Abstract
This study investigated the influence of three high-fat diets (HFDs), differing in the percentage of total calories from saturated fat (SF) (6%, 12%, 24%) but identical in total fat (40%), for a 16-week period in mice on a variety of tissue-specific cellular processes believed to be at the root of obesity-related diseases. Specifically, we examined ectopic lipid accumulation, oxidative capacity [peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) mRNA and protein; mtDNA; Cox IV and cytochrome C protein; citrate synthase activity; and gene expression of fission 1, mitofusin (Mfn) 1 and Mfn2], oxidative stress (4-hydroxy-2-nonenal), endoplasmic reticulum (ER) stress (binding immunoglobulin protein, activating transcription factor 6-p50, p-eukaryotic initiation factor 2 alpha and x-box binding protein 1 spliced protein), inflammatory [p-c-Jun N-terminal kinase (JNK), p-nuclear factor kappa-B, p-p38 mitogen-activated protein kinase) and insulin signaling (p-Akt), and inflammation [tumor necrosis factor-alpha, monocyte chemotactic protein-1, interleukin-6, F4/80, toll-like receptor (TLR)2 and TLR4 gene expression] in various tissues, including the adipose tissue, liver, skeletal muscle and heart. In general, adipose and hepatic tissues were the only tissues which displayed evidence of dysfunction. All HFDs down-regulated adipose, cardiac and hepatic PGC-1α mRNA and hepatic citrate synthase activity, and induced adipose tissue oxidative stress, whereas only the 6%-SF and 12%-SF diet produced hepatic steatosis. However, compared to the 6%-SF and 24%-SF diets, consumption of the 12%-SF diet resulted in the greatest degree of dysregulation (hepatic ER and oxidative stress, JNK activation, increased F4/80 gene expression and down-regulation of adipose tissue Akt signaling). These findings suggest that the saturated fatty acid composition of an HFD can greatly influence the processes responsible for obesity-related diseases — nonalcoholic fatty liver disease, in particular — as well as provide further evidence that the mechanisms at the root of these diseases are diet and tissue sensitive.
Keywords: Saturated fat, High-fat diet, ER and oxidative stress, Inflammatory and insulin signaling, Mitochondria
1. Introduction
Humans face an obesity epidemic; in the United States alone, more than 30% of the adult population is obese, with 50% of adults projected to become obese by 2030 [1]. The medical consequences of obesity are serious, as obese individuals are at an increased risk of developing multiple life-threatening conditions including type 2 diabetes mellitus, cardiovascular disease and cancer [2]. The fundamental cause of these afflictions is related to various molecular, endocrine and metabolic changes resulting primarily from a prolonged over-indulgence in energy-dense, high-fat foods without a sufficient matching of energy expenditure.
The utilization of high-fat-diet (HFD)-induced obesity animal models in clinical research has shed light onto the possible culprits responsible for obesity-related diseases: ectopic lipid accumulation, mitochondrial dysfunction, oxidative and endoplasmic reticulum (ER) stress, insulin resistance (IR) and inflammation [3–8]. However, while all of these factors are likely to play a role in obesity-related diseases, there is currently very little evidence on the tissue-specific pathogenesis of these processes, the extent to which each one of these variables is interlinked and the degree to which lipid composition may influence these factors.
Saturated fatty acids (SFAs) have been shown to greatly influence obesity progression and associated molecular disturbances; they not only possess proinflammatory properties, known to promote IR through activation of nuclear factor kappa-β (NFκβ) and c-Jun N-terminal kinase (JNK), but overexposure of SFAs to various cell types has been shown to induce apoptosis as well as ER and oxidative stress more so than unsaturated fatty acids (USFAs) [9–12]. Furthermore, long-chain saturated fatty acids (LCSFAs, >C12:0) are not as efficiently oxidized as USFAs and thus are more likely to promote adiposity and ectopic lipid accumulation [13,14]. We have recently reported that adiposity, macrophage behavior, inflammation and IR can be greatly affected by dietary SF content [15]. However, it was found that these outcomes are not necessarily proportional to the percentage of SF in the diet; a diet most closely mimicking the standard American diet (12% and 40% of overall calories from saturated fat and total fat, respectively) led to the greatest adiposity (body weight, body fat percentage, fat mass and adipocyte size), macrophage infiltration into adipose tissue (expression of F4/80 and CD11c), inflammation (NFκB activation) and IR, whereas diets composed of 6% (6%-SF) and 24% (24%-SF) of total calories from SF, but an equivalent level of overall calories from fat (40%), produced lower levels of these variables, with the 24%-SF diet resulting in the least degree of IR [15]. We felt that these results warranted a more thorough examination into the influence that HFDs, of varying SF content, have on several high-fat-feeding-induced molecular changes believed to be at the core of the underlying mechanisms responsible for the illnesses stemming from obesity.
Therefore, the purpose of this investigation was to extend the findings from our previous study by examining the effects of these three HFDs, differing in the percentage of total calories from SF (6%, 12%, and 24% of total caloric intake) but identical in total fat (40%), on several molecular, endocrine and metabolic changes in multiple tissues, including adipose, hepatic, skeletal muscle and cardiac. This was done using tissues collected during the previous investigation [15]. To our knowledge, this is the first study to examine the tissue-specific pathogenesis of these processes in a variety of tissues and under the same investigation, and the first to assess the extent to which dietary SF content may influence these responses.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice were bred and cared for in the animal facility at the University of South Carolina. They were housed four to five per cage, maintained on a 12:12-h light–dark cycle in a low-stress environment (22°C, 50% humidity, low noise), and given food and water ad libitum. Principles of laboratory animal care were followed, and the Institutional Animal Care and Usage Committee of the University of South Carolina approved all experiments.
It was not possible to calculate individual food intake, as mice were housed four to five per cage. However, in general, we did not observe any differences in weekly food intake (food consumed by mice in each cage/number of mice in cage) among the HFD-fed mice over the course of the study.
2.2. Diets
At 4 weeks of age, mice were randomly assigned to one of five treatment diets (n=8–9/group): two control diets (AIN-76A, AIN-76A Mod) and three HFDs (6%-SF, 12%-SF and 24%-SF) (BioServ, Frenchtown, NJ, USA) [15]. Diets were administered for a period of 16 weeks (i.e., 4–20 weeks of age). The percentage of calories provided by each of the three macronutrients, the ratio of monounsaturated:polyunsaturated FAs (MUFA: PUFA) and the omega-6:omega-3 were identical for the HFDs and were designed to be similar to the standard American diet [16,17]. The second control diet (AIN-76A Mod) was used in order to match the MUFA:PUFA and the omega-6:omega-3 of the HFDs. Additional detail on these diets is provided by Enos et al. [15] and in Table 1.
Table 1.
Diet composition of treatment diets
| AIN-76A | AIN-76A modified | 6% SF fat diet | 12% SF diet | 24% SF diet | |
|---|---|---|---|---|---|
| Ingredient (g/kg) | |||||
| Casein | 200 | 200 | 165 | 165 | 165 |
| DL-Methionine | 3 | 3 | 3 | 3 | 3 |
| Lard | 0 | 0 | 3.3 | 35.4 | 68.6 |
| Coconut oil | 0 | 0 | 1 | 30 | 96.7 |
| Corn oil | 50 | 15.6 | 62.5 | 49.9 | 25.2 |
| Soybean oil | 0 | 3.6 | 14.1 | 9.3 | 2.5 |
| Olive oil | 0 | 30.9 | 122.1 | 78.4 | 10 |
| Corn starch | 150 | 80 | 50 | 50 | 50 |
| Maltodextrin | 0 | 100 | 100 | 100 | 100 |
| Sucrose | 500 | 469.5 | 381.5 | 381.5 | 381.5 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| Vitamin mix (AIN-76A) | 10 | 10 | 10 | 10 | 10 |
| Mineral mix (AIN-76A) | 35 | 35 | 35 | 35 | 35 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Energy (kcal/g) | 3.79 | 3.79 | 4.57 | 4.57 | 4.57 |
| Energy (% kcal) | |||||
| Carbohydrate | 68.8 | 68.7 | 47 | 47 | 47 |
| Fat | 12.2 | 12.2 | 40 | 40 | 40 |
| Protein | 19.0 | 19.1 | 13 | 13 | 13 |
| Fatty acid profile (g/kg) | |||||
| Caprylic acid (C8:0) | 0 | 0 | .075 | 2.3 | 7.3 |
| Capric acid (C10:0) | 0 | 0 | .063 | 1.8 | 5.9 |
| Lauric acid (C12:0) | 0 | 0 | .45 | 13.5 | 43.3 |
| Myristic acid (C14:0) | 0 | .004 | .23 | 5.5 | 17.1 |
| Palmitic acid (C16:0) | 5.3 | 5.5 | 22.7 | 26 | 28.3 |
| Palmitoleic acid (C16:1) | 0 | .4 | 1.7 | 2 | 2 |
| Stearic acid (C18:0) | 0 | 1.05 | 4.6 | 8.5 | 12.7 |
| Oleic acid (C18:1) | 13.7 | 27.1 | 108.7 | 88 | 48.5 |
| Linoleic acid (C18:2) | 26.8 | 13.2 | 52.9 | 43.2 | 24.5 |
| α-Linolenic acid (C18:3) | 0.6 | .66 | 2.6 | 2.2 | 1.2 |
| % of total calories from SFAs | 1.4% | 1.7% | 6% | 12% | 24% |
| % of total calories from MCFAs (C6:0–C12:0) | - | - | .1% | 3.6% | 11.8% |
| % of total calories from LCSFAs (C14:0–C18:0) | 1.4% | 1.7% | 5.9% | 8.4% | 12.2% |
| % of total calories from LCFAs (C14:0–C-18:3) | 10.8% | 10.5% | 39.9% | 36.4% | 28.2% |
| % of total calories from USFAs | 10.8% | 10.5% | 34% | 28% | 16% |
| % of total calories from MUFAs | 3.6% | 7% | 22.6% | 18.6% | 10.6% |
| % of total calories from PUFAs | 7.2% | 3.5% | 11.4% | 9.4% | 5.4% |
| % of total calories from n-3 FAs | .15% | .16% | .53% | .45% | .25% |
| % of total calories from n-6 FAs | 7.0% | 3.2 % | 10.8% | 8.9% | 5.1% |
| Cholesterol (mg/kg) | 0 | 0 | 3 | 34 | 65 |
| Ratio: MUFA:PUFA | 1:2 | 2:1 | 2:1 | 2:1 | 2:1 |
| Ratio: n-6:n-3 FA | 45:1 | 20:1 | 20:1 | 20:1 | 20:1 |
2.3. Tissue collection
At 20 weeks of age and following 16 weeks of dietary intervention, mice were sacrificed for tissue collection. The epididymal fat pad, liver, left and right gastrocnemius, and heart were removed, cleaned and immediately snap-frozen in liquid nitrogen and stored at −80°C until analysis.
2.4. Oil red O staining and tissue lipid accumulation
Frozen 10-μm liver sections were cut using a cryostat (Leica Biosystems, Germany) set at −20°C. Hematoxylin and eosin (H&E) and oil red O (Sigma, St. Louis, MO, USA) staining of the liver was performed as previously described [18]. Lipids were isolated from the liver, heart and gastrocnemius utilizing the Folch extraction method and were quantified gravimetrically [19].
2.5. Quantification of mitochondrial DNA/nuclear DNA
A QIAamp DNA mini kit (Qiagen, Valencia, CA, USA) was used for DNA isolation from the epididymal adipose tissue, liver, heart and left gastrocnemius as previously described [20]. Isolated DNA was subjected to quantitative real-time polymerase chain reaction (PCR) (100 ng/reaction) carried out with Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Comparison of kinetics of amplification of β-actin [Endogenous Control (VIC)/MGB Probe, catalog no. 4352341E] (Applied Biosystems) and cytochrome b (forward: TATTCCTTCATGTCGGACGA; reverse: AAATGCTGTGGCTATGACTG; probe: ACCTGAAACATTGGAGTACTTCTACTG) was used to determine the relative amounts of nuclear and mtDNA.
2.6. Citrate synthase activity
Citrate synthase activity was determined in the epididymal adipose tissue, liver, heart and left gastrocnemius as previously described [21]. Briefly, samples were diluted in homogenization medium (50 mM Tris, 0.15 M KCL, pH 7.4), homogenized and centrifuged at 1000g at 4°C for 10 min. The protein concentration of the resultant supernatant was determined by the Bradford method [22]. Subsequently, each sample was freeze-thawed (×3) before being analyzed for enzyme activity. Maximal citrate synthase activity was determined spectrophotometrically at 25°C by measuring the rate of disappearance of acetyl-CoA at 232 nm in an assay reagent (100 mM Tris, pH 8.1) with excess acetyl CoA (0.2 mM) and oxaloacetate (0.17 mM) (Sigma) over a 5-min interval. Enzyme activity was normalized to the amount of sample protein loaded to the assay reagent. The two control diets (AIN-76A and AIN-76A Mod) were combined to represent the “control” diets for these analyses.
2.7. Western blots
Epididymal adipose tissue, liver, half of the right gastrocnemius and heart were homogenized in radioimmunoprecipitation buffer (Sigma), which included a protease inhibitor cocktail (Sigma), 1% glycerophosphate (100×), 0.5% sodium orthovanadate (1 mM) and 1% sodium fluoride (5 mM). The protein concentration was determined by the Bradford method. Western blots were performed as previously described using primary antibodies for Cox IV, cytochrome C, GAPDH, β-actin, phosphorylated (Ser536) and total NFkB p65, phosphorylated (Thr183/Tyr185) and total JNK, phosphorylated (Ser473 and Thr308) and total Akt, binding immunoglobulin protein (BiP), phosphorylated (Ser51) and total eukaryotic initiation factor 2 alpha (EIF2α), phosphorylated p-38 mitogen-activated protein kinase (MAPK) (Thr180/Tyr182) (Cell Signaling, Danvers, MA, USA), 4-hydroxy-2-nonenal (4-HNE) (Alpha Diagnostics International, San Antonio, TX, USA), activating transcription factor 6 (ATF6)-p50 (Imgenex, San Diego, CA, USA), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) along with a positive control (sc-2209), total p-38 MAPK, as well as x-box binding protein 1 spliced (XBP-1s) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) [17]. As there were no differences in the activation of any of the measured proteins between the two control diets (AIN-76A and AIN-76A Mod), these samples were combined to represent the “control” diets for these analyses.
2.8. Gene expression
Epididymal adipose tissue, liver, gastrocnemius and heart were homogenized under liquid nitrogen using a polytron, and total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and RNeasy mini-spin columns (Qiagen) (adipose tissue only). Quantification of hepatic, skeletal muscle and cardiac gene expression for F4/80, monocyte chemotactic protein-1 (MCP-1), interleukin 6 (IL-6), toll-like receptor (TLR)-4, TLR-2, tumor necrosis factor-alpha (TNF-α), PGC-1α (including adipose tissue) as well fission 1 (Fis1), mitofusin (Mfn)1 and Mfn2 mRNA in adipose and hepatic tissues was performed as previously described [20] using Applied Biosystems reagents. Analysis of inflammatory mediators in adipose tissue is published elsewhere and therefore was not repeated in this current investigation [15]. Similar to citrate synthase activity analyses, the two control diets (AIN-76A and AIN-76A Mod) were combined to represent the “control” diets for Fis1, Mfn1 and Mfn2 gene expression quantification.
2.9. Statistical analysis
Data were analyzed using a one-way analysis of variance using commercial software (SigmaStat, SPSS, Chicago, IL, USA). Student–Newman–Keuls test was used for all post hoc analyses. Analyses that did not meet normal assumptions (equal variance and normal distribution) were repeated using a nonparametric multiple comparison procedure (Dunn’s test). Statistical significance was set with an alpha value of P≤.05. Data are presented as mean (±S.E.M.).
3. Results
3.1. Excess lipid accumulation is evident in hepatic tissue of 6%-SF- and 12%-SF-fed mice
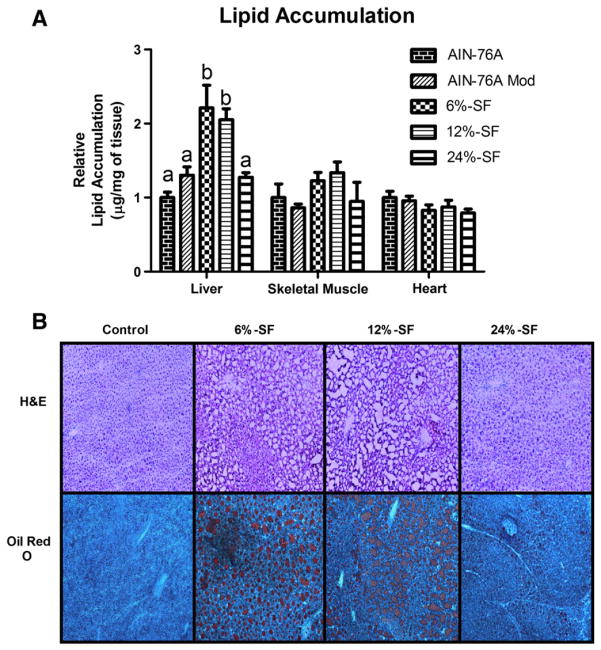
Of the nonadipose tissues analyzed, we show a significant accumulation of excess lipids in the liver as a result of HFD consumption. Interestingly, only the 6%-SF and 12%-SF diets produced this effect; the 24%-SF diet resulted in a similar level of hepatic lipid accumulation as the control diets (P≤.05) (Fig. 1A–B). On the other hand, there were no differences in lipid accumulation across the diets in the heart or gastrocnemius (Fig. 1A).
Fig. 1.
Ectopic lipid accumulation. Relative lipid accumulation (n=5–9/group) in (A) liver, gastrocnemius and heart and representative images of H&E and (B) oil red O staining of liver shot at a magnification of 10×. Diets not sharing a common letter differ significantly from one another (P≤.05).
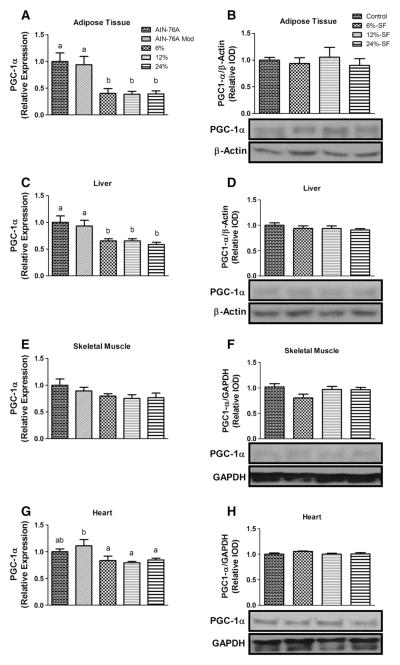
3.2. HFDs down-regulate PGC-1α gene expression in adipose, hepatic and cardiac tissues and modulate citrate synthase activity in the liver without affecting PGC-1α protein concentration or mitochondrial content
All HFDs elicited a significant down-regulation in PGC-1α gene expression in adipose, hepatic and cardiac tissues compared to one or both of the control diets (P≤.05) (Fig. 2A, C, G). Interestingly, however, no differences were found in PGC-1α protein concentration across the diets in any of the tissues examined (Fig. 2B, D, F, H).
Fig. 2.
Mitochondrial biogenesis. Relative mRNA expression (n=8–9/group) and representative Western blots (n=6–7/group) of PGC-1α in (A, B) adipose tissue, (C, D) liver, (E, F) skeletal muscle and (G, H) heart. Diets not sharing a common letter differ significantly from one another (P≤.05). Western blots presented as relative intensity of densitometry (IOD).
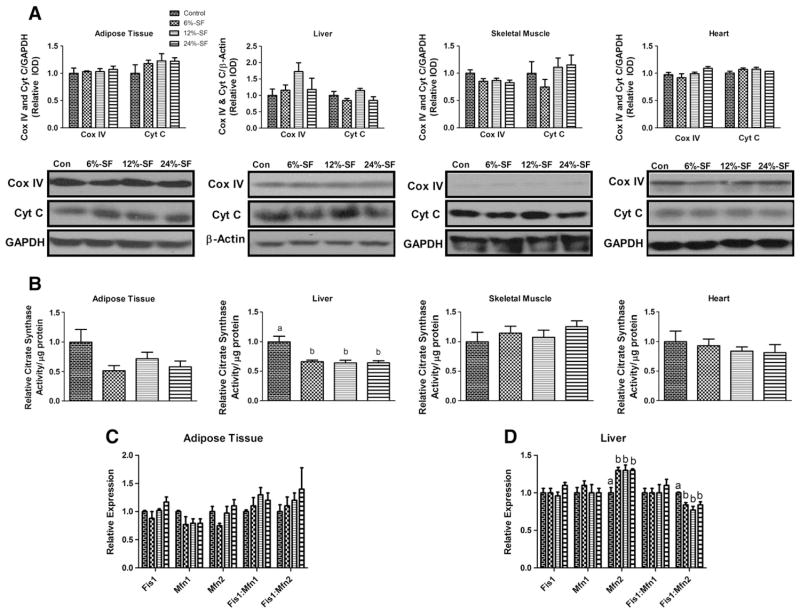
Mitochondrial content was assessed by quantification of mtDNA copy number relative to nuclear DNA (data not shown) and Cox IV and cytochrome C protein concentrations (Fig. 3A). None of the HFD treatments elicited any changes in mitochondrial content in any of tissues analyzed.
Fig. 3.
Oxidative capacity. Representative Western blots (n=6–7/group) of (A) Cox IV and cytochrome C and (B) relative citrate synthase activity per μg of protein (n=8–9/group) in adipose tissue, liver, skeletal muscle and heart as well as relative gene expression of Fis1, Mfn1, Mfn2, Fis1:Mfn1 and Fis1:Mfn2 in (C) adipose tissue and (D) liver. Diets not sharing a common letter differ significantly from one another (P≤.05). Western blots presented as relative IOD.
Despite the fact that there were no differences in mitochondrial density, there was a significant decrease in citrate synthase activity/μg of protein for all three HFDs in the liver only (P≤.05) (Fig. 3B). We next examined the gene expression of proteins involved in mitochondrial fission (Fis1) and fusion (Mfn1 and Mfn2) as well as the relative ratio of Fis1:Mfn1 and Fis1:Mfn2 mRNA, as an imbalance between these two mitochondrial processes can negatively impact oxidative capacity [23]. No differences were found in the gene expression of Fis1, Mfn1, Mfn2 or the ratio between these fission and fusion markers in the adipose tissue (Fig. 3C). On the other hand, in the liver, although consumption of each of the HFDs did not influence the Fis1 or Mfn1 mRNA expression, it did increase Mfn2 gene expression, significantly reducing the relative Fis1:Mfn2 mRNA ratio for all HFD mice compared to control-diet mice (P≤.05) (Fig. 3D).
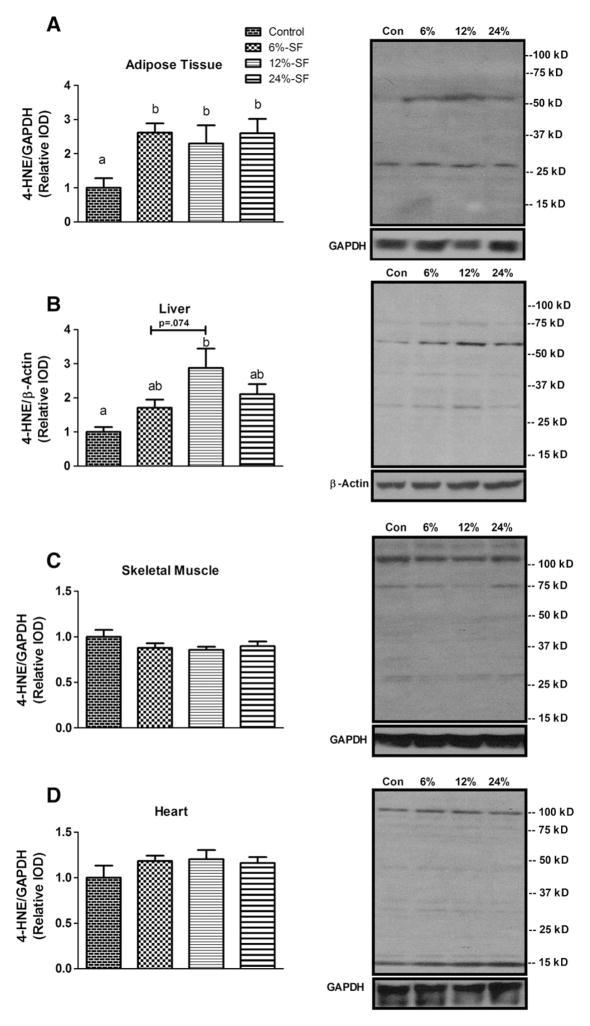
3.3. All HFDs induce oxidative stress in the adipose tissue, but only the 12%-SF diet does so in the liver
We assessed oxidative stress by examining the tissue concentration of the PUFA peroxidation product, 4-HNE, a common marker of oxidative damage [24]. 4-HNE was found to be elevated in the adipose tissue of all three HFD groups (P≤.05) (Fig. 4A) and in the liver of the 12%-SF-fed mice (P≤.05) (Fig. 4B). Additionally, there was a trend for the 12%-SF diet to increase hepatic lipid peroxidation compared to the 6%-SF diet (P=.074). Conversely, HFD consumption had no effect on 4-HNE concentrations in the skeletal muscle or the heart.
Fig. 4.
Oxidative stress. Representative Western blots of 4-HNE in (A) adipose tissue, (B) liver, (C) skeletal muscle and (D) heart (n=6–7/group). Diets not sharing a common letter differ significantly from one another (P≤.05). Western blots presented as relative IOD.
3.4. ER stress is evident in the liver following consumption of the 12%-SF diet
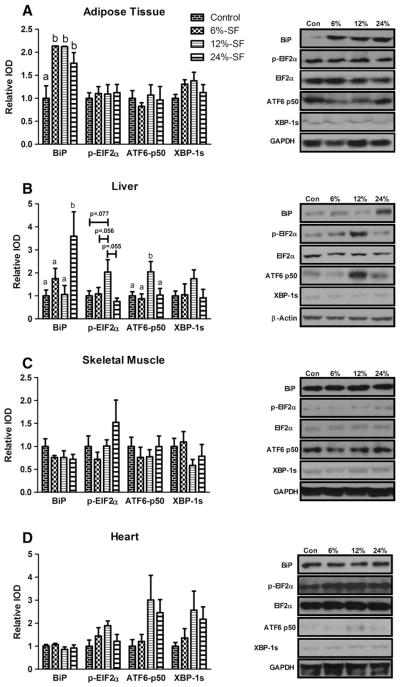
In addition to measuring the protein concentration of BiP, we assessed the activation of each of the three ER-stress pathways by examining the protein concentrations of ATF6-p50 (ATF6 pathway), p-EIF2α (PERK pathway) and XBP-1s (IRE1 pathway).
It was found that all three HFDs increased adipose tissue BiP protein concentration compared to control-fed mice (P≤.05) (Fig. 5A). However, there was no evidence of activation of any of the three ER-stress pathways in the adipose tissue. Such evidence was found only in the liver of the 12%-SF-fed mice, as there were a significant increase in ATF6-p50 protein concentration compared to all other diets (P≤.05) (Fig. 5B) and a trend for an increase in p-EIF2α compared to the 6%-SF (P=.056), 24%-SF (P=.055) and control-fed mice (P=.077). Interestingly, however, we found the quantity of BiP protein to be significantly increased in only the liver of the 24%-SF fed mice compared to all other diets (P≤.05) (Fig. 5B). There were no changes in any of the ER stress markers in the skeletal muscle or the heart.
Fig. 5.
ER stress. Representative Western blots of BiP, p-EIF2α, ATF6-p50 and XBP-1s in (A) adipose tissue, (B) liver, (C) skeletal muscle and (D) heart (n=6–7/group). Diets not sharing a common letter differ significantly from one another (P≤.05). Western blots presented as relative IOD.
3.5. Up-regulation of inflammatory signaling and inflammation is nonexistent or minimal in the skeletal muscle and heart but is evident in the liver
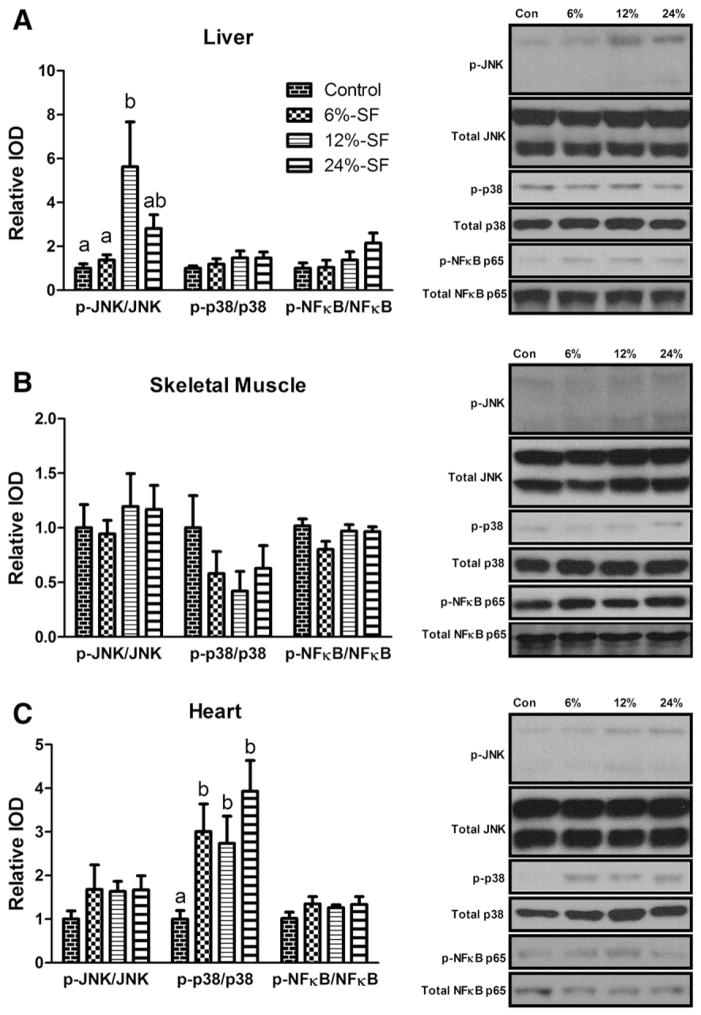
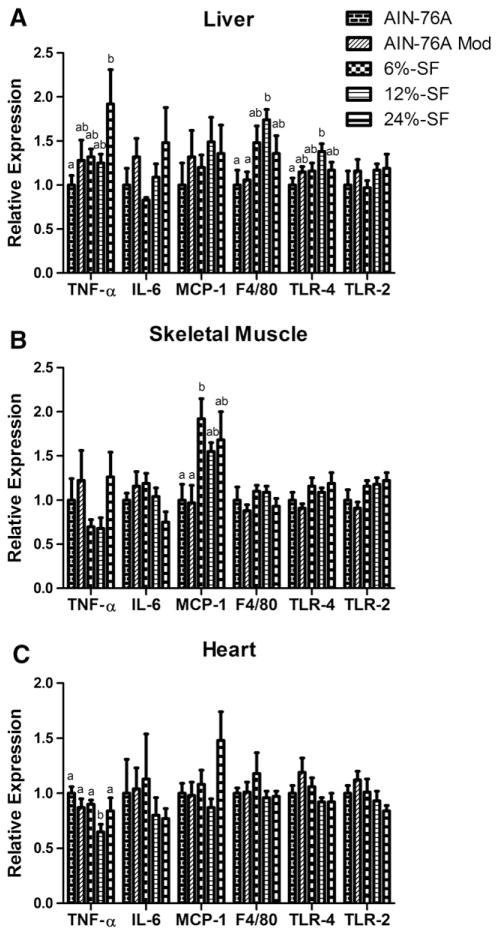
Hepatic phosphorylated-JNK (p-JNK) and cardiac phosphorylated p-38 MAPK (p-p38) were the only inflammatory signaling molecules found to be up-regulated in any of the tissues analyzed; p-JNK was found to be significantly elevated in the 12%-SF diet compared to the control- and 6%-SF-fed mice (P≤.05) (Fig. 6A), whereas p-p38 was found to be elevated in all HFD-fed mice compared to control-fed mice (P≤.05) (Fig. 6C). With regard to inflammatory markers, the 12%-SF diet increased hepatic F4/80 and TLR-4 expression compared to both control diets and the AIN-76A diet, respectively (P≤.05) (Fig. 7A). However, the 12%-SF diet did not significantly influence skeletal muscle inflammatory markers (Fig. 7B) and surprisingly reduced cardiac TNF-α expression in the heart compared to control diets (P≤.05) (Fig. 7C). Regarding the 6%-SF and 24%-SF diets, they elicited minimal inflammation in the skeletal muscle and liver, respectively; the 6%-SF diet induced a significant increase in skeletal muscle MCP-1 expression compared to control-fed mice (P≤.05) (Fig. 7B), and the 24%-SF-fed mice displayed an increase in hepatic TNF-α expression compared to the AIN-76A-fed mice (P≤.05) (Fig. 7A).
Fig. 6.
Inflammatory signaling. Representative Western blots of p-JNK, p-p38 MAPK and p-NFκB p65 in (A) liver, (B) skeletal muscle and (C) heart (n=6–7/group). Diets not sharing a common letter differ significantly from one another (P≤.05). Western blots presented as relative IOD.
Fig. 7.
Inflammation. Relative mRNA expression of TNF-α, IL-6, MCP-1, F4/80, TLR-4 and TLR-2 in (A) liver, (B) skeletal muscle and (C) heart (n=8–9/group). Diets not sharing a common letter differ significantly from one another (P≤.05).
3.6. Adipose tissue basal Akt activation is diminished in adipose tissue of 6%-SF and 12%-SF mice
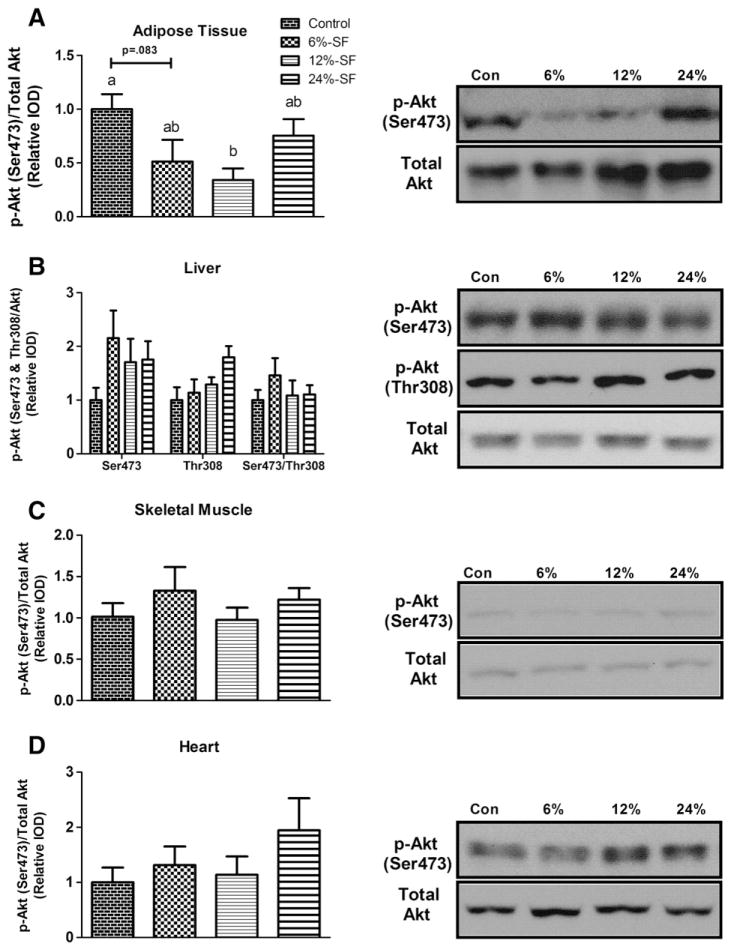
Under basal conditions, we found phosphorylated-Akt (p-Akt) (Ser473) to be significantly down-regulated in the adipose tissue of 12%-SF-fed mice (P≤.05) and a trend to be lower in the 6%-SF-fed mice (P=.083) compared to control-fed mice (Fig. 8A). In contrast, we found no difference in basal p-Akt (Ser473 and/or Thr308) in any other tissue between any of the treatment groups (Figs. 8B–D) or in the hepatic relative ratio of p-Akt Ser473:Thr308 (Fig. 8B).
Fig. 8.
Akt activation. Representative Western blots of p-Akt (Ser473) as well as p-Akt (Thr308) and p-Akt (Ser473)/p-Akt (Thr308) (liver only) in (A) adipose tissue, (B) liver, (C) skeletal muscle and (D) heart (n=6–7/group). Diets not sharing a common letter differ significantly from one another (P≤.05). Western blots presented as relative IOD.
4. Discussion
Obesity is characterized by the accumulation of excess body fat and an increased risk of disease resulting from a complex network of interrelated molecular perturbations; ectopic lipid accumulation, mitochondrial dysfunction, oxidative and ER stress, inflammation and IR have all been singled out as possible culprits. Because the majority of the studies examining these processes do so in isolated models and/or while utilizing a single HFD, it is difficult to comprehend the extent to which these manifestations are intertwined and the degree to which they are influenced by lipid composition. Thus, in this investigation, we examined the effect of three HFDs differing in the percentage of total calories from SF (6%, 12% and 24%) but identical in total fat (40%) on each of these cellular and molecular changes in a variety of tissues, including the adipose and hepatic tissue, skeletal muscle and heart. This was a follow-up to our previous investigation in which we surprisingly found that the diet most closely mimicking the standard American diet (12% of calories from SF) led to the greatest adiposity, macrophage infiltration into adipose tissue, inflammation and IR, whereas diets composed of 6% and 24% of total calories from SF produced lower levels of these variables, with the 24%-SF diet resulting in the least degree of IR [15].
Ectopic fat accumulation is a common outcome associated with obesity. Large lipid depositions in the liver (hepatic steatosis) and in the skeletal muscle have been shown to play a prominent role in the development of metabolic dysfunction [25,26]. Consumption of the 6%-SF and 12%-SF diets resulted in elevated levels of lipid accumulation compared to the control or 24%-SF diets. Furthermore, the excessive hepatic lipid aggregation preceded any significant accumulation in either the skeletal muscle or cardiac tissue as supported by others [27].
Because ectopic lipid accumulation is strongly associated with changes in mitochondrial capacity, we next examined markers of mitochondrial biogenesis, content and function. HFD consumption reduced the mRNA expression of PGC-1α, a marker of mitochondrial biogenesis, in the adipose tissue, liver and heart, but not in the skeletal muscle. Interestingly, however, we did not see any statistically significant changes in PGC-1α protein levels or mitochondrial content in any of the tissues analyzed. This may be explained by the fact that PGC-1α is known to be posttranslationally modified [28]. However, the factors that regulate this process in an obese model are not fully understood, as previous work shows that obesity may result in either a decrease or increase in PGC-1α protein concentration without any variation in PGC-1α mRNA expression [29–31]. Furthermore, while a large number of studies have investigated the influence of obesity on PGC-1α, they have failed to measure both PGC-1α gene expression and protein concentration together [32–39].
Hepatic citrate synthase activity, on the other hand, was decreased with each of the HFDs, suggesting that HFD-induced decrements in mitochondrial function occur in the liver prior to any such diminishments in other tissues. The fact that the 24%-SF-fed mice exhibited a decrease similar to those consuming the 6%-SF and 12%-SF diets is interesting, as this reduction in citrate synthase activity occurred despite the fact that they had similar hepatic lipid levels to control-fed mice. This suggests that mitochondria dysfunction may precede ectopic lipid accumulation as reported by others [40]. However, this is not true in all cases, as others have shown no impairment in mitochondrial function, yet ectopic fat accumulates [41].
A possible rationale for the reduced citrate synthase activity may be related to changes in the equilibrium between mitochondrial fission and fusion, as an imbalance between these two processes can lead to mitochondria dysfunction [23]. We did see a significant increase in the mRNA expression of the fusion marker Mfn2 for all HFDs resulting in an unbalanced ratio of relative Fis1:Mfn2 gene expression. However, it is uncertain whether changes in the balance between fission and fusion preceded the decrements in citrate synthase activity or vice versa. In addition, it is interesting to note that hepatic Mfn2 gene expression was up-regulated in all HFD-fed mice, as others have reported Mfn2 mRNA expression to be reduced in skeletal muscle of obese individuals and Mfn2 overexpression in rats has been shown to reverse IR [42–44].
It is also conceivable that oxidative and/or ER stress may have contributed to the decrements in citrate synthase activity. Both oxidative stress and ER stress are known to influence various processes including inflammation, insulin signaling and mitochondrial capacity [3,45–47]. Although we did not see any significant changes in citrate synthase activity in the adipose tissue, all three HFDs induced adipose tissue oxidative stress. On the other hand, all HFDs significantly decreased hepatic citrate synthase activity, yet only the 12%-SF diet exhibited a significant increase in oxidative stress. Thus, oxidative stress, in the form of lipid peroxidation, is not necessarily paired to diminishments in oxidative capacity.
In order to establish a relationship between oxidative and ER stress, we measured the protein concentrations of p-EIF2α, ATF6 p50 and XBP-1s, as well as the molecular chaperone BiP. Activation of one or more of the ER-stress pathways was only evident in the liver of the 12%-SF-fed mice as substantiated by a trend towards an increase in p-EIF2α and an elevation in ATF6-p50. We thought it perplexing that BiP was elevated in the adipose tissue of all three HFDs and in the liver of the 24%-SF mice without evidence for activation of any of the three ER-stress pathways. It is known that BiP resides on the luminal side of the ER membrane bound to each of the three ER-stress-response inducers (PERK, IRE1α, ATF6-α) [45]. Once misfolded proteins aggregate, BiP is released from its bound form, initiating the activation of the ER stress transducers. Thus, it is also puzzling that BiP was not elevated in the liver of the 12%-SF diet — the diet shown to promote hepatic steatosis and activation of at least one of the ER stress pathways — yet was elevated in the liver 24%-SF diet — the diet resulting in no significant hepatic lipid accumulation or activation of any of the ER-stress pathways. These observations imply that BiP expression may be regulated via a pathway independent of the three unfolded-protein response signaling branches. In addition, given that current knowledge regarding ER stress and BiP behavior is largely based upon in vitro studies, it may be that BiP regulation is slightly different in the context of HFD-induced obesity and in vivo models.
Because oxidative stress and ER stress have been tightly linked to inflammatory processes [46,48], we next examined inflammatory signaling molecules as well as markers of inflammation. We have previously reported that adipose tissue concentration of p-JNK was significantly increased in all three of these HFDs, whereas only the 12%-SF-fed mice exhibited a significantly higher degree of NFκB p65 activation compared to control-fed mice [15]. Of the three inflammatory pathways examined, only hepatic p-JNK was elevated in 12%-SF-fed mice compared to control and 6%-SF-fed mice. This was no surprise given that ER and oxidative stress are two known activators of JNK [49]. Upon activation, JNK can produce IR via serine phosphorylation of IRS-1 [49]. The 12%-SF mice also exhibited increased expression of the macrophage marker F4/80 and TLR-4. Based upon the results of this study, it seems that hepatic impairments in mitochondrial function, ER and oxidative stress, JNK activation and accumulation of macrophages precede the full progression into steatohepatitis. The finding that the consumption of the 6%-SF and 24%-SF diets resulted in elevated skeletal muscle MCP-1 and hepatic TNF-α gene expression, respectively, without any significant change in phosphorylated JNK, p38 MAPK and NFκB-p65 indicates that other signaling pathways are likely involved in influencing the expression of these markers. Of further interest is the observation that p38 MAPK activation was significantly up-regulated in the heart of all HFD-fed mice with no apparent concomitant change in inflammation — save for a decrease in TNF-α gene expression in the 12%-SF-fed mice. Although p38 MAPK is classically known as a proinflammatory signaling protein, it has also been shown to play an important role in cardiac hypertrophy and dysfunction [50] — common side effects of HFD-induced obesity [51].
In our previous investigation, we reported that mice consuming the 12%-SF diet experienced the most severe IR, followed by the 6%-SF and 24%-SF diets, as assessed by the homeostasis model assessment index [15]. Past research indicates that HFD-induced IR is a tissue-sensitive process, occurring first in the liver, followed by white adipose tissue and skeletal muscle, respectively [7,52,53]. Given this, we expected lower basal levels of hepatic p-Akt (Ser473) in addition to down-regulation of p-Akt (Ser473) in the adipose tissue of the HFD-fed mice. However, this was not the case — only adipose tissue concentration of p-Akt (Ser 473) was found to be significantly down-regulated, or trending to be, in the 12%-SF and 6%-SF mice, respectively. In addendum to hepatic p-Akt (Ser473), we also investigated hepatic Akt phosphorylation at Thr308 and the ratio between these two phosphorylated residues. Others have shown that ER stress can differentially regulate Akt activation at these two sites, and the ratio of these two residues can ultimately influence downstream substrate activity [54]. Yet, we did not see any difference in these outcomes between any of the groups. It should be noted that, although we did not see any basal changes in hepatic p-Akt, it does not mean that the HFD-fed mice did not exhibit hepatic IR. Others have shown, despite IR, basal levels of p-Akt in hepatic, adipose and/or skeletal muscle tissue to be similar between low-fat-diet-fed and HFD-fed mice; however, when stimulated by insulin, the low-fat-diet-fed mice exhibited a greater degree of Akt activation than the HFD-fed mice [55]. With this being said, it is intriguing to see reduced basal activation of Akt in the adipose tissue of the 6%-SF- and 12%-SF-fed mice without any changes in hepatic Akt activation for any of the treatment diets. These results provide evidence of the incomplete understanding of the factors that manipulate Akt activation in the settings of obesity and IR.
In general, it is evident that the hepatic and adipose tissue displayed a greater level of dysfunction than the skeletal muscle and heart, which seemed to be dependent on the diet consumed. Specifically, the 24%-SF diet failed to produce hepatic steatosis or down-regulation of adipose tissue p-Akt, whereas consumption of the 6%-SF diet resulted in hepatic steatosis but did not induce characteristics of deregulatory processes similar to the 12%-SF diet. An explanation for the observed similarities in basal levels of adipose tissue p-Akt and hepatic lipid content between the 24%-SF diet and the control diets relative to the 6%-SF and 12%-SF diets may be the increased medium-chain fatty acid (MCFA) content of the 24%-SF diet (12% vs. 4% and .1% of total calories as MCFAs for the 24%-SF, 12%-SF and 6%-SF diets, respectively). These variations exist as it was not possible for the composition of the SF in each of the HFDs to be consistent while utilizing various lipid-rich ingredients and simultaneously controlling for the omega-6:omega-3 and MUFA:PUFA ratios. Given their shorter chain length, MCFAs are much more efficiently oxidized than LCFAs [13,56,57]. Indeed, recently, it was shown that MCFAs dose-dependently prevent hepatic steatosis in a rat model of nonalcoholic fatty liver disease (NAFLD) [58]. Another plausible rationale for the lack of hepatic lipid accumulation produced by the 24%-SF diet compared to the 6%-SF and 12%-SF diets may be the lower MUFA content of the 24%-SF diet (11%, 19% and 23% of total calories from MUFAs in 24%-SF, 12%-SF and 6%-SF diets, respectively). It has been reported that MUFAs, when provided in significant amounts, promote SFA esterification into triglycerides [59,60]. Thus, the significantly lower content of MUFAs in the 24%-SF diet may have led to the partitioning of the LCSFAs into phospholipids or to serve as precursors for intracellular lipid intermediates such as diacylglycerols and ceramides. Integration of SFAs into phospholipids can stiffen the cellular membrane and negatively impact membrane-associated functions [12], while diacylglycerols and ceramides can function as secondary messengers within the cell and have been linked to metabolic dysfunction [25]. This may explain why the 24%-SF diet, comprised of more LCSFAs — the FAs thought to be largely responsible for SF’s detrimental impact on cellular processes — than either the 12%-SF or 6%-SF diets (12.2% versus 8.4% and 5.9%, respectively), up-regulated hepatic BiP concentration and TNF-α gene expression. Even so, the ability to quickly metabolize the significant amount of MCFAs comprising the 24%-SF diet — thus minimizing their capacity to be stored, incorporated into phospholipids and/or serve as substrates for lipid intermediates — likely protected against hepatic lipotoxicity, thus minimizing hepatic perturbations.
It is important to point out that the MCFA argument may not be valid when explaining the similar degree of hepatic steatosis but distinct differences in hepatic dysfunction between the 12%-SF and 6%-SF diets. It is likely that the MCFA caloric content of the diet would need to be higher to produce similar effects generated by the 24%-SF diet. Thus, a possible explanation for these outcomes may be due to the difference in absolute SFA content between the 6%-SF and 12%-SF diets (in particular, the differences in LCSFAs — 5.9% versus 8.4% of total calories for the 6%-SF and 12%-SF diets, respectively) coupled with the greater level of MUFAs in the 6%-SF versus the 12%-SF diets (23% versus 19% of total calories from MUFAs). These differences likely influenced the lipid composition of the cellular membrane and/or the hepatic FA depositions. In support of this, Wang et al., through the utilization of two HFDs, one composed largely of SFAs and the other composed primarily of PUFAs, showed that, despite a similar degree of hepatic steatosis, the SFA-laden diet resulted in a greater concentration of hepatic SFA accumulation producing ER stress and apoptosis — two perturbations not induced by the PUFA-rich diet [61]. Interestingly, however, both HFDs elicited the same degree of hepatic mitochondrial dysfunction, independent of the SFA composition of the lipid deposition. Although 2.5% and 4% variations in LCSFA and MUFA content between the 6%-SF and 12%-SF diets, respectively, seem like modest differences, others have shown that supplementing an HFD with 2.5% of a given fat source leading to a minimal change in LCFA composition can greatly impact the development of NAFLD [62]. It is evident that the divergent findings reported in the study, as well as our previous investigation, suggest that not one species of FAs is responsible for the given outcomes, but rather an ensemble of variations in several classes of FAs.
In conclusion, this is the first study to examine the influence of three HFDs differing in the percentage of total calories from saturated fat (6%, 12%, 24%) but identical in total fat (40%) on a plethora of tissue-specific cellular processes underlying the obese condition. Our results suggest that high-fat feeding induces adipose tissue and liver perturbations prior to any significant initiation of cellular distress in the skeletal muscle and heart. Additionally, findings from this study paired with those from our previous study [15] show that the SFA composition of an HFD can greatly influence the processes responsible for obesity-related diseases, as well as provide further evidence that the mechanisms at the root of these diseases are diet and tissue sensitive. In the future, timeline and dietary manipulation studies should be employed to better understand the tissue-specific pathology of dysfunctional cellular processes and the influence that dietary manipulation has on these outcomes so that therapeutic modalities may be conceived to more efficiently combat the obesity epidemic.
Acknowledgments
The authors would like to thank Kei Lam for her technical support.
Abbreviations
- 4-HNE
4-hydroxy-2-nonenal
- ATF6
activating transcription factor 6
- BiP
binding immunoglobulin protein
- EIF2α
eukaryotic initiation factor 2 alpha
- FAs
fatty acids
- Fis1
fission 1
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- IRE1
inositol-requiring enzyme 1
- IL-6
interleukin-6
- IR
insulin resistance
- JNK
c-Jun N-terminal kinase
- LCFAs
long-chain fatty acids
- LCSFAs
long-chain saturated fatty acids
- MAPK
mitogen-activated protein kinase
- MCFAs
medium-chain fatty acids
- MCP-1
monocyte chemotactic protein-1
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- MUFA
monounsaturated fatty acid
- NAFLD
nonalcoholic fatty liver disease
- NFκB
nuclear factor kappa-B
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PUFA
polyunsaturated fatty acid
- SF
saturated fat
- SFAs
saturated fatty acids
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-alpha
- USFAs
unsaturated fatty acids
- XBP-1s
x-box binding protein 1 spliced
Footnotes
Funding: This work was supported by grants from the National Institutes of Health (National Cancer Institute R21CA167058 to E.A.M.; National Institute of General Medical Sciences P20GM103641) and the University of South Carolina (Advanced Support Programs for Innovative Research Excellence to E.A.M.).
References
- 1.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–30. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clin Dermatol. 2004;22:296–302. doi: 10.1016/j.clindermatol.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Pagliassotti MJ. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr. 2012;32:17–33. doi: 10.1146/annurev-nutr-071811-150644. [DOI] [PubMed] [Google Scholar]
- 4.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 5.Coletta DK, Mandarino LJ. Mitochondrial dysfunction and insulin resistance from the outside in: extracellular matrix, the cytoskeleton, and mitochondria. Am J Physiol Endocrinol Metab. 2011;301:E749–55. doi: 10.1152/ajpendo.00363.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68:701–11. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- 7.Kleemann R, van Erk M, Verschuren L, van den Hoek AM, Koek M, Wielinga PY, et al. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS One. 2010;5:e8817. doi: 10.1371/journal.pone.0008817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittendorfer B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr Opin Clin Nutr Metab Care. 2011;14:535–41. doi: 10.1097/MCO.0b013e32834ad8b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev. 2010;68:38–61. doi: 10.1111/j.1753-4887.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- 12.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2012;52:165–74. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72:905–11. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- 14.Leyton J, Drury PJ, Crawford MA. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr. 1987;57:383–93. doi: 10.1079/bjn19870046. [DOI] [PubMed] [Google Scholar]
- 15.Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, et al. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54:152–63. doi: 10.1194/jlr.M030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 17.Grotto D, Zied E. The standard American diet and its relationship to the health status of Americans. Nutr Clin Pract. 2010;25:603–12. doi: 10.1177/0884533610386234. [DOI] [PubMed] [Google Scholar]
- 18.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–8. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol. 2011;111:1066–71. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 21.Mineo PM, Cassell EA, Roberts ME, Schaeffer PJ. Chronic cold acclimation increases thermogenic capacity, non-shivering thermogenesis and muscle citrate synthase activity in both wild-type and brown adipose tissue deficient mice. Comp Biochem Physiol A Mol Integr Physiol. 2011;161:395–400. doi: 10.1016/j.cbpa.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–8. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–33. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perseghin G. Viewpoints on the way to a consensus session: where does insulin resistance start? The liver Diabetes Care. 2009;32(Suppl 2):S164–7. doi: 10.2337/dc09-S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonkers RA, van Loon LJ, Nicolay K, Prompers JJ. In vivo postprandial lipid partitioning in liver and skeletal muscle in prediabetic and diabetic rats. Diabetologia. 2013;56:618–26. doi: 10.1007/s00125-012-2792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamovich Y, Shlomai A, Tsvetkov P, Umansky KB, Reuven N, Estall JL, et al. The protein level of PGC-1alpha, a key metabolic regulator, is controlled by NADH-NQO1. Mol Cell Biol. 2013;33(13):2603–13. doi: 10.1128/MCB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmstrom MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 2012;302:E731–9. doi: 10.1152/ajpendo.00159.2011. [DOI] [PubMed] [Google Scholar]
- 30.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105:7815–20. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu HY, Hong T, Wen GB, Han J, Zuo D, Liu Z, et al. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab. 2009;297:E898–906. doi: 10.1152/ajpendo.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, et al. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:15439–50. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 33.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–92. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 34.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–60. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 35.Nagatomo F, Fujino H, Kondo H, Takeda I, Tsuda K, Ishihara A. High-fat diet-induced reduction of peroxisome proliferator-activated receptor-gamma coacti-vator-1alpha messenger RNA levels and oxidative capacity in the soleus muscle of rats with metabolic syndrome. Nutr Res. 2012;32:144–51. doi: 10.1016/j.nutres.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Nadal-Casellas A, Amengual-Cladera E, Proenza AM, Llado I, Gianotti M. Long-term high-fat-diet feeding impairs mitochondrial biogenesis in liver of male and female rats. Cell Physiol Biochem. 2010;26:291–302. doi: 10.1159/000320552. [DOI] [PubMed] [Google Scholar]
- 37.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carabelli J, Burgueno AL, Rosselli MS, Gianotti TF, Lago NR, Pirola CJ, et al. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J Cell Mol Med. 2011;15:1329–38. doi: 10.1111/j.1582-4934.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab. 2010;35:151–62. doi: 10.1139/h09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raffaella C, Francesca B, Italia F, Marina P, Giovanna L, Susanna I. Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity (Silver Spring) 2008;16:958–64. doi: 10.1038/oby.2008.10. [DOI] [PubMed] [Google Scholar]
- 41.Flamment M, Arvier M, Gallois Y, Simard G, Malthiery Y, Ritz P, et al. Fatty liver and insulin resistance in obese Zucker rats: no role for mitochondrial dysfunction. Biochimie. 2008;90:1407–13. doi: 10.1016/j.biochi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, et al. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33:645–51. doi: 10.2337/dc09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gan KX, Wang C, Chen JH, Zhu CJ, Song GY. Mitofusin-2 ameliorates high-fat diet-induced insulin resistance in liver of rats. World J Gastroenterol. 2013;19:1572–81. doi: 10.3748/wjg.v19.i10.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 46.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 48.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 49.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–20. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behr TM, Nerurkar SS, Nelson AH, Coatney RW, Woods TN, Sulpizio A, et al. Hypertensive end-organ damage and premature mortality are p38 mitogen-activated protein kinase-dependent in a rat model of cardiac hypertrophy and dysfunction. Circulation. 2001;104:1292–8. doi: 10.1161/hc3601.094275. [DOI] [PubMed] [Google Scholar]
- 51.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008;295:H1206–15. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 53.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56(7):1638–48. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 54.Yung HW, Charnock-Jones DS, Burton GJ. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One. 2011;6:e17894. doi: 10.1371/journal.pone.0017894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, et al. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60:784–96. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Alexandrou E, Herzberg GR, White MD. High-level medium-chain triglyceride feeding and energy expenditure in normal-weight women. Can J Physiol Pharmacol. 2007;85:507–13. doi: 10.1139/y07-034. [DOI] [PubMed] [Google Scholar]
- 57.Bach AC, Ingenbleek Y, Frey A. The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res. 1996;37:708–26. [PubMed] [Google Scholar]
- 58.Ronis MJ, Baumgardner JN, Sharma N, Vantrease J, Ferguson M, Tong Y, et al. Medium chain triglycerides dose-dependently prevent liver pathology in a rat model of non-alcoholic fatty liver disease. Exp Biol Med (Maywood) 2013;238:151–62. doi: 10.1258/ebm.2012.012303. [DOI] [PubMed] [Google Scholar]
- 59.Nolan CJ, Larter CZ. Lipotoxicity: why do saturated fatty acids cause and monounsaturates protect against it? J Gastroenterol Hepatol. 2009;24:703–6. doi: 10.1111/j.1440-1746.2009.05823.x. [DOI] [PubMed] [Google Scholar]
- 60.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–40. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–51. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 62.Ferramosca A, Conte A, Burri L, Berge K, De Nuccio F, Giudetti AM, et al. A krill oil supplemented diet suppresses hepatic steatosis in high-fat fed rats. PLoS One. 2012;7:e38797. doi: 10.1371/journal.pone.0038797. [DOI] [PMC free article] [PubMed] [Google Scholar]