Abstract
Background
Prostate and thyroid cancers represent two of the most overdiagnosed tumors in the U.S. Hypothesizing that patients diagnosed with one of these malignancies were more likely to be diagnosed with the other, we examined the coupling of diagnoses of prostate and thyroid cancer in a large U.S. administrative dataset.
Methods
The SEER database was used to identify men diagnosed with clinically localized CaP or thyroid cancer between 1995 and 2010. SEER*stat software was used to estimate multivariable adjusted standardized incidence ratios (SIR’s) and investigate the rates of subsequent malignancy diagnosis. Additional non-urologic cancer sites were added as control groups.
Results
Patients with thyroid cancer were much more likely to be diagnosed with prostate cancer than patients in the SEER control group (SIR 1.28 [CI 1.1–1.5]; p<0.05). Similarly, the observed incidence of thyroid cancer was significantly higher in patients with CAP when compared with SEER controls (SIR 1.30 [CI 1.2–1.4]; p<0.05). When stratified by follow-up interval, the observed thyroid cancer diagnosis rate among men with CAP was significantly higher than expected at 2–11 (SIR 1.83 [CI 1.4–2.4]), 12–59 (SIR 1.24 [CI 1.0–1.5]), and 60–119 (SIR 1.25 [CI 1.0–1.5]) months of follow-up. There was no increased risk of CAP or thyroid cancer diagnosis among patients with non-urologic malignancies.
Conclusions
There is a significant association of diagnoses with prostate and thyroid cancer in the U.S. In the absence of a known biological link between these tumors, these data suggest that diagnosis patterns for prostate and thyroid malignancies are linked.
Keywords: prostate cancer, thyroid cancer, overdiagnosis, incidence
Introduction
While prostate cancer (CAP) is the most common noncutaneous cancer in the United States,1 the merits of widespread PSA-based screening are contested.2,3 CAP screening has led to a potential reduction in advanced disease and prostate cancer specific mortality,3 but diagnosis and overtreatment of clinically insignificant malignancy is a tremendous challenge for primary care providers, specialists, and men who undergo PSA-screening.4 Depending on the method of assessment, estimates of overdiagnosis range from 1.7% to 67%,4 and overtreatment is associated with a well-documented and significant burden of morbidity that affects quality of life.5–7 A similar trend exists for thyroid cancer, as diagnosis of small and indolent tumors has steadily risen. 8,9 Indeed, incidence of thyroid cancer has nearly tripled since 1975, while the mortality rate is largely unchanged.9
Second-primary malignancies account for approximately 18% of incident cancers in the United States,10 and the number of individuals who have undergone cancer treatment at some point in their lives is growing by 2% annually.11 The development of subsequent cancers can be attributed to a number of potential risk factors, which include receipt of radiation or chemotherapy, genetic predisposition, environmental exposures, endocrinologic alterations, and compromise of immune function.12,13 Increased long-term surveillance of individuals who have undergone cancer treatments influences subsequent cancer detection – a phenomenon known as surveillance bias.12–14,15–17 The type of care an individual receives may influence the likelihood of future cancer detection. For instance, exposure to urologic care for treatment of non-prostate-related malignancy significantly increases the likelihood of CAP detection, but not prostate cancer death.18
Because prostate and thyroid cancer are two of the most over-diagnosed malignancies in the United States, 4,19 it is possible that type, patterns, and intensity of care leading patients to be diagnosed with one of these cancers may result in discovery of the other. Hence we assessed the association of diagnoses of prostate and thyroid cancer in the U.S. using a large administrative dataset.
Material and Methods
The Surveillance Epidemiology and End Results (SEER) database of the National Cancer Institute was used to identify men diagnosed with clinically localized CAP or thyroid cancer between 1995 and 2010.20 SEER collects data on all individuals diagnosed with cancer residing in several geographically defined regions of the United States.21 We chose 12 registries based upon those with data available for all years of interest. The SEER registries include a broad spectrum of geographic regions and population densities within the United States, and include Detroit, Connecticut, Hawaii, Iowa, San Francisco-Oakland, Seattle, New Mexico, Utah, Atlanta, San Jose-Monterey, Los Angeles, and rural Georgia.
To measure the relative risk for prostate and thyroid cancer in patients treated for localized prostate or thyroid cancer compared with the general SEER registry population, we calculated standardized incidence ratio’s (SIRs) for each type of second and higher primary cancer (i.e. observed/expected) along with its 95% confidence interval (CI). The SEER*Stat Multiple Primary-SIR program (version 8.1.2)22 was used to calculate the SIRs.23 The SIR estimates were obtained by using the MP-SIR macro in SEER*Stat. SIRs greater and less than 1 reflect an increase and decrease in tumor incidence compared to what would be expected in the general population (after multivariable adjustment for gender, year of diagnosis, age, and race). Additional cancer diagnoses included as control groups to assess the generalizability of findings included Hodgkin and Non-Hodgkin lymphoma, colon/rectal, pancreas, lung/bronchus, bladder, and kidney cancers. To compute the excess risk of second cancers, that is, the average additional number of cancer patients per 10,000 cancer survivors per year, the expected number was subtracted from the number of observed cases, and the difference divided by the Person-years (PY) at risk. The number of excess cases was then expressed per 10,000 PY.
Patients with a malignancy who survived at least 2 months after initial diagnosis are included in the present analyses; second cancers identified within the first 2 months after diagnosis were excluded as these likely represent latent lesions present prior to diagnosis of the index cancer. PY at risk in the study cohort were accumulated by 5-year age groups and latency periods (2–11, 12–59, 60–119, and ≥ 120 months) after the date of diagnosis of the first cancer (i.e. index cancer) to the date of either their diagnosis of the targeted second cancer, last known follow-up, death, or December 31, 2010, whichever occurred first. We used varying time windows because later diagnoses were more likely to represent incident cases rather than latent ones that existed prior to the index cancer. Site-specific cancer incidence rates from the U.S. population were obtained from SEER by ethnic group, sex, 5-year age groups, and 5-year calendar periods, and were multiplied by the accumulated PY in the study cohort to estimate the expected number of cancer cases. To calculate the excess risks of second cancers, the expected number was subtracted from the number of observed cases, and the difference divided by the PY at risk. The number of excess cases was then expressed per 10,000 PY. P values <0.05 were considered statistically significant for all comparisons.
Results
A total of 330,079 and 5,399 patients diagnosed with primary clinically localized prostate and thyroid cancers, respectively, were identified from the SEER database between 1995 and 2010 (Table 1). Patients with primary prostate and thyroid cancer were monitored for a second primary cancer for 2,097,867 and 34,992 PY’s of follow-up, respectively. 23,226 second primary cancers were diagnosed during the observation period. In patients with CAP, the risk of diagnosis with one of the second primary cancers examined was 6.9%. During the total duration of follow-up, patients with CAP had significantly elevated risks of subsequent diagnosis with thyroid (SIR 1.3 [95% CI 1.16–1.44]; p<0.05), kidney (SIR 1.25 [95% CI 1.19–1.31]; p<0.05), and bladder (SIR 1.10 [95% CI 1.07–1.14]; p<0.05) cancers following adjustment for age, gender, race and year of diagnosis. There was no significant increase in the diagnosis of leukemia, Hodgkin or Non-Hodgkin lymphoma, esophageal, colorectal, lung/bronchus, and pancreatic cancer among patients with primary CAP (Table 2).
Table 1.
Cohort demographic data.
| Prostate Cancer n (%) | Thyroid Cancer n (%) | |
|---|---|---|
|
| ||
| Total Patients | 330,079 | 5,399 |
|
| ||
| Age at Diagnosis (yrs) (mean ± SD) | 66.7±9.4 | 50.3±14.2 |
|
| ||
| Marital Status | ||
| Single | 30,425 (9.2) | 981 (18.2) |
| Married | 230,739 (69.9) | 3,835 (71.0) |
| Divorced/Widowed | 38,038 (11.5) | 362 (6.7) |
| Unknown | 30,877 (9.4) | 221 (4.1) |
|
| ||
| Year of Diagnosis | ||
| 1995–1998 | 71,996 (21.8) | 887 (16.4) |
| 1999–2002 | 88,330 (26.8) | 1,256 (23.3) |
| 2003–2006 | 85,539 (25.9) | 1,493 (27.7) |
| 2007–2010 | 84,214 (25.5) | 1,763 (32.7) |
|
| ||
| Race | ||
| Caucasian | 258,786 (78.4) | 4,547 (84.2) |
| African American | 42,853 (13.0) | 255 (4.7) |
| Other | 20,944 (6.4) | 534 (9.9) |
| Unknown | 7,496 (2.3) | 63 (1.2) |
|
| ||
| Gleason Score | n/a | |
| 6 | 66,633 (46.1) | |
| 7 | 58,150 (40.3) | |
| 8 | 10,360 (7.2) | |
| 9 | 6,532 (4.5) | |
| 10 | 598 (0.41) | |
Table 2.
Standardized incidence ratios of secondary malignancy in patients diagnosed with primary clinically localized CAP, stratified by follow-up intervals after diagnosis of primary malignancy. Bold typeface indicates p<0.05.
| Time after diagnosis of primary malignancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–11 months | 12–59 months | 60–119 months | ≥120 months | Total | ||||||
| Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | |
| Thyroid | 54/29.5 | 1.83 (1.4–2.4) | 146/118 | 1.24 (1.0–1.5) | 109/87 | 1.25 (1.0–1.5) | 29/26 | 1.11 (0.7–1.6) | 338/261 | 1.30 (1.2–1.4) |
| Kidney | 399/160 | 2.49 (2.3–2.8) | 712/653 | 1.09 (1.01–1.17) | 534/491 | 1.09 (1–1.2) | 177/152 | 1.16 (1–1.4) | 1822/1457 | 1.25 (1.19–1.31) |
| Urinary Bladder | 552/390 | 1.41 (1.3–1.5) | 1812/1673 | 1.08 (1.03–1.13) | 1401/1360 | 1.03 (0.98–1.09) | 523/466 | 1.12 (1.0–1.2) | 4288/3891 | 1.10 (1.07–1.14) |
| Colorectal | 595/592 | 1.01 (0.9–1.1) | 2308/2384 | 0.97 (0.93–1.01) | 1507/1718 | 0.88 (0.83–0.92) | 478/530 | 0.90 (0.8–0.99) | 4888/5223 | 0.94 (0.91–0.96) |
| Lung and Bronchus | 767/849 | 0.90 (0.84–.097) | 2717/3446 | 0.79 (0.76–0.82) | 2075/2550 | 0.81 (0.78–0.85) | 631/803 | 0.79 (0.73–0.85) | 6190/7648 | 0.81 (0.79–0.83) |
| Esophagus | 72/82 | 0.88 (0.69–1.11) | 262/332 | 0.79 (0.7–0.89) | 221/246 | 0.9 (0.79–1.03) | 64/76 | 0.84 (0.65–1.08) | 619/736 | 0.84 (0.78–0.91) |
| Pancreas | 132/140 | 0.95 (0.8–1.1) | 561/586 | 0.96 (0.88–1.0) | 407/464 | 0.88 (0.79–0.97) | 147/156 | 0.95 (0.8–1.11) | 1247/1345 | 0.93 (0.88–0.98) |
| Hodgkin’s Lymphoma | 16/12 | 1.37 (0.8–2.2) | 37/46 | 0.8 (0.6–1.1) | 32/33 | 0.97 (0.66–1.4) | 7/10 | 0.68 (0.3–1.4) | 92/101 | 0.91 (0.73–1.12) |
| Non-Hodgkin’s Lymphoma | 284/213 | 1.34 (1.2–1.5) | 871/887 | 0.98 (0.92–1.1) | 706/692 | 1.02 (0.95–1.1) | 226/227 | 0.99 (0.87–1.1) | 2087/2019 | 1.03 (0.99–1.08) |
| Leukemia | 133/150 | 0.88 (0.74–1.1) | 545/633 | 0.86 (0.79–0.94) | 476/500 | 0.95 (0.87–1.04) | 184/168 | 1.09 (0.94–1.26) | 1338/1451 | 0.92 (0.87–0.97) |
Patients with known prostate cancer were 30% more likely to be diagnosed with thyroid cancer (1.5% of secondary cancer diagnoses) compared to the general U.S. population. Examination of the latency trends revealed that 4 of 10 evaluated cancer types (thyroid, kidney, bladder, and Non-Hodgkin lymphoma) had significantly elevated SIR’s at 2–11 months following CAP diagnosis (Fig 1). Beyond this period, only the increased risk of subsequent thyroid cancer persisted at 2–11 (SIR 1.83 [95% CI 1.38–2.39]; p<0.05), 12–59 (SIR 1.24 [95% CI 1.04–1.45]; p<0.05) and 60–119 (SIR 1.25 [95% CI 1.03–1.51]; p<0.05) months. The risk of bladder cancer detection remained persistently elevated 120 months after CAP diagnosis (SIR 1.12 [95% CI 1.03–1.22]; p<0.05), but no significant association was observed between 60–119 months.
Figure 1.
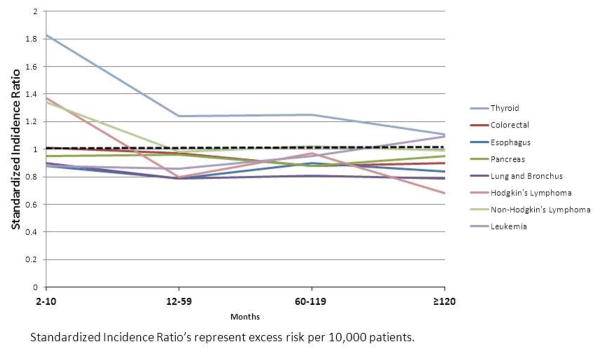
Latency course of risk of second primary non-urologic tumor diagnosis among men with clinically localized prostate cancer.
In patients with primary localized thyroid carcinoma, CAP was the most common second malignancy diagnosed (45.4%). Thyroid cancer patients had a 28% higher than expected overall risk of subsequent CAP diagnosis (SIR 1.28 [95% CI 1.08–1.5]; p<0.05). When stratified by follow-up interval, the observed CAP diagnosis rate among men with thyroid cancer was significantly higher than expected at 12–59 months of follow-up (SIR 1.42 [CI 1.1–1.79]; p<0.05). Most cases of subsequent CAP occurred within 5 (62.5%) and 10 (95.1%) years of thyroid cancer diagnosis, whereas the risk of CAP detection (SIR 0.52 [95%CI 0.21–1.07]) was no longer statistically significant among long-term (≥ 10 years) survivors of thyroid cancer. The overall risks of subsequent kidney cancer (SIR 2.53 [95% CI 1.71–3.62]; p>0.05) and non-Hodgkin lymphoma (SIR 1.61 [95% CI 1.03–2.39]; p<0.05) were also increased (Table 3). There was, however, a latency effect on the diagnosis of both cancers, with a sharp increase in kidney cancer risk at 60–119 months of follow-up (SIR 4.49 [95% CI 2.66–7.1]; p<0.05), and at 12–59 months for non-Hodgkin’s lymphoma (SIR 2.0 [95% CI 1.07–3.42]; p<0.05). The aggregate excess risk of prostate cancer was 9.28/10,000 PY, however the most pronounced excess was for CAP’s detected in the first 1 (18.1/10,000 PY) to 5 (13.1/10,000 PY) years following primary cancer diagnosis.
Table 3.
Standardized incidence ratios of secondary malignancy in patients diagnosed with primary thyroid cancer, stratified by follow-up intervals after diagnosis of primary malignancy. Bold typeface indicates p<0.05.
| Time after diagnosis of primary malignancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–11 months | 12–59 months | 60–119 months | ≥120 months | Total | ||||||
| Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | Obs/Exp | SIR (95% CI) | |
| Prostate | 20/12 | 1.64 (1–2.5) | 70/49 | 1.42 (1.1–1.8) | 47/38 | 1.25 (0.9–1.7) | 7/13 | 0.52 (0.2–1.1) | 144/113 | 1.28 (1.1–1.5) |
| Urinary Bladder | 4/2 | 1.75 (0.5–4.5) | 8/10 | 0.84 (0.4–1.7) | 5/8 | 0.65 (0.2–1.5) | 2/3 | 0.69 (0.1–2.5) | 19/22 | 0.84 (0.5–1.3) |
| Kidney | 2/1 | 1.59 (0.2–5.8) | 8/5 | 1.57 (0.7–3.1) | 18/4 | 4.49 (2.7–7.1) | 2/1.5 | 1.36 (0.2–4.9) | 30/12 | 2.53 (1.7–3.6) |
| Colorectal | 2/4 | 0.55 (0.1–2.0) | 19/15 | 1.29 (0.8–2.0) | 3/11 | 0.27 (0.1–0.8) | 5/3.9 | 1.28 (0.4–3.0) | 29/33 | 0.87 (0.6–1.3) |
| Lung and Bronchus | 3/5 | 0.62 (0.1–1.8) | 18/19 | 0.93 (0.6–1.5) | 15/15 | 1.01 (0.6–1.7) | 4/5 | 0.75 (0.2–1.9) | 40/44 | 0.9 (0.6–1.2) |
| Esophagus | 2/0.5 | 3.71 (0.5–13) | 0/2 | 0 (0–1.7) | 3/2 | 1.76 (0.4–5.2) | 0/0.6 | 0 (0–6.0) | 5/5.1 | 0.99 (0.3–2.3) |
| Pancreas | 2/0.9 | 2.3 (0.3–8) | 2/4 | 0.55 (0–2) | 5/3 | 1.73 (0.6–4.0) | 2/1 | 1.84 (0.2–6.6) | 11/8.5 | 1.3 (0.7–2.3) |
| Hodgkin’s Lymphoma | 1/0.16 | 6.18 (0.2–34) | 0/0.6 | 0 (0–6) | 0/0.4 | 0 (0–9) | 0/0.1 | 0 (0–28) | 1/1.3 | 0.77 (0–4.3) |
| Non-Hodgkin’s Lymphoma | 4/1.6 | 2.48 (0.7–6.4) | 13/7 | 2.00 (1.0–3.4) | 6/5 | 1.2 (0.4–2.6) | 1/1.8 | 0.55 (0.0–3.1) | 24/14.9 | 1.61 (1.0–2.4) |
| Leukemia | 0/0.99 | 0 (0–3.7) | 9/4 | 2.21 (1.0–4.2) | 4/3 | 1.26 (0.3–3.2) | 1/1.2 | 0.86 (0.0–4.8) | 14/9 | 1.49 (0.8–2.5) |
Discussion
Large national registry data demonstrate an association of prostate and thyroid cancer diagnoses, regardless of which cancer was diagnosed first. Patients with CAP had a 30% excess risk of subsequent thyroid cancer, while those with primary thyroid cancer had a similar 28% increase in subsequent CAP diagnosis when compared to the general population (controls). In both cases, the increased risk of identifying second primary malignancy was greatest shortly after the primary cancer diagnosis, with a lower-than-expected risk found at later follow-up. Other studies have evaluated the risk of second primary malignancy in prostate cancer patients;24–27 however, this report is novel in that it focuses on the short and long-latency of the risks rather than aggregate hazards alone and examines the role of diagnosis and surveillance bias in associated cancers.
Several hypotheses can explain the apparent association between two cancer diagnoses. For instance, bidirectional associations between malignancies raise the possibility of shared genetic or environmental risk factors, or a treatment effect (but an increase in treatment-related cancers usually only becomes apparent years after the first primary cancer).28 Meanwhile, the unidirectional association trends, as observed between thyroid and prostate cancer in our study, are most likely to indicate an effect of treatment or surveillance bias.28
Surveillance bias, a nonrandom type of information bias, refers to the notion that “the more you look, the more you find”.16 This phenomenon occurs when some patients are followed more closely or have more diagnostic tests performed than others, which leads to a more frequent diagnosis assignment in the more closely monitored group.15 Although overall population-wide rates of recommended screening are low,14 cancer survivors are more likely to undergo cancer screening compared to the general population.29–31 Among cancer survivors, increased understanding of the disease, risk perception, and mode of primary cancer detection are associated with screening for second primary cancers, and screen-detected cancer survivors are approximately twice as likely to receive all appropriate second primary cancer surveillance, even after controlling for other covariates known to affect cancer screening behaviors.14 Heightened detection efforts may increase compliance with screening guidelines, but could also result in diagnosis of cancers of limited clinical significance. Such surveillance bias likely contributes to diagnosis of secondary malignancy in patients with thyroid and prostate cancer.
Of course, cancer survivors are not solely responsible for second malignancy screening; providers play a large role in directing follow-up care. Characteristics of primary-care providers affect patient participation in cancer screening.32,33 Importantly, some physicians are “screening-prone.” Systematic recommendation for both breast and prostate cancer screening are strongly linked with systematic recommendation for colorectal cancer screening.33 Cancer preventive services are more likely to be provided when an oncologist and a primary care physician manage a survivor together (rather than pursuing follow-up with a single provider alone).32 Coordination between urology, oncology, and primary care providers has been identified as a metric of quality cancer survivorship care and has been suggested as a mechanism to improve appropriate screening utilization.32
Heightened diagnostic focus may explain the increase in second primary cancers after CAP.25 For all malignant cancers diagnosed between 1989 and 2006 in the Netherlands, 7% of all men with CAP developed additional malignancies; urinary cancers (27%) represented the most common cancer diagnoses after CAP.34 In patients diagnosed with primary cancers of the urinary system, CAP was the most commonly diagnosed second primary cancer (30%).34 Similarly, a 2-fold increased risk of a second neoplasm was reported among men with CAP in the Netherlands, Belgium and the United Kingdom.25 The risk of second primary urologic cancers among men with CAP suggests a strong detection bias for urologic cancers.18,27,35 In this study’s cohort, the increased rates of kidney and bladder cancer detected 2–11 months following CAP diagnosis are consistent with these prior findings.
For cancers of the prostate and thyroid, the increased risk of secondary diagnosis was observed regardless of which tumor type was diagnosed first. However, in a Swedish cohort where systematic CAP screening has not been adopted, no association between thyroid and prostate cancer diagnosis appears to exist.35 Thyroid and prostate cancer are arguably the two most overtreated malignancies, and the observed relationship may be explained by coupling of screening for both cancers in the United States.8 In the U.S., the incidence of thyroid cancer has tripled in the past 30 years, making it one of the fastest growing diagnoses; in contrast, the incidence in Sweden, Japan and China has increased only minimally, with no significant change in mortality.19 Ready access to portable ultrasound machines, wider access to healthcare, financial incentives, and greater use of new imaging technology for other indications have fueled an 80% increase in neck imaging36 and have produced a 2.4-fold increase in the reported incidence of thyroid nodules over the past 30 years. Yet, thyroid cancer-specific mortality has remained static.19 Similar trends can be seen in the prostate cancer with the exception that CAP-specific mortality has significantly decreased over the last 2 decades. Nevertheless, some estimates of overtreatment in response to early diagnosis suggest rates that exceed 50%, and validated risk-based CAP screening strategies are urgently needed.4,37
Among patients with primary CAP, the incidence of second primary lung/bronchus and colorectal cancers was significantly lower than expected. Some long-term follow-up studies have reported a modestly increased risk of colorectal cancer in men who have received external-beam radiation therapy for CAP,38 but the observation time in the current study probably is too short to reflect such an association, as the latency period for second primary cancer development following CAP treatment is typically at least 5 to 10 years and may well exceed 15 years.39 The decreased lung cancer incidence may indicate selection, as the high morbidity in patients with a history of smoking presents as a contraindication for definitive prostate cancer treatment.27 Patients with primary thyroid and CAP not only face overtreatment risks from the primary malignancy, but also appear subject to similar challenges from a second cancer. This study highlights the importance of careful patient selection for screening and reduction of overdiagnosis to preserve the benefits and reduce the harms of cancer screening.4
Some methodological limitations warrant consideration. The data suggest strong trends over time but it is not possible to make firm conclusions surrounding causality using them. Alternative mechanisms could explain the increased detection of additional cancers after a diagnosis of prostate or thyroid cancer within this limited observational cohort. An unequal distribution of risk factors between groups could have biased the results. Observational studies are further prone to the possibility of residual confounding by unmeasured or imperfectly measured confounders. Furthermore, underestimation of second cancers can result from migration of subjects from SEER Program areas, and, despite the large study population, the relative rarity of some cancers included in the analysis reduces the precision of some hazard estimates presented. Nevertheless, this study is strengthened by a large patient population with extensive follow-up and in-depth evaluation of the latency course of risks. The work incorporates SIR and multivariable methodologies rather than solely an assessment of overall risks. Moreover, our findings may have potential implications for thyroid and prostate cancer screening policies.
Conclusions
This monograph reports a significant association between prostate and thyroid cancer diagnoses in the U.S. The data suggest that patients with these malignancies are not only exposed to challenges of overtreatment of the primary cancer, but also face long-term risks of overtreatment of a secondary low risk malignancy.
Acknowledgments
Funding/Support and role of the sponsor: This publication was supported in part by grant number P30 CA006927 from the National Cancer Institute and by the Department of Defense, Physician Research Training Award (AK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the Department of Defense. Additional funds were provided by Fox Chase Cancer via institutional support of the Kidney Cancer Keystone Program.
Footnotes
All authors listed contributed to, read, and approved the submitted manuscript. Furthermore, the authors have no conflicts of interest to report.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. Journal of the National Cancer Institute. 2012 Jan 18;104(2):125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. The New England journal of medicine. 2012 Mar 15;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and Overtreatment of Prostate Cancer. European urology. 2014 Jan 9; doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivarajan G, Prabhu V, Taksler GB, Laze J, Lepor H. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. European urology. 2014 Jan;65(1):58–65. doi: 10.1016/j.eururo.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Prabhu V, Sivarajan G, Taksler GB, Laze J, Lepor H. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. European urology. 2014 Jan;65(1):52–57. doi: 10.1016/j.eururo.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam RK, Cheung P, Herschorn S, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. The lancet oncology. 2014 Feb;15(2):223–231. doi: 10.1016/S1470-2045(13)70606-5. [DOI] [PubMed] [Google Scholar]
- 8.Welch HG, Black WC. Overdiagnosis in cancer. Journal of the National Cancer Institute. 2010 May 5;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 9.Davies L, Welch HG. Current Thyroid Cancer Trends in the United States. JAMA otolaryngology-- head & neck surgery. 2014 Feb 20; doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 10.Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nature reviews. Clinical oncology. 2013 May;10(5):289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 11.Cancer survivors: living longer, and now better. Lancet. 2004 Dec 18–31;364(9452):2153–2154. doi: 10.1016/S0140-6736(04)17601-0. [DOI] [PubMed] [Google Scholar]
- 12.Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Seminars in radiation oncology. 2010 Jan;20(1):67–78. doi: 10.1016/j.semradonc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng AK, Travis LB. Subsequent malignant neoplasms in cancer survivors. Cancer journal. 2008 Nov-Dec;14(6):429–434. doi: 10.1097/PPO.0b013e31818d8779. [DOI] [PubMed] [Google Scholar]
- 14.Suh B, Shin DW, Kim SY, et al. Mode of primary cancer detection as an indicator of screening practice for second primary cancer in cancer survivors: a nationwide survey in Korea. BMC cancer. 2012;12:557. doi: 10.1186/1471-2407-12-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA: the journal of the American Medical Association. 2011 Jun 15;305(23):2462–2463. doi: 10.1001/jama.2011.822. [DOI] [PubMed] [Google Scholar]
- 16.Pierce CA, Haut ER, Kardooni S, et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. The Journal of trauma. 2008 Apr;64(4):932–936. doi: 10.1097/TA.0b013e318166b808. discussion 936–937. [DOI] [PubMed] [Google Scholar]
- 17.Craig SL, Feinstein AR. Antecedent therapy versus detection bias as causes of neoplastic multimorbidity. American journal of clinical oncology. 1999 Feb;22(1):51–56. doi: 10.1097/00000421-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran AT, Smaldone MC, Egleston BL, et al. Comparison of prostate cancer diagnosis in patients receiving unrelated urological and non-urological cancer care. BJU international. 2013 Jul;112(2):161–168. doi: 10.1111/bju.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. Bmj. 2013;347:f4706. doi: 10.1136/bmj.f4706. [DOI] [PubMed] [Google Scholar]
- 20.Knops AM, Legemate DA, Goossens A, Bossuyt PM, Ubbink DT. Decision aids for patients facing a surgical treatment decision: a systematic review and meta-analysis. Annals of surgery. 2013 May;257(5):860–866. doi: 10.1097/SLA.0b013e3182864fd6. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. [Accessed February 11, 2014];SEER registry groupings for analyses. 2013 Available at: http://www.seer.cancer.gov/registries/terms.html.
- 22. [Accessed January 15, 2014];Surveillance Research Program, National Cancer Institute SEER*Stat software. 2014 Version 8.1.2: www.seer.cancer.gov/seerstat.
- 23.Lal G, Groff M, Howe JR, Weigel RJ, Sugg SL, Lynch CF. Risk of subsequent primary thyroid cancer after another malignancy: latency trends in a population-based study. Annals of surgical oncology. 2012 Jun;19(6):1887–1896. doi: 10.1245/s10434-011-2193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Kestin LL, Ye H, Wallace M, Martinez AA, Vicini FA. Analysis of second malignancies after modern radiotherapy versus prostatectomy for localized prostate cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011 Jan;98(1):81–86. doi: 10.1016/j.radonc.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Kellen E, Zeegers MP, Dirx M, et al. Occurrence of both bladder and prostate cancer in five cancer registries in Belgium, The Netherlands and the United Kingdom. European journal of cancer. 2007 Jul;43(11):1694–1700. doi: 10.1016/j.ejca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone PA, Powell CR, Riffenburgh R, Rohde DC, Kane CJ. Second primary malignancies in T1-3N0 prostate cancer patients treated with radiation therapy with 10-year followup. The Journal of urology. 1998 Mar;159(3):946–949. [PubMed] [Google Scholar]
- 27.Hinnen KA, Schaapveld M, van Vulpen M, et al. Prostate brachytherapy and second primary cancer risk: a competitive risk analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011 Dec 1;29(34):4510–4515. doi: 10.1200/JCO.2011.35.0991. [DOI] [PubMed] [Google Scholar]
- 28.Sandeep TC, Strachan MW, Reynolds RM, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. The Journal of clinical endocrinology and metabolism. 2006 May;91(5):1819–1825. doi: 10.1210/jc.2005-2009. [DOI] [PubMed] [Google Scholar]
- 29.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005 Dec 1;23(34):8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 30.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003 Apr 15;21(8):1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 31.Trask PC, Rabin C, Rogers ML, et al. Cancer screening practices among cancer survivors. American journal of preventive medicine. 2005 May;28(4):351–356. doi: 10.1016/j.amepre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.McCabe MS, Partridge AH, Grunfeld E, Hudson MM. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Seminars in oncology. 2013 Dec;40(6):804–812. doi: 10.1053/j.seminoncol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisinger F, Pivot X, Coscas Y, et al. Impact of general practitioners’ sex and age on systematic recommendation for cancer screening. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 2011 Jan;20( Suppl 1):S39–41. doi: 10.1097/01.cej.0000391570.71877.18. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, de Vries E, Louwman M, et al. Prevalence of multiple malignancies in the Netherlands in 2007. International journal of cancer. Journal international du cancer. 2011 Apr 1;128(7):1659–1667. doi: 10.1002/ijc.25480. [DOI] [PubMed] [Google Scholar]
- 35.Van Hemelrijck M, Drevin L, Holmberg L, Garmo H, Adolfsson J, Stattin P. Primary cancers before and after prostate cancer diagnosis. Cancer. 2012 Dec 15;118(24):6207–6216. doi: 10.1002/cncr.27672. [DOI] [PubMed] [Google Scholar]
- 36.Leenhardt L, Bernier MO, Boin-Pineau MH, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. European journal of endocrinology/European Federation of Endocrine Societies. 2004 Feb;150(2):133–139. doi: 10.1530/eje.0.1500133. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Albertsen PC, Andriole GL, Roobol MJ, Schroder FH, Vickers AJ. Risk-based prostate cancer screening. European urology. 2012 Apr;61(4):652–661. doi: 10.1016/j.eururo.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margel D, Baniel J, Wasserberg N, Bar-Chana M, Yossepowitch O. Radiation therapy for prostate cancer increases the risk of subsequent rectal cancer. Annals of surgery. 2011 Dec;254(6):947–950. doi: 10.1097/SLA.0b013e3182382fd5. [DOI] [PubMed] [Google Scholar]
- 39.Suit H, Goldberg S, Niemierko A, et al. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiation research. 2007 Jan;167(1):12–42. doi: 10.1667/RR0527.1. [DOI] [PubMed] [Google Scholar]


