Abstract
Objective
In order to better identify melanoma patients who are, at the time of primary melanoma diagnosis, at high risk of developing brain metastases, primary melanoma characteristics were examined as risk factors for brain metastasis development.
Methods
In a study of two patient cohorts, clinicopathological characteristics prospectively collected at primary cutaneous melanoma diagnosis for patients with/without brain metastasis were assessed in univariate and multivariate analyses using data from two prospectively-collected databases: Melanoma Cooperative Group (MCG) (1972–1982), and Interdisciplinary Melanoma Cooperative Group (IMCG) (2002–2009). Candidate risk factors were evaluated in association with time to brain metastasis via either the log-rank test or Cox proportional hazards regression analysis with/without considering competing risks.
Results
Out of 2341 total patients included in the study, 222 (9.5%) developed brain metastases (median follow-up: 98 months). The median time to brain metastases was 30.5 months, and the median survival time after brain metastases was 4 months. Increased hazard ratios (HR) for brain metastasis were found among thicker (logarithmic value in mm) (MCG – HR=1.97, P<0.0001; IMCG – HR=1.31, P=0.018), ulcerated (MCG –HR=1.93, P=0.01; IMCG – HR=3.14, P<0.0001), and advanced-stage (MCG – HR=2.08, P=0.008; IMCG – HR=2.56, P=0.0002) primary melanomas based on multivariate Cox regression analysis assuming the presence of competing risks.
Conclusions
Primary cutaneous melanoma thickness, ulceration, and stage were identified and validated as risk factors associated with time to melanoma brain metastasis.
Keywords: melanoma, neoplasm metastasis, brain neoplasms, competing risk
INTRODUCTION
Melanoma is the third most common cause of brain metastasis, surpassed only by lung and breast cancer [1]. Lung and breast cancer mortality rates have both decreased [2], yet there continues to be an increase in the melanoma death rate with brain metastases contributing to half of all melanoma-related deaths [3]. The clinical presentation of melanoma brain metastasis differs from that of lung and breast cancer brain metastasis as there is a higher incidence of multiple intracranial lesions and a greater hemorrhagic tendency [4,5]. Therefore, many melanoma patients with brain metastasis are not candidates for either surgical resection or stereotactic radiosurgery despite the potential survival benefits [6]. Even with optimal treatment, however, patients with melanoma brain metastasis have a median survival of only 3 to 6 months [7,8]. It is therefore critical to identify primary melanoma patients at high risk of progressing to brain metastasis early, preferably at the time of initial melanoma diagnosis.
Previous studies examining risk factors for melanoma brain metastasis development have suffered from various limitations. Studies, for example, which included melanoma brain metastasis patients treated predominantly by surgery may have resulted in selection bias given that the best surgical candidates have limited intracranial involvement, controlled extracranial disease, and a good performance status. Differences in the time point: primary [7–10] vs. metastatic [11] melanoma diagnosis at which potential risk factors were evaluated may have contributed to the divergent findings as well. Research conducted using population-based regional or national registries, in contrast, has the potential to produce more generalizable results; yet while demographic and primary melanoma characteristics are available, registries like the Surveillance, Epidemiology, and End Results (SEER) program do not collect data on metastasis status or metastatic sites following pathological diagnosis.
In this study of two patient cohorts, we attempted to minimize the limitations encountered by previous studies by identifying clinicopathological characteristics at initial melanoma diagnosis that are risk factors for brain metastasis development. We did so using multivariate survival models applied to prospectively-collected, individual patient data from over 2000 cutaneous melanoma patients. In addition to evaluating the robustness of selected brain metastasis risk predictors, we also sought to test their discriminatory power in distinguishing short vs. long time to brain metastasis from primary melanoma diagnosis.
PATIENTS AND METHODS
Study population and data
Our study cohorts were comprised of prospectively-accrued, cutaneous melanoma patients at New York University Medical Center, enrolled in either the Melanoma Cooperative Group (MCG) (November 1972–November 1982) [12] or the Interdisciplinary Melanoma Cooperative Group (IMCG) (August 2002–December 2009) [13]. 18 patients were accrued at Bellevue Hospital, while the remaining patients were accrued at NYU Clinical Cancer Center. MCG and IMCG patients were followed through October 1993 and December 2011, respectively. Informed consent was obtained from all patients at the time of enrollment.
Demographic and clinicopathologic information collected for all melanoma patients included age at pathological diagnosis, gender, primary tumor thickness (mm), ulceration status, mitosis (absent vs. present), histotype, anatomic site, first recurrence date and site, presence and date of brain metastasis and extracranial visceral metastases, and disease/vital status at last follow-up. All patients were then re-staged according to the 2009 American Joint Committee on Cancer (AJCC) guidelines [14].
Statistical analyses
All patients from the two cohorts with informed consent were eligible and included in the statistical analysis. Continuous variables were summarized using median and range and compared across groups using the Wilcoxon-Mann-Whitney test, and categorical variables were summarized by counts and proportions and compared using the Chi-square test. Time-to-brain metastasis was calculated from the date of initial melanoma diagnosis. Each potential risk factor was evaluated in association with brain metastasis-free survival via either the log-rank test or Cox proportional hazards regression analysis. Categorical variables included gender, primary tumor ulceration status, mitosis: present vs. absent, histologic subtype: nodular vs. superficial spreading/other, anatomic site: head/neck vs. other locations, and AJCC stage at pathological diagnosis: III/IV vs. I/II. Continuous variables included age at initial melanoma diagnosis and primary tumor thickness (logarithmic of mm). All potential risk factors eligible to be included in the multivariate Cox proportional hazards model were evaluated and selected by minimizing Akaike’s information criterion through a backward-forward selection. To evaluate the robustness of selected risk factors, the linear combination of model predictors weighted by regression coefficients in Cox models were used as a risk score for brain metastasis-free survival, defined as time to brain metastasis from primary melanoma diagnosis. An optimal cut-off point was selected to classify IMCG (discovery cohort) patient risk scores into low vs. high groups whereby the log-rank test statistic contrasting the two groups would be maximized [15,16]. The regression coefficients for brain metastasis-free survival and the cut-off point both obtained from the IMCG cohort were used to separate MCG (validation cohort) patient risk scores into low vs. high groups. Kaplan-Meier curves and the log-rank test were used to assess the differential brain metastasis-free survival profiles of the two groups.
Survival analysis was also performed assuming the presence of competing risks, defined as deaths not related to melanoma brain metastasis. Cumulative incidence functions between groups stratified by each potential risk factor were compared using Gray’s test [17], which accounts for competing risks. Multivariate evaluation of risk factors was performed using a semi-parametric Cox proportional hazards model for the subdistribution proposed by Fine and Gray [18]. Statistical analyses were performed using SAS (version 9.2) and R (version 2.13.1).
RESULTS
Demographic and primary tumor characteristics for MCG and IMCG patients are presented in Table 1, stratified by database and brain metastasis status. Brain metastasis patients similarly comprise 9% (90/1043) and 10% (132/1298) of patients in the MCG and IMCG cohorts, respectively at last follow–up (MCG median 4261 days, IMCG median 1362 days). In the MCG dataset, 88 of 90 (97.8%) patients with brain metastasis and 128 of 153 (83.7%) patients with other metastasis died during the follow up time. In the IMCG dataset, 121 of 132 (91.7%) patients with brain metastasis and 127 of 242 (52.5%) patients with other metastasis died during the follow-up time. In the two datasets, the median time to brain metastases was 30.5 months, and the median survival time after brain metastases was 4 months.
Table 1.
Demographic and primary tumor characteristics of 2341 cutaneous melanoma patients stratified by NYU melanoma database and brain metastasis status.
| Characteristic | MCG (1972–1982)
|
P c | IMCG (2002–2009)
|
P c | ||
|---|---|---|---|---|---|---|
| Brain metastasis (N = 90) | No brain metastasis (N = 953) | Brain metastasisa (N = 132) | No brain metastasisb (N = 1,166) | |||
| N (%) | N (%) | N (%) | N (%) | |||
| Age at pathological diagnosis (years) | .24 | .85 | ||||
| Median (Range) | 54 (16–88) | 52 (4–91) | 57 (27–86) | 59 (6–98) | ||
| Gender | .03 | .16 | ||||
| Male | 54 (60%) | 459 (48%) | 80 (61%) | 631 (54%) | ||
| Female | 36 (40%) | 494 (52%) | 52 (39%) | 535 (46%) | ||
| Primary tumor thickness (mm) | <.0001 | <.0001 | ||||
| Median (Range) | 2.70 (0.50–15) | 1.20 (0.10–14) | 2.24 (0.21–30) | 0.90 (0.10–30) | ||
| ≤1.00 | 12 (13%) | 418 (44%) | 19 (17%) | 638 (56%) | ||
| 1.01–4.00 | 54 (60%) | 447 (47%) | 67 (58%) | 405 (36%) | ||
| >4.00 | 24 (27%) | 88 (9%) | 29 (25%) | 89 (8%) | ||
| Primary tumor ulceration status | <.0001 | <.0001 | ||||
| Absent | 43 (48%) | 736 (79%) | 54 (48%) | 952 (84%) | ||
| Present | 46 (52%) | 191 (21%) | 59 (52%) | 180 (16%) | ||
| Primary tumor mitosis | .002 | <.0001 | ||||
| Absent | 19 (23%) | 353 (42%) | 18 (16%) | 446 (41%) | ||
| Present | 62 (77%) | 497 (58%) | 93 (84%) | 651 (59%) | ||
| Primary tumor histotype | .003 | <.0001 | ||||
| Superficial spreading | 52 (68%) | 685 (80%) | 40 (36%) | 678 (61%) | ||
| Nodular | 19 (25%) | 98 (11%) | 59 (53%) | 274 (25%) | ||
| Other | 5 (7%) | 75 (9%) | 12 (11%) | 154 (14%) | ||
| Primary tumor anatomic site | .24 | .0003 | ||||
| Head/neck | 13 (14%) | 136 (14%) | 33 (29%) | 171 (15%) | ||
| Trunk | 42 (47%) | 364 (38%) | 44 (38%) | 435 (38%) | ||
| Extremity | 35 (39%) | 453 (48%) | 38 (33%) | 527 (47%) | ||
| AJCC stage at pathological diagnosis | <.0001 | <.0001 | ||||
| I | 26 (29%) | 608 (64%) | 34 (26%) | 801 (69%) | ||
| II | 34 (38%) | 246 (26%) | 35 (27%) | 196 (17%) | ||
| III | 29 (33%) | 88 (9%) | 46 (35%) | 157 (13%) | ||
| IV | 0 (0%) | 4 (<1%) | 17 (13%) | 12 (1%) | ||
Abbreviations: MCG, Melanoma Cooperative Group; IMCG, Interdisciplinary Melanoma Cooperative Group; AJCC, American Joint Committee on Cancer
Number of patients may not sum to total due to unclassified/unavailable data.
17 unknown primaries
33 unknown primaries
By the Wilcoxon-Mann-Whitney test for continuous variables and the Chi-square test for categorical variables
The clinicopathological features of primary melanoma patients who later developed brain metastasis were compared with those of patients who did not develop brain metastasis. There was no statistically significant difference in age at pathological diagnosis between patients with and without brain metastasis in either the MCG or IMCG (P>0.05). Brain metastasis patients in the MCG and IMCG, however, presented with thicker primary melanomas as compared to non-brain metastasis patients in their respective cohort (P<0.0001, <0.0001). In particular, 27% and 25% of MCG and IMCG brain metastasis patients had primary tumors greater than 4.00 mm in thickness, respectively. Only 9% and 8% of MCG and IMCG patients without brain metastasis, in contrast, had primary melanomas thicker than 4.00 mm. Ulceration was more frequently present in the primary tumors of patients who progressed to brain metastasis as compared to those who did not develop brain metastasis in the MCG and IMCG (P<0.0001, <0.0001). 52% of the brain metastasis patients in both the MCG and IMCG had ulcerated tumors, whereas ulceration was present in just 21% and 16% of the primary tumors in non-brain metastasis patients from the MCG and IMCG, respectively. Statistically significant differences in AJCC stage at pathological diagnosis (P<0.0001, <0.0001), primary tumor mitosis (P=0.002, <0.0001) and histotype (P=0.003, <0.0001) were also observed between patients with and without brain metastasis in both the MCG and IMCG.
Univariate and multivariate Cox proportional hazards (PH) models were constructed using data from the MCG and IMCG. On univariate Cox PH analysis, primary tumor thickness (logarithmic value in mm), ulceration status, mitosis, histotype, anatomic site, and AJCC stage at pathological diagnosis were found to be statistically significant predictors for brain metastasis development among both MCG and IMCG patients (Table 2). Multivariate analysis of MCG and IMCG data continued to support the strong association between progression to brain metastasis and primary tumor thickness (logarithmic value in mm), ulceration status, and AJCC stage at pathological diagnosis (Table 3). Increased hazard ratios (HR) for brain metastasis development on multivariate analysis were observed for thicker (logarithmic thickness value) (MCG –HR=2.22, 95% CI 1.61–3.08, P<0.0001; IMCG – HR=1.40, 95% CI 1.11–1.76, P=0.004), ulcerated (MCG – HR=2.12, 95% CI 1.30–3.44, P=0.002; IMCG – HR=2.92, 95% CI 1.89–4.50, P<0.0001), and advanced-stage (MCG – HR=2.62, 95% CI 1.57–4.35, P=0.002; IMCG – HR=2.70, 95% CI 1.73–4.21, P<0.0001) primary melanomas. Of note, primary tumors located on the head/neck were associated with a statistically significant increased risk of brain metastasis when compared to those on other locations among IMCG patients only (HR=3.12, 95% CI 2.03–4.80, P<0.0001).
Table 2. Univariate analysis of clinicopathological variables at primary melanoma diagnosis associated with brain metastasis-free survival.
Cox proportional hazards analysis assuming the absence (A) and presence (B) of competing risks.
| Variable | Cox PH Analysis
|
Cox PH Analysis Under Competing Risk
|
||||||
|---|---|---|---|---|---|---|---|---|
| MCG | IMCG | MCG | IMCG | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR(95% CI) | P | HR(95% CI) | P | |
| Primary tumor thickness (mm)a | 3.29 (2.50–4.33) | <.0001 | 2.21 (1.78–2.54) | <.0001 | 2.81(2.2–3.58) | <.0001 | 1.99(1.69–2.34) | <.0001 |
| Primary tumor ulceration status | ||||||||
| Present vs. absent | 4.49(2.91–6.93) | <.0001 | 5.58(3.47–7.28) | <.0001 | 3.96(2.57–6.1) | <.0001 | 5(3.4–7.36) | <.0001 |
| Primary tumor mitosis | ||||||||
| Present vs. absent | 2.53 (1.48–4.35) | 0.0008 | 2.84 (.178–4.88) | <.0001 | 2.37(1.38–4.05) | 0.002 | 2.67(1.61–4.44) | 0.0002 |
| AJCC stage at pathological diagnosis | ||||||||
| III/IV vs. I/II | 5.91(3.76–9.30) | <.0001 | 5.63 (3.72–7.39) | <.0001 | 4.63(2.94–7.29) | <.0001 | 4.67(3.25–6.71) | <.0001 |
| Primary tumor histotype | ||||||||
| Nodular vs. superficial spreading/other | 2.97 (1.76–5.04) | <.0001 | 3.06 (2.01–4.24) | <.0001 | 2.74(1.62–4.64) | <.0001 | 2.83(1.91–4.19) | <.0001 |
| Primary tumor anatomic site | ||||||||
| Head/neck vs. others | 1.18(0.65–2.13) | 0.59 | 2.16(1.44–3.24) | 0.0004 | 1.11(0.61–2.00) | 0.74 | 2.03(1.33–3.10) | 0.001 |
| Gender | ||||||||
| Male vs. female | 1.88 (1.20–2.94) | 0.005 | 1.26(0.89–1.79) | 0.200 | 1.81 (1.16–2.82) | 0.009 | 1.24(0.87–1.77) | 0.24 |
Table 3. Multivariate analysis of clinicopathological characteristics at primary melanoma diagnosis associated with brain metastasis-free survival.
Cox proportional hazards analysis assuming the absence (A) and presence (B) of competing risks.
| Variable | Cox PH Analysis
|
Cox PH Analysis Under Competing Risk
|
||||||
|---|---|---|---|---|---|---|---|---|
| MCG | IMCG | MCG | IMCG | |||||
| HR(95% CI) | P | HR(95% CI) | P | HR | P | HR | P | |
| Primary tumor thickness (mm)a | 2.22(1.61–3.08) | <.0001 | 1.40(1.11–1.76) | 0.004 | 1.97(1.47–2.65) | <.0001 | 1.31(1.05–1.63) | 0.018 |
| Primary tumor ulceration status | ||||||||
| Present vs. absent | 2.12(1.30–3.44) | 0.002 | 2.92(1.89–4.50) | <.0001 | 1.93(1.16–3.21) | 0.01 | 3.14(2.02–4.89) | <.0001 |
| AJCC stage at pathological diagnosis | ||||||||
| III/IV vs. I/II | 2.62(1.57–4.35) | 0.0002 | 2.70(1.73–4.21) | <.0001 | 2.08(1.21–3.58) | 0.008 | 2.56(1.57–4.19) | 0.0002 |
| Primary tumor anatomic site | ||||||||
| Head/neck vs. others | 1.23(0.68–2.23) | 0.50 | 3.12(2.03–4.80) | <.0001 | 1.19(0.65–2.16) | 0.58 | 2.88(1.82–4.56) | <.0001 |
We further evaluated model robustness and predictive power through Kaplan-Meier analysis constructed using cutoff scores obtained from the Cox analysis. Specifically, to evaluate the robustness of selected predictors in multivariate models and the discriminatory power in distinguishing short vs. long brain metastasis-free survival based on clinicopathological characteristics at primary melanoma diagnosis, the linear combination of model predictors weighted by regression coefficients was used as a risk score. Of note, brain metastasis-free survival was used as a time-to-event outcome measure. A high vs. a low risk score corresponds to a short vs. a long time to brain metastasis, respectively from the time of initial melanoma diagnosis. We used the IMCG as the discovery cohort and the MCG as the independent validation cohort. A cut-off point was first selected to divide IMCG patient risk scores into low and high groups such that the log-rank test statistic for time to brain metastasis between the two groups was maximized. The Kaplan-Meier curves in Fig. 1A displayed a clear, marked separation and showed that the low-risk score group in the IMCG had longer brain metastasis-free survivals as compared to the high-risk score group (log-rank test, P<0.0001). The risk score method was then applied to separate MCG patients (validation cohort) into high and low risk score groups using the same regression coefficients from the Cox model and the same cut-off value from the IMCG. The resultant Kaplan-Meier curves (Fig. 1B) were also distinctly separated, demonstrating that the low-risk score group in the validation cohort had significantly longer time to brain metastasis when compared to the high-risk score group (P<0.0001).
Figure 1. Primary melanoma patients with high risk scores for developing brain metastasis based on demographic and primary tumor characteristics have worse brain metastasis-free survival.
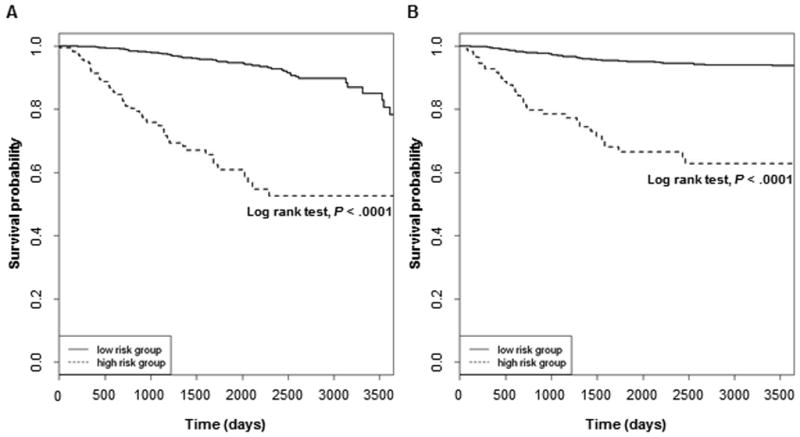
Kaplan-Meier estimates of brain metastasis-free survival for primary melanoma patients with low vs. high risk scores for brain metastasis development by the Cox proportional hazards model in the (A) IMCG (Discovery Cohort) and (B) MCG (Validation Cohort).
Cox regression analysis was also performed incorporating potential competing risk, namely death due to causes other than melanoma brain metastasis. On univariate analysis, most clinicopathological covariates remained strongly associated with brain metastasis development in the context of competing risks: logarithmic primary tumor thickness (MCG: HR=2.81, 95% CI 2.2–3.58, P<0.0001; IMCG: HR=1.99, 95% CI 1.69–2.34, P<0.0001), ulceration status: present vs. absent (MCG: HR=3.96, 95% CI 2.57–6.1, P<0.0001; IMCG: HR=5.00, 95% CI 3.4–7.36, P<0.0001), mitosis: present vs. absent (MCG: HR=2.37, 95% CI 1.38–4.05, P=0.002; IMCG: HR=2.67, 95% CI 1.61–4.44, P=0.0002), AJCC stage at initial melanoma diagnosis: III/IV vs. I/II (MCG: HR=4.63, 95% CI 2.94–7.29, P<0.0001; IMCG: HR=4.67, 95% CI 3.25–6.71, P<0.0001), and histotype: nodular vs. superficial spreading/other (MCG: HR=2.74, 95% CI 1.62–4.64, P<0.0001; IMCG: HR=2.83, 95% CI 1.91–4.19, P<0.0001) (Table 2). IMCG data, furthermore, supported the association between primary tumor anatomic site and brain metastasis development assuming the presence of competing risks (Table 2). On multivariate analysis assuming presence of competing risks, thicker (logarithmic value) (MCG – HR=1.97, 95% CI 1.47–2.65, P<0.0001; IMCG – HR=1.31, 95% CI 1.05–1.63, P=0.018), ulcerated (MCG – HR=1.93, 95% CI 1.16–3.21, P=0.01; IMCG – HR=3.14, 95% CI 2.02–4.89, P<0.0001), and advanced-stage (MCG – HR=2.08, 95% CI 1.21–3.58, P=0.008; IMCG – HR=2.56, 95% CI 1.57–4.19, P=0.0002) primary melanomas were jointly associated with increased risk of brain metastasis development (Table 3).
DISCUSSION
Early identification of primary melanoma patients at increased risk of brain metastasis is an ongoing prognostic challenge. Prior studies investigating melanoma brain metastasis risk factors have all suffered from limitations inherent to their study design. To our knowledge, this is the first cohort-based study to both identify and validate melanoma brain metastasis risk factors with multivariate models using prospectively-collected individual patient data from two independent cohorts of over 1000 primary melanoma patients each. On both univariate and multivariate analyses, we identified nearly the same statistically significant risk factors from the two independent datasets. The multivariate models also yielded essentially the same significant risk factors (predictors) with or without considering competing risks. To further evaluate the robustness of selected predictors in the multivariate Cox models, we examined the utility of linear predictors of Cox models to discriminate short vs. long time to brain metastasis based on clinicopathological characteristics at primary melanoma diagnosis using the IMCG as a discovery cohort and the MCG as the independent validation cohort. Even though MCG patients with brain metastasis were diagnosed more than two decades earlier than IMCG patients, the linear predictor of the multivariate Cox model obtained from IMCG patients performed well in discriminating patients with short from those with long time to brain metastasis profiles in the MCG cohort. This indicates the robustness of the selected risk factors and the existence of discriminatory power of the multivariate Cox models that are based on clinicopathological characteristics present at initial melanoma diagnosis.
Data from the IMCG but not the MCG supported the head/neck location as a risk factor for brain metastasis development when compared to other locations. A potential contributing factor is the temporal division between these two groups, the former including patients treated during the 2000s–2010s and the latter including patients treated during the 1970s–1990s. With the introduction of the sentinel lymph node biopsy in 1990, the standard of care for primary melanoma patients changed dramatically. Patients who previously underwent a lymph node dissection were now selected to undergo a staging procedure associated with less morbidity. Sentinel lymph node biopsies in head and neck melanomas, however, have a false-negative rate of 30% given the complexity of the lymphatic drainage patterns [19], and subsequent management decisions in these cases are based on inaccurate staging results with the potential to negatively affect patient outcomes like disease-free survival, overall survival, and even brain metastasis-free survival. Changes in the standard of care for primary melanoma patients may therefore have confounded our evaluation of the head/neck site as a risk factor for melanoma brain metastasis development.
Of the clinicopathological characteristics present at initial melanoma diagnosis, ulceration was shown to be a strong risk factor for brain metastasis development. Although ulceration status is correlated with primary tumor thickness and AJCC stage at pathological diagnosis, it remained statistically significant in multivariate models from both the MCG and IMCG that included primary tumor thickness, AJCC stage at pathological diagnosis, and primary tumor anatomic site as covariates. This suggests that, even though ulceration (as well as thickness) is partially factored into the AJCC staging, it remains an independent risk factor. Indeed, ulceration status also continued to be a significant predictor of brain metastasis development with similar hazard ratios in multivariate Cox regression models with or without considering potential competing risks, and there is evidence to suggest that ulcerated primary melanomas have distinct biological implications [20], especially for patients who present with tumors greater than 4 mm in thickness [21]. In a study of over 250 patients with thick (>4 mm) melanomas, not only did patients with ulcerated primaries have a significantly worse five-year overall survival rate compared to those with non-ulcerated tumors (34% vs. 54%), but also this significant difference persisted after controlling for thickness, mitotic rate, histotype, vascular involvement, and lymph node status [21].
Our study provides the foundation for a future, comprehensive brain metastasis-risk prediction assessment tool potentially incorporating baseline clinicopathological, molecular, genetic, and other predictors. Such a model would have major clinical significance in that it could help melanoma clinicians identify high-risk patients and plan treatment accordingly. In particular, there are efforts currently underway to develop and test targeted therapies specifically for patients with brain metastases [22], and a risk assessment model that can be applied at the time of primary diagnosis would help identify patients in need of such targeted therapies in the adjuvant setting. Our study also suggests a use for future research in this area, such as studies that identify clinicopathological differences in brain metastasis and other organ metastasis patients at the time of primary diagnosis.
In summary, our results showed that primary cutaneous melanoma thickness, ulceration, and stage were independently associated with time to melanoma brain metastasis. These findings demonstrate that melanoma brain metastasis risk models can be built using primary tumor characteristics with certain power to discriminate patients with short vs. long brain metastasis-free survival at the time of initial melanoma diagnosis.
Acknowledgments
Source of funding: This work was supported in part by the Department of Defense Peer Reviewed Cancer Research Program (W81XWH-10-1-0804), the NYU Cancer Institute Cancer Center Support Grant (5P30CA016087), and the Marc Jacobs Campaign to support melanoma research.
Footnotes
Conflicts of interest: None declared
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamberlain MC. Brain metastases: a medical neuro-oncology perspective. Expert Rev Neurother. 2010;10:563–573. doi: 10.1586/ern.10.30. [DOI] [PubMed] [Google Scholar]
- 4.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1:313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]
- 5.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210–216. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 6.Eigentler TK, Figl A, Krex D, Mohr P, Mauch C, Rass K, et al. Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer. 2011;117:1697–1703. doi: 10.1002/cncr.25631. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 8.Zakrzewski J, Geraghty LN, Rose AE, Christos PJ, Mazumdar M, Polsky D, et al. Clinical variables and primary tumor characteristics predictive of the development of melanoma brain metastases and post-brain metastases survival. Cancer. 2011;117:1711–1720. doi: 10.1002/cncr.25643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn-Cedermark G, Månsson-Brahme E, Rutqvist LE, Larsson O, Johansson H, Ringborg U. Central nervous system metastases of cutaneous malignant melanoma – a population-based study. Acta Oncol. 1998;37:463–470. doi: 10.1080/028418698430412. [DOI] [PubMed] [Google Scholar]
- 10.Daryanani D, Plukker JT, de Jong MA, Haaxma-Reiche H, Nap R, Kuiper H, et al. Increased incidence of brain metastases in cutaneous head and neck melanoma. Melanoma Res. 2005;15:119–124. doi: 10.1097/00008390-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Bedikian AY, Wei C, Detry M, Kim KB, Papadopoulos NE, Hwu WJ, et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. Am J Clin Oncol. 2011;34:603–610. doi: 10.1097/COC.0b013e3181f9456a. [DOI] [PubMed] [Google Scholar]
- 12.Kopf AW, Gross DF, Rogers GS, Rigel DS, Hellman LJ, Levenstein M, et al. Prognostic index for malignant melanoma. Cancer. 1987;59:1236–1241. doi: 10.1002/1097-0142(19870315)59:6<1236::aid-cncr2820590634>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Wich LG, Hamilton HK, Shapiro RL, Pavlick A, Berman RS, Polsky D, et al. Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research. Am J Transl Res. 2009;1:35–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman EB, Shang S, Miera EV, Fog JU, Teilum MW, Ma MW, et al. Serum microRNAs as biomarkers for recurrence in melanoma. J Transl Med. 2012;10:155. doi: 10.1186/1479-5876-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satzger I, Mattern A, Kuettler U, Weinspach D, Voelker B, Kapp A, et al. MicroRNA-15b represents an independent prognostic parameter and is correlated with tumor cell proliferation and apoptosis in malignant melanoma. Int J Cancer. 2010;126:2553–2562. doi: 10.1002/ijc.24960. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Saltman BE, Ganly I, Patel SG, Coit DG, Brady MS, Wong RJ, et al. Prognostic implication of sentinel lymph node biopsy in cutaneous head and neck melanoma. Head Neck. 2010;32:1686–1692. doi: 10.1002/hed.21390. [DOI] [PubMed] [Google Scholar]
- 20.Eggermont AM, Spatz A, Lazar V, Robert C. Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol. 2012;24:137–140. doi: 10.1097/CCO.0b013e32834fcb0d. [DOI] [PubMed] [Google Scholar]
- 21.Zettersten E, Sagebiel RW, Miller JR, 3rd, Tallapureddy S, Leong SP, Kashani-Sabet M. Prognostic factors in patients with thick cutaneous melanoma (> 4 mm) Cancer. 2002;94:1049–1056. [PubMed] [Google Scholar]
- 22.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]


