ssDNA complexes with wtSSB formed in 10 mM NaCl at less than saturating protein to DNA ratios (Fig. 4b-i). At a low protein to DNA ratio (R35=0.3), where R35 is the fraction of ssDNA saturated by SSB if it were all to bind in its (SSB)35 binding mode, the c(s) profile shows a bimodal distribution of ssDNA, with ~16% of the ssDNA having a sedimentation coefficient ~53 S, indicating a high SSB binding density, whereas ~53% of the DNA sediments with s~20–24 S indicating little bound SSB (Table S1). The remainder of the ssDNA sediments between these two peaks reflecting a wide variation in the amount of SSB bound. At saturating concentrations of wtSSB, the ssDNA moves with a sedimentation coefficient of the high molecular weight complex, ~54 S (90% at R35=0.8). These results are qualitatively consistent with the EMSA results (Fig. 4A-i).
We next examined the SSB linker variants at a protein to ssDNA ratio of R35=0.3 at 10 mM NaCl. Fig. 4b-ii shows that the ssDNA bound with either SSB-GG (no linker) or SSB-EcPfEc (Pf-linker), sediments as single peaks with s ~24 S and ~30 S, respectively indicating the absence of high cooperativity. The SSBΔ130-166 and SSBΔ120-166 variants missing 37 and 47 amino acids from the linker, respectively (Fig. 4b-iii) display bimodal c(s) profiles similar to wtSSB indicating high cooperativity. The shift in peak position for the highly cooperative complexes of SSBΔ130-166 (43 S) and SSBΔ120-166 (40 S) relative to wtSSB (~53 S) reflects the lower molecular mass of the SSB due to the partial deletion of the linkers. We also examined the SSBΔC8 variant. The c(s) profile indicates that binding is cooperative at 10 mM NaCl (Fig. 4b-iv), however, the c(s) distribution is clearly less cooperative than wtSSB (the fraction of the highly cooperative species decreases from 16% to ~7%, Table S1). Hence, the acidic tip also contributes to cooperative binding.
We have previously described SSB variants in which either two subunits (SSB linked dimer-Drl) or all four subunits (SSB linked tetramer-Drl) are covalently linked [24]. The amino acid linker connecting the OB-folds in these constructs is from the D. radiodurans SSB, hence these variants are designated SSB-LD-Drl and SSB-LT-Drl, respectively [24]. These variants possess only two (SSB-LD-Drl) or one (SSB-LT-Drl) C-terminal tails, respectively [24] and we examined their cooperative binding at 10 mM NaCl (R35 = 0.3). Figure 4B-iii shows that the LD-Drl variant with two tails displays the bimodal ssDNA profile indicative of high cooperativity similar to wtSSB (16% highly cooperative species, Table S1). However, the LT-Drl variant with only one C-terminal tail does not display bimodal behavior, although it still binds with more cooperativity than SSB-GG (Fig. 4b-iv, Table S1). Hence, reducing the number of C-terminal tails to one significantly reduces cooperative binding to ssDNA.
In vivo complementation and UV sensitivity of SSB linker variants
We examined the ability of SSB variants to function in E. coli by testing their ability to complement the loss of wtSSB in vivo by using a complementation assay [24, 45]. Our results indicate that the genes expressing all of the SSB variants functionally complement the wtSSB gene in vivo, with the exception of SSBΔC8 as shown previously [46]. Therefore it appears that the presence of a C-terminal linker is not essential for E. coli growth. These results also indicate that highly cooperative binding of SSB to ssDNA is not essential for E. coli survival.
We also tested E. coli expressing the SSB variants for their ability to recover from DNA damage. E. coli cells expressing SSB linker variants were exposed to varying degrees of UV irradiation (see Materials and Methods). Exposure to UV irradiation leads to formation of DNA breaks and base damage including crosslinks [47]. Interestingly, the ability of cells to recover after UV-induced damage depends on the length of the linker (Fig. 5). While the cells expressing SSB variants with partial linker deletions (SSBΔ151-166, SSBΔ130-166 and SSBΔ120-166) show behavior similar to wtSSB, cells expressing SSB-GG or SSB-EcPfEc are much more sensitive to UV irradiation. This indicates that a linker of sufficient length and composition is important for some DNA repair pathways. Whether this is due to a need for cooperative binding or if the phenotype reflects a need for a minimum length tether remains to be determined.
Fig. 5. Sensitivity of E. coli strains carrying the SSB linker variants to UV irradiation.
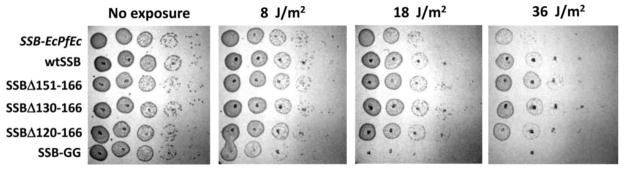
The sensitivity to uv irradiation of E. coli cells carrying the SSB linker deletion variants SSBΔ151-166, SSBΔ130-166 and SSBΔ120-166 is very similar to wtSSB, although sensitivity increases for the SSB-EcPfEc variant and even more for the SSB-GG variant.
Discussion
Cooperative binding to ssDNA of SSB proteins involved in DNA replication was first demonstrated for the phage T4 gene 32 protein [18–20, 48] and subsequently for the E. coli SSB protein [12, 42–44]. However, the regions of SSB protein responsible for highly cooperative binding remained unidentified, although it was hypothesized based on crystal structures that interactions between the L45 loops might be involved [15].
Although the DNA binding domain (OB-fold) and the 9 amino acid tip are essential for E. coli survival there has been little investigation of the function or properties of the 56 amino acid linker that connects the OB-fold and the tip. Our studies reveal several surprising results. First, complete elimination of the linker (SSB-GG) eliminates highly cooperative binding of SSB to ssDNA in vitro. Second, removal of the acidic C-terminal tip (last 9 amino acids) reduces, but does not eliminate cooperative binding. Third, an SSB variant with two C-terminal tails retains cooperative binding, whereas a one tail variant shows a reduction in cooperative binding. Fourth, replacement of the 56 amino acid EcSSB linker with the 80 amino acid Pf-linker (SSB-EcPfEc) eliminates the (SSB)35 binding mode and thus also cooperativity.
Based on these results, three components appear to influence highly cooperative binding of SSB to ssDNA. 1. It is necessary but not sufficient for SSB tetramers to be bound in the (SSB)35 mode; 2. The number of IDLs and their amino acid compositions are important; 3. the acidic tips of SSB tetramers also facilitate cooperative binding. Although the one tail variant loses its ability to bind with high cooperativity, it still shows some residual cooperativity. Interestingly, unlike the two-tailed variant, expression of the single-tailed SSB variant shows a dominant lethal phenotype. Single tailed SSB also shows defects in coupled leading and lagging strand replication and does not support replication restart in vivo [24].
The length of the linker region seems less important since linkers with lengths of 9, 19 or 40 amino acids still display highly cooperative binding. It is important to note that the internal deletions do not just reduce the length. They also change the amino acid composition although this does not impact the predilection for globular conformations as is evidenced by the localization of the sequences to the R1 region of the diagram-of-states shown in Fig. S4. The analysis of sequences from orthologs that is shown in Figure S4 indicates that there are several IDL sequences that are of similar length to that of the E. coli IDL albeit with increasing fraction of charged residues (FCR). The presence of these sequences affords the opportunity to assess the impact of increased FCR, and hence diminished stability of globules, on the cooperativity of ssDNA binding while keeping the tip sequence and the length of the IDL fixed. We find that the two-tailed SSB linked dimer, retains high cooperativity.
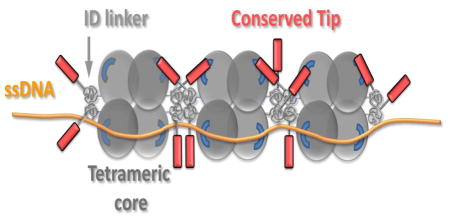
Previous studies have shown that at low to moderate salt concentrations, the 9 amino acid tips of the C-termini of SSB can interact with the ssDNA binding sites within the DNA binding core (OB-fold) and compete weakly for ssDNA binding [25, 26]. In the (SSB)35 binding mode an average of two ssDNA binding sites are unoccupied, hence, one possible mechanism for cooperative binding, depicted in Fig. 6, is that the 9 amino acid tip(s) from one (SSB)35-bound tetramer, interacts with a ssDNA binding site in an adjacent (SSB)35-bound tetramer, thus promoting clustering of SSB on the ssDNA. However, we also show that an SSB in which only the last 8 amino acids of the tip are deleted (SSBΔC8) still binds cooperatively, although it is reduced. Thus, the acidic tip and an IDL of appropriate amino acid composition contribute to cooperative ssDNA binding. The fact that an SSB with no linker, but retaining the acidic tip (SSB-GG) does not show cooperative binding may indicate the need for a minimal length IDL to allow the tip to reach an unoccupied ssDNA site on an adjacent SSB tetramer on the ssDNA. The fact that a one-tailed variant loses high cooperativity supports this proposal. Although E. coli can survive with an SSB lacking the linkers as long as the 9 amino acid tip is retained, our studies indicate that E. coli sensitivity to UV irradiation increases when the SSB linker is removed or its composition is changed. Hence, the linker composition/conformation is important for some DNA repair processes.
Figure 6. Model for highly cooperative ssDNA binding in the (SSB)35 mode.
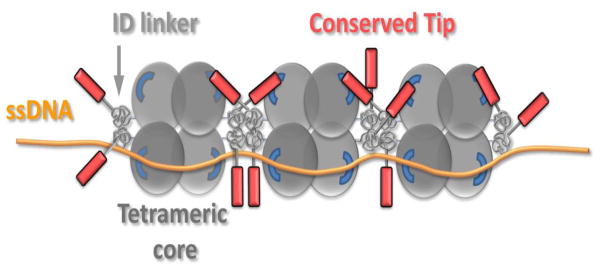
Binding of SSB to ssDNA in its (SSB)35 binding mode leaves DNA binding sites unoccupied in two subunits. Negatively charged tips of two C-terminal tails bind to the unoccupied subunits of adjacent tetramers. Positive cooperativity is enhanced due to inter-chain interactions between globular IDLs of adjacent tetramers.
Our computational studies suggest that the sequence of the disordered linker plus tip from E. coli should form heterogeneous distributions of globules and this is supported by hydrodynamic measurements. For strong polyampholytes such as the C-terminal linker plus tip from P. falciparum, the patterning of oppositely charged residues gives rise to conformations whose statistical properties are congruent with those of Flory random coils. Our survey of 134 bacterial SSB proteins indicates that the lengths of C-terminal linkers plus tips vary from 25 to 135 residues, with the large majority being 55–65 residues long. Based on their amino acid compositions, a majority of these sequences are classified as globule formers according to the diagram-of-states derived from recent studies that classify IDPs based on their amino acid compositions [39] (Fig. S4). We therefore expect that variations in linker lengths will not lead to significant changes in the overall dimensions of globule forming sequences. Further, since globule formation is a proxy for strong self-interactions [49], globule, as opposed to coil-forming linkers should promote intra- and inter-tetramer interactions involving IDLs that in turn promote positive cooperativity in ssDNA binding. We propose a model where self-associating linkers with acidic tips can mediate two types of interactions between adjacent SSBs (Fig. 6). These include interactions between globular linkers, which should be encouraged by high local concentrations, and previously documented interactions between acidic tips and OB cores. Upon ssDNA binding, the linker and tip-mediated interactions between SSBs might serve as “links in a chain” that facilitate the formation of high molecular weight clusters. The links, we propose, are strengthened by increasing the numbers of tails and this is consistent with data we report for the wtSSB versus the two- and one-tailed variants.
Although the SSB linker deletion variants examined here are not naturally occurring in E. coli, the C-terminal tails of bacterial SSB proteins have lengths that vary from ~25 to 135 amino acids (see Fig. S4), hence these deletion variants are of interest for the behavior of SSB proteins from those bacteria. Previous studies have indicated that the binding of the SSB interacting proteins (SIPs), PriC and PriA, destabilize the (SSB)65 binding mode in favor of the (SSB)35 binding mode [27, 28]. Based on the results reported here, it seems likely that the binding of SIPs to the SSB tails might tend to shift binding away from a cooperative mode, based on our result that reducing the number of tails to one reduces highly cooperative binding. However an SSB with four tails could still bind SIPs via two tails and retain highly cooperative binding to ssDNA.
Materials and Methods
Reagents and buffers
All buffers were prepared with reagent grade chemicals and water treated with a Milli Q (Millipore, Bedford, MA) water purification system. Buffer T is 10 mM Tris, pH 8.1 (25°C), 0.1 mM Na3EDTA. The gel electrophoresis buffer is 20 mM Tris-Acetate (pH 8.3), 0.5 mM Na3EDTA (Sigma-Aldrich, T4038-1L).
DNA and SSB tail variants
Oligodeoxynucleotides were synthesized and purified to ≥ 98% purity as described [14]. The poly(dT) (Midland certified reagent company, Midland, TX((Catalog #P-2004, Lot number 071308)), had an average length of ~ 1000 (Midland Certified Reagent Company). DNA samples were dialyzed extensively before use. Single stranded circular M13 mp18 DNA was purchased from New England Biolabs (Catalog #N4040S). DNA concentrations were determined spectrophotometrically: poly(dT), dT35 and dT70: ε260 = 8.1×103 M−1 (nucleotide) cm−1 [19]; M13 DNA: ε259 = 7370 M−1 cm−1 (nucleotide) [50].
SSBΔ151-166, SSBΔ130-166, SSBΔ120-166 and SSB-GG (see sequences in Supplemental Information) were generated with the Agilent Lightning Site-Directed Mutagenesis Kit using E. coli wtSSB with the native operon (cloned into pET21a) as the template. The SSB-EcPfEc construct was generated using the E. coli or P. falciparum SSB as a template. Mutants were expressed using an auto-induction protocol [51]. All variants were purified (>98% pure) as described [52, 53] and concentrations determined spectrophotometrically [6] (buffer T, 0.2M NaCl): ε280=1.13 × 105 M−1 cm−1 for wtSSB, SSBΔC8, SSBΔ151-166 and ε280=8.98 × 104 M−1 cm−1 for SSBΔ130-166, SSBΔ120-166, SSB-GG and SSB-EcPfEc.
Agarose gel electrophoresis
Agarose gel electrophoresis to examine the cooperativity of SSB-ssM13 DNA complexes was performed as described [12] using 0.5% agarose gels (14 cm horizontal gels). The DNA concentration was held constant (0.8–1.5 nmole nucleotides in 30μL of the sample), while varying the protein concentration under the solution conditions given in the text. Electrophoresis was carried out at room temperature (22° C) at constant voltage (~1 V/cm) for 3 to 3.5 h, after which the gel was soaked in a 1 M NaCl buffer T to dissociate bound SSB and the DNA was visualized by staining with ethidium bromide (2 μg/ml solution) and destained for 2–3 hrs at 4° C in buffer T without ethidium bromide.
Fluorescence measurements
Titrations of SSB constructs with ssDNA were performed by monitoring the intrinsic tryptophan fluorescence and analyzed as described [54].
Analytical sedimentation
Sedimentation velocity experiments were performed using an Optima XL-A analytical ultracentrifuge and an An50Ti rotor (Beckman Instruments, Inc., Fullerton, CA) as described [29]. Protein samples, 1.5 – 3 μM tetramer, were dialyzed versus appropriate buffer before scans. Experimental runs were performed at 42000 RPM (25°C) while monitoring absorbance at 280 nm (or 230–235 nm for low concentration samples). The traces were analyzed using the program SEDFIT [35] to obtain c(s) distributions and frictional coefficient ratios. The values of ῡi were calculated from the amino acid compositions using the program SEDINTERP. Values of s20,w were converted from fitted experimental values of s25,salt using corresponding values of viscosity and density and assuming that υ is constant.
The sedimentation velocity experiments with M13 phage ssDNA were performed at 15000 RPM (25°C) at constant DNA concentration (25 or 50 μM nucleotides) while varying protein concentration and monitoring absorbance at 260 nm. Protein/DNA ratios were calculated assuming a 35 nucleotide site size (R35=[P]/[M13]nts×35). The contribution of SSB absorbance at R35=0.3 is < 5%. Data were analyzed using SEDFIT [35].
In Vivo complementation experiments
SSB complementation experiments were performed as described previously [24, 45]. All experiments were repeated at least twice and plasmids were sequenced to confirm that no mutations occurred.
UV sensitivity experiments
RDP317 containing SSB mutants after the above passage series were tested for UV sensitivity. A colony was picked from a freshly streaked plate and grown overnight. Serial 10-fold dilutions were made and 5μ of the 10−2 through 10−6 were spotted onto LB agar plates containing kanamycin and ampicillin and allowed to dry into the plate before being exposed to UV. Plates were exposed to shortwave light (254nm, Mineral Light Lamp Model UVGL-25) set to 4 inches above for 6, 14 and 28 s or non-exposed. UV doses in Fig. 5 (8, 18 and 36 j/m2) were calculated from the exposure times based on an applied power of 1.3 W/m2, measured for a distance of 4 inches between the irradiated surface and the UV lamp.
Atomistic simulations
All simulations were performed using the CAMPARI modeling package (http://campari.sourceforge.net). The forcefield parameters were obtained from the abs_3.2_opls.prm file. All polypeptide atoms and solution ions were modeled in atomic detail. The sequences used for the simulations are:
AcGGRQGGGAPAGGNIGGGQPQGGWGQPQQPQGGNQFSGGAQSRPQQSAPAAPSNEPPMDFDDDIPFNme designated as EcEc;
AcGGRQGGGAPAGGNIGGGQPQGGWGQPQQPQGGNQFSGGPPMDFDDDIPFNme designated as EcEc(Δ151-166);
AcGGRQGGGAPAGGNIGGGPPMDFDDDIPFNme designated as EcEc(Δ130-166);
AcGGRQGGGPPMDFDDDIPFNme designated as EcEc(Δ120-166); and
AcDDKRNFNQRNNSNNINSENQQHINNEHINNNNINNGNDFMPLNSNDKIIEDKEFTDRLDDNNEENNFQSNSETFDKQEGIYDKMNVQEFEENme designated as PfPf.
Here, Ac and Nme refer to N-acetyl and N′-methylamide, which we use as capping groups for the N- and C-termini, respectively. Parameters for the mobile Na+ and Cl− ions are those of Mao and Pappu [55] The screening model was set to mode 2 and the long-range electrostatics correction model designated by the keyword LRER_MC was set to mode 1 for the PP sequence given its high FCR and this parameter for was set to mode 3 for the E. coli linker plus tip sequences. The degrees-of-freedom for the Metropolis Monte Carlo simulation were the backbone and side chain torsion angles, and rigid body translations and rotations of the proteins and solution ions. The effects of solvent-mediated interactions were modeled using the ABSINTH implicit solvation model and force field [36]. Spherical cutoffs of 10 Å and 14 Å were used for Lennard-Jones interactions and electrostatic interactions between atoms bearing partial charges that belong to neutral charge groups. No cutoffs were deployed for calculating the electrostatic interaction between atoms belonging to charge groups bearing a net charge.
In each simulation, the polypeptide plus solution ions were part of a spherical droplet and the diameter of the droplet was set to be equal to the contour length of the chain, which ensures the avoidance of confinement or finite size artifacts. Neutralizing counterions and excess ion pairs were included to mimic a NaCl concentration of 15 mM. To enhance conformational sampling, we used thermal replica exchange [56] with a schedule that is similar to that used in previous work [39]. For each sequence, we performed three independent replica exchange simulations. Each simulation comprises 6.1×107 independent Monte Carlo moves, with the results of the initial 106 moves being set aside as equilibration. Swaps between neighboring thermal replicas were attempted once every 5×104 steps. Snapshots were saved once every 5×104 steps whereas the polymeric properties were computed once every 500 steps and saved to disk once every 2×104 steps.
Supplementary Material
Highlights.
The length, composition and number of SSB C-termini affect ssDNA binding
C-termini are involved in highly cooperative binding to ssDNA
Intrinsically disordered linkers and tips of the C termini regulate cooperativity
Intrinsically disordered linkers of SSB adopt compact (globular) conformations.
Linker deletion increases E. coli sensitivity to UV DNA damage
Acknowledgments
We thank T. Ho for synthesis and purification of the oligodeoxynucleotides. This research was supported in part by the NIH (GM030498 to TML) and NSF (MCB 1121867 to RVP).
Abbreviations
- ssDNA
single stranded DNA
- SSB
single stranded DNA binding protein
- IDP
intrinsically disordered protein
- IDL
intrinsically disordered linker
- SIP
SSB interacting protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chase JW, Williams KR. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–36. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- 2.Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990;54:342–80. doi: 10.1128/mr.54.4.342-380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohman TM, Ferrari ME. Escherichia coli single-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527–70. doi: 10.1146/annurev.bi.63.070194.002523. [DOI] [PubMed] [Google Scholar]
- 4.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Shereda RD, Kozlov AG, Lohman TM, Cox MM, Keck JL. SSB as an organizer/mobilizer of genome maintenance complexes. Crit Rev Biochem Mol Biol. 2008;43:289–318. doi: 10.1080/10409230802341296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohman TM, Overman LB. Two binding modes in Escherichia coli single strand binding protein-single stranded DNA complexes. Modulation by NaCl concentration. J Biol Chem. 1985;260:3594–603. [PubMed] [Google Scholar]
- 7.Bujalowski W, Lohman TM. Escherichia coli single-strand binding protein forms multiple, distinct complexes with single-stranded DNA. Biochemistry. 1986;25:7799–802. doi: 10.1021/bi00372a003. [DOI] [PubMed] [Google Scholar]
- 8.Chrysogelos S, Griffith J. Escherichia coli single-strand binding protein organizes single-stranded DNA in nucleosome-like units. Proc Natl Acad Sci U S A. 1982;79:5803–7. doi: 10.1073/pnas.79.19.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith JD, Harris LD, Register J., 3rd Visualization of SSB-ssDNA complexes active in the assembly of stable RecA-DNA filaments. Cold Spring Harb Symp Quant Biol. 1984;49:553–9. doi: 10.1101/sqb.1984.049.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Bujalowski W, Overman LB, Lohman TM. Binding mode transitions of Escherichia coli single strand binding protein-single-stranded DNA complexes. Cation, anion, pH, and binding density effects. J Biol Chem. 1988;263:4629–40. [PubMed] [Google Scholar]
- 11.Roy R, Kozlov AG, Lohman TM, Ha T. Dynamic structural rearrangements between DNA binding modes of E. coli SSB protein. J Mol Biol. 2007;369:1244–57. doi: 10.1016/j.jmb.2007.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohman TM, Overman LB, Datta S. Salt-dependent changes in the DNA binding co-operativity of Escherichia coli single strand binding protein. J Mol Biol. 1986;187:603–15. doi: 10.1016/0022-2836(86)90338-4. [DOI] [PubMed] [Google Scholar]
- 13.Overman LB, Bujalowski W, Lohman TM. Equilibrium binding of Escherichia coli single-strand binding protein to single-stranded nucleic acids in the (SSB)65 binding mode. Cation and anion effects and polynucleotide specificity. Biochemistry. 1988;27:456–71. doi: 10.1021/bi00401a067. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari ME, Bujalowski W, Lohman TM. Co-operative binding of Escherichia coli SSB tetramers to single-stranded DNA in the (SSB)35 binding mode. J Mol Biol. 1994;236:106–23. doi: 10.1006/jmbi.1994.1122. [DOI] [PubMed] [Google Scholar]
- 15.Raghunathan S, Kozlov AG, Lohman TM, Waksman G. Structure of the DNA binding domain of E-coli SSB bound to ssDNA. Nature Structural Biology. 2000;7:648–52. doi: 10.1038/77943. [DOI] [PubMed] [Google Scholar]
- 16.Roy R, Kozlov AG, Lohman TM, Ha T. SSB protein diffusion on single-stranded DNA stimulates RecA filament formation. Nature. 2009;461:1092–7. doi: 10.1038/nature08442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou R, Kozlov AG, Roy R, Zhang J, Korolev S, Lohman TM, et al. SSB functions as a sliding platform that migrates on DNA via reptation. Cell. 2011;146:222–32. doi: 10.1016/j.cell.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberts B, Frey L, Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972;68:139–52. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- 19.Kowalczykowski SC, Lonberg N, Newport JW, von Hippel PH. Interactions of bacteriophage T4-coded gene 32 protein with nucleic acids. I. Characterization of the binding interactions. J Mol Biol. 1981;145:75–104. doi: 10.1016/0022-2836(81)90335-1. [DOI] [PubMed] [Google Scholar]
- 20.Giedroc DP, Keating KM, Williams KR, Konigsberg WH, Coleman JE. Gene 32 protein, the single-stranded DNA binding protein from bacteriophage T4, is a zinc metalloprotein. Proc Natl Acad Sci U S A. 1986;83:8452–6. doi: 10.1073/pnas.83.22.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman TM, Bujalowski W, Overman LB. E. coli single strand binding protein: a new look at helix-destabilizing proteins. Trends Biochem Sci. 1988;13:250–5. [PubMed] [Google Scholar]
- 22.Kozlov AG, Lohman TM. Kinetic mechanism of direct transfer of Escherichia coli SSB tetramers between single-stranded DNA molecules. Biochemistry. 2002;41:11611–27. doi: 10.1021/bi020361m. [DOI] [PubMed] [Google Scholar]
- 23.Lee KS, Marciel AB, Kozlov AG, Schroeder CM, Lohman TM, Ha T. Ultrafast Redistribution of E. coli SSB along Long Single-Stranded DNA via Intersegment Transfer. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antony E, Weiland E, Yuan Q, Manhart CM, Nguyen B, Kozlov AG, et al. Multiple C-terminal tails within a single E. coli SSB homotetramer coordinate DNA replication and repair. J Mol Biol. 2013;425:4802–19. doi: 10.1016/j.jmb.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlov AG, Cox MM, Lohman TM. Regulation of single-stranded DNA binding by the C termini of Escherichia coli single-stranded DNA-binding (SSB) protein. J Biol Chem. 2010;285:17246–52. doi: 10.1074/jbc.M110.118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su XC, Wang Y, Yagi H, Shishmarev D, Mason CE, Smith PJ, et al. Bound or free: interaction of the C-terminal domain of Escherichia coli single-stranded DNA-binding protein (SSB) with the tetrameric core of SSB. Biochemistry. 2014;53:1925–34. doi: 10.1021/bi5001867. [DOI] [PubMed] [Google Scholar]
- 27.Wessel SR, Marceau AH, Massoni SC, Zhou R, Ha T, Sandler SJ, et al. PriC-mediated DNA replication restart requires PriC complex formation with the single-stranded DNA-binding protein. J Biol Chem. 2013;288:17569–78. doi: 10.1074/jbc.M113.478156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharyya B, George NP, Thurmes TM, Zhou R, Jani N, Wessel SR, et al. Structural mechanisms of PriA-mediated DNA replication restart. Proc Natl Acad Sci U S A. 2014;111:1373–8. doi: 10.1073/pnas.1318001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antony E, Weiland EA, Korolev S, Lohman TM. Plasmodium falciparum SSB tetramer wraps single-stranded DNA with similar topology but opposite polarity to E. coli SSB. J Mol Biol. 2012;420:269–83. doi: 10.1016/j.jmb.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antony E, Kozlov AG, Nguyen B, Lohman TM. Plasmodium falciparum SSB tetramer binds single-stranded DNA only in a fully wrapped mode. J Mol Biol. 2012;420:284–95. doi: 10.1016/j.jmb.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams KR, Spicer EK, LoPresti MB, Guggenheimer RA, Chase JW. Limited proteolysis studies on the Escherichia coli single-stranded DNA binding protein. Evidence for a functionally homologous domain in both the Escherichia coli and T4 DNA binding proteins. J Biol Chem. 1983;258:3346–55. [PubMed] [Google Scholar]
- 32.Raghunathan S, Ricard CS, Lohman TM, Waksman G. Crystal structure of the homo-tetrameric DNA binding domain of Escherichia coli single-stranded DNA-binding protein determined by multiwavelength x-ray diffraction on the selenomethionyl protein at 2.9-A resolution. Proc Natl Acad Sci U S A. 1997;94:6652–7. doi: 10.1073/pnas.94.13.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savvides SN, Raghunathan S, Futterer K, Kozlov AG, Lohman TM, Waksman G. The C-terminal domain of full-length E-coli SSB is disordered even when bound to DNA. Protein Science. 2004;13:1942–7. doi: 10.1110/ps.04661904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arif SM, Vijayan M. Structural diversity based on variability in quaternary association. A case study involving eubacterial and related SSBs. Methods Mol Biol. 2012;922:23–35. doi: 10.1007/978-1-62703-032-8_2. [DOI] [PubMed] [Google Scholar]
- 35.Dam J, Schuck P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Methods Enzymol. 2004;384:185–212. doi: 10.1016/S0076-6879(04)84012-6. [DOI] [PubMed] [Google Scholar]
- 36.Vitalis A, Pappu RV. ABSINTH: a new continuum solvation model for simulations of polypeptides in aqueous solutions. J Comput Chem. 2009;30:673–99. doi: 10.1002/jcc.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao AH, Crick SL, Vitalis A, Chicoine CL, Pappu RV. Net charge per residue modulates conformational ensembles of intrinsically disordered proteins. Proc Natl Acad Sci U S A. 2010;107:8183–8. doi: 10.1073/pnas.0911107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao AH, Lyle N, Pappu RV. Describing sequence-ensemble relationships for intrinsically disordered proteins. Biochem J. 2013;449:307–18. doi: 10.1042/BJ20121346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das RK, Pappu RV. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proc Natl Acad Sci U S A. 2013;110:13392–7. doi: 10.1073/pnas.1304749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bujalowski W, Lohman TM. Negative co-operativity in Escherichia coli single strand binding protein-oligonucleotide interactions. II. Salt, temperature and oligonucleotide length effects. J Mol Biol. 1989;207:269–88. doi: 10.1016/0022-2836(89)90455-5. [DOI] [PubMed] [Google Scholar]
- 41.Lohman TM, Bujalowski W. Effects of base composition on the negative cooperativity and binding mode transitions of Escherichia coli SSB-single-stranded DNA complexes. Biochemistry. 1994;33:6167–76. doi: 10.1021/bi00186a016. [DOI] [PubMed] [Google Scholar]
- 42.Sigal N, Delius H, Kornberg T, Gefter ML, Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972;69:3537–41. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruyechan WT, Wetmur JG. Studies on the cooperative binding of the Escherichia coli DNA unwinding protein to single-stranded DNA. Biochemistry. 1975;14:5529–34. doi: 10.1021/bi00696a023. [DOI] [PubMed] [Google Scholar]
- 44.Schneider RJ, Wetmur JG. Kinetics of transfer of Escherichia coli single strand deoxyribonucleic acid binding protein between single-stranded deoxyribonucleic acid molecules. Biochemistry. 1982;21:608–15. doi: 10.1021/bi00533a002. [DOI] [PubMed] [Google Scholar]
- 45.Porter RD, Black S. The single-stranded-DNA-binding protein encoded by the Escherichia coli F factor can complement a deletion of the chromosomal ssb gene. J Bacteriol. 1991;173:2720–3. doi: 10.1128/jb.173.8.2720-2723.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curth U, Genschel J, Urbanke C, Greipel J. In vitro and in vivo function of the C-terminus of Escherichia coli single-stranded DNA binding protein. Nucleic Acids Res. 1996;24:2706–11. doi: 10.1093/nar/24.14.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonura T, Smith KC. Enzymatic Production of Deoxyribonucleic-Acid Double-Strand Breaks after Ultraviolet-Irradiation of Escherichia-Coli K-12. Journal of Bacteriology. 1975;121:511–7. doi: 10.1128/jb.121.2.511-517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lohman TM. Kinetics and Mechanism of Dissociation of Cooperatively Bound T4-Gene-32-Protein Single-Stranded Nucleic-Acid Complexes. 1. Irreversible Dissociation Induced by Sodium-Chloride Concentration Jumps. Biochemistry. 1984;23:4656–65. doi: 10.1021/bi00315a022. [DOI] [PubMed] [Google Scholar]
- 49.Pappu RV, Wang X, Vitalis A, Crick SL. A polymer physics perspective on driving forces and mechanisms for protein aggregation. Arch Biochem Biophys. 2008;469:132–41. doi: 10.1016/j.abb.2007.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berkowitz SA, Day LA. Molecular weight of single-stranded fd bacteriophage DNA. High speed equilibrium sedimentation and light scattering measurements. Biochemistry. 1974;13:4825–31. doi: 10.1021/bi00720a022. [DOI] [PubMed] [Google Scholar]
- 51.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–34. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Lohman TM, Green JM, Beyer RS. Large-scale overproduction and rapid purification of the Escherichia coli ssb gene product. Expression of the ssb gene under lambda PL control. Biochemistry. 1986;25:21–5. doi: 10.1021/bi00349a004. [DOI] [PubMed] [Google Scholar]
- 53.Bujalowski W, Lohman TM. Monomer-tetramer equilibrium of the Escherichia coli ssb-1 mutant single strand binding protein. J Biol Chem. 1991;266:1616–26. [PubMed] [Google Scholar]
- 54.Kozlov AG, Galletto R, Lohman TM. SSB-DNA binding monitored by fluorescence intensity and anisotropy. Methods Mol Biol. 2012;922:55–83. doi: 10.1007/978-1-62703-032-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao AH, Pappu RV. Crystal lattice properties fully determine short-range interaction parameters for alkali and halide ions. J Chem Phys. 2012;137:064104. doi: 10.1063/1.4742068. [DOI] [PubMed] [Google Scholar]
- 56.Mitsutake A, Sugita Y, Okamoto Y. Replica-exchange multicanonical and multicanonical replica-exchange Monte Carlo simulations of peptides. II. Application to a more complex system. Journal of Chemical Physics. 2003;118:6676–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.