Abstract
The optimal combination of galactomannan index (GMI) testing for the diagnosis of invasive pulmonary aspergillosis (IPA) remains unclear. For diagnostic approaches that are triggered by clinical signs and symptoms in high-risk patients, institutional variation remains, with some centers routinely relying on only serum GMI or bronchoalveolar lavage (BAL) GMI testing. In addition, use of mold-active agents before diagnosis of IPA is becoming increasingly common, and understanding the effect of these drugs on test yield is important when making time-critical treatment decisions. In a single-center cohort of 210 allogeneic hematopoietic cell transplant (HCT) recipients, we found that serum and BAL GMI testing contributed independently to IPA diagnosis, supporting the practice of sending both tests simultaneously to ensure a timely diagnosis of IPA. BAL GMI sensitivity was not affected by receipt of mold-active therapy in our cohort.
Keywords: Aspergillus, invasive pulmonary aspergillosis, IPA, diagnosis, mold-active therapy, galactomannan, BAL, bronchoalveolar lavage
Invasive pulmonary aspergillosis (IPA) remains a significant cause of morbidity and mortality in hematopoietic cell transplant (HCT) recipients (1), and poor outcomes have been linked to delays in diagnosis and treatment. The fungal polysaccharide galactomannan is a biomarker for invasive aspergillosis in both the serum and bronchoalveolar lavage (BAL) fluid, and has been a valuable adjunct to traditional culture and histopathology-based methods of detection (2–6). However, diagnosis of IPA remains challenging: diagnostic approaches vary between institutions, and the best combination of tests to maximize diagnostic yield remains unclear. Many institutions perform either serum galactomannan index (GMI) or BAL GMI testing, but may not perform both tests. In addition, receipt of mold-active therapy (MAT) during workup for IPA has been shown to decrease the yield of the serum GMI (7). The effects of MAT on BAL GMI test performance are less clear. Recent studies showed no change in BAL GMI sensitivity with <24 h (8) or <3 days of MAT (2), but the impact of MAT, when administered for a longer period before BAL GMI testing, remains unclear. In this study, we examined the relative contributions of the serum and BAL GMI on the diagnosis of IPA in patients who have undergone allogeneic HCT at Fred Hutchinson Cancer Research Center (FHCRC) and who have a high pre-test probability of IPA, and we calculated the effects of MAT on BAL GMI performance.
Patients and methods
We retrospectively analyzed a cohort of adult patients who underwent BAL evaluation for suspected pulmonary disease within 100 days of their first allogeneic HCT between 2004 and 2010 at FHCRC. Based on standard institutional practice, patients who developed any clinical sign or symptom compatible with IPA (i.e., cough, chest or pleuritic discomfort, dyspnea, hypoxemia, or fever >38.0°C, unresponsive to broad-spectrum antibacterial antibiotics) underwent chest computed tomography (CT) analysis followed by bronchoscopy, if a radiographic abnormality was detected. All patients included in the study had a chest CT, BAL cultures, BAL GMI testing, and serum GMI testing performed. Patients were excluded from the study if they were diagnosed with IPA pre-transplantation, or if evidence of disseminated aspergillosis was present at the time of diagnosis; the latter included proven Aspergillus from sites outside the lungs, or neuroimaging showing lesions in the context of a probable IPA diagnosis. Piperacillin-tazobactam is not used as the empiric treatment of fever and neutropenia or for neutropenic prophylaxis at our institution.
Out of 210 patients, 182 had serum GMI testing performed at the time of their workup. For patients who underwent BAL GMI testing, but did not have a concurrent serum GMI within 7 days of bronchoscopy (n = 28), we performed retrospective GMI testing using a prospectively collected biorepository of serum samples. For this subset of patients, we tested serum samples that fell within a 7-day interval of the matched BAL procedure (median was 1 day before BAL). GMI testing was performed according to the manufacturer’s insert (Bio-Rad Laboratories, Inc., Hercules, California, USA) and a GMI of ≥ 0.5 with a confirmatory index was considered positive in both serum and BAL samples.
We extracted information on radiographs, transplant details, and baseline characteristics of our patients from a clinical database and via chart review. Unpaired t-tests were used to compare GMI means (Stata: release 12, 2011; StataCorp., College Station, Texas USA). All patients provided written consent, and this study was approved by our institutional review board.
When examining the contribution and overlap of each test to the diagnosis of IPA, we used the 2008 revised EORTC/MSG criteria in defining possible, probable, proven, and no IPA (9). When calculating the BAL GMI sensitivity, we removed BAL GMI as a microbiologic criterion for probable IPA, and included only a positive serum GMI and/or growth of Aspergillus in culture of BAL fluid as criteria for probable cases. We then examined the proportion with BAL GMI positivity and calculated the sensitivity of the assay among these probable cases. We further categorized patients based on receipt of MAT, calculating the sensitivity of the BAL GMI in serum GMI and culture-probable cases who received ≤ 1 day of MAT versus ≥ 5 days of MAT before BAL. Patients who received between 2 and 4 days of MAT were excluded from this part of the analysis. MAT was defined as mold-active azole, polyene, or echinocandin drugs. We did not calculate specificity of the BAL GMI because, in accordance with the 2008 EORTC/MSG criteria, we did not consider the included BAL GMI-positive cases to be false positives, and we included these patients when examining the contribution of serum GMI, BAL GMI, and BAL culture in the diagnosis of IPA in our cohort.
Results
We identified 210 patients between 2004 and 2010 who met inclusion criteria. All patients met the host criterion for IPA as described in the EORTC/MSG criteria (9) because of receipt of an allogeneic HCT. A total of 100 patients were diagnosed with probable IPA (using the microbiologic criteria of a positive BAL GMI, a positive serum GMI, or a positive BAL culture), and 110 patients were diagnosed with either possible (n = 106) or no IPA (n = 4). Among the 100 patients with probable IPA, 18 (18%) had a positive BAL culture for an Aspergillus species (15 Aspergillus fumigatus, 1 Aspergillus versicolor, 1 Aspergillus ustus, and 1 Aspergillus niger), 68 (68%) had a positive BAL GMI, and 53 (53%) had a positive serum GMI; 25 patients (25%) were positive for both serum and BAL GMI. Baseline characteristics are shown in Table 1.
Table 1.
Patient characteristics by diagnosis of probable or possible/no invasive pulmonary aspergillosis (IPA)
| Patient characteristics, N=210 | IPA*, N= 100 Number (%) |
Possible/No IPA, N=110, Number (%) | P-value |
|---|---|---|---|
| Median age in years (range) | 52 (23–74) | 50 (18–75) | NS |
|
| |||
| Female gender | 39 (39) | 41 (37) | NS |
|
| |||
| Underlying diagnosis | NS | ||
| Acute leukemia | 46 (46) | 55 (50) | |
| CML/CLL | 9 (9) | 13 (12) | |
| MDS | 20 (20) | 13 (12) | |
| Non-Hodgkin’s | 14 (14) | 15 (14) | |
| Other | 11 (11) | 14 (12) | |
|
| |||
| Related donor | 32 (32) | 40 (36) | NS |
|
| |||
| HLA-matched | 69 (69) | 88 (80) | NS |
|
| |||
| Myeloablative | 70 (70) | 67 (61) | NS |
IPA defined using culture, serum GMI, and BAL GMI.
N, number; NS, not significant; CML/CLL, chronic myeloid leukemia/chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; HLA, human leukocyte antigen; GMI, galactomannan index, BAL, bronchoalvolar lavage.
No patients were considered proven at the time of diagnosis because no biopsies were performed; however, 3 patients later underwent autopsies confirming IPA. The GMIs for these patients and time of death after diagnosis of IPA are as follows: serum GMI 0.812, BAL GMI 0.275, 71 days; serum GMI 3.04, BAL GMI 1.58, 20 days; and serum GMI 2.74, BAL GMI 0.6, 7 days.
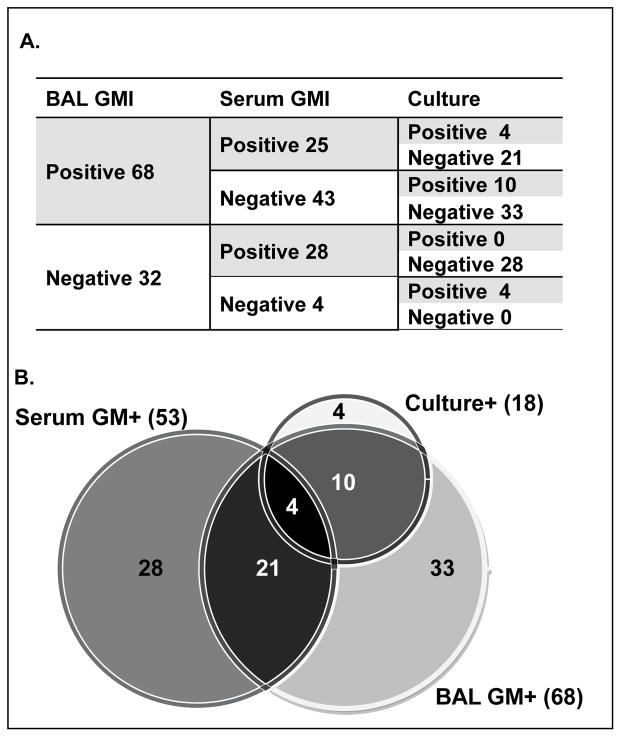
We examined the overlapping and independent contributions of the BAL culture, BAL GMI, and serum GMI to IPA diagnosis. Although the majority of the BAL culture-positive cases were also positive by BAL GMI (14/18; 78%), the BAL GMI captured an additional 54 cases. Less overlap was seen between serum GMI and BAL GMI: only 25/68 (36.8%) of BAL GMI-positive cases and 25/53 (47.2%) of serum GMI-positive cases were also positive by the other GMI test (Fig. 1). Patients who had retrospective serum GMI testing were not significantly more or less likely to have a positive result (32% vs. 25%, P = 0.36) than those who underwent concurrent serum GMI testing.
Fig. 1.
Numerical breakdown (A) and Venn diagram (B) of the specific contributions of BAL culture, BAL GMI, and serum GMI test results in the diagnosis of IPA. IPA, invasive pulmonary aspergillosis; BAL, bronchoalveolar lavage; GMI, galactomannan index.
Also our institution considers a BAL GMI level of ≥ 0.5 as positive, some institutions use a cutoff of ≥ 0.8 instead. We therefore looked at the subset of 19 patients whose BAL GMI levels were between 0.5 and 0.8 in our cohort. Of these 19 patients, 5 were also positive by serum GMI at the time of diagnosis, 3 were positive by BAL culture, 1 had mold on BAL pathology consistent with Aspergillus, 2 had a serum GMI that became positive in the future (3 weeks and 5 weeks later), and the remaining 8 patients had clinical and radiographic evidence for IPA (i.e., halo sign with radiographic improvement following voriconazole treatment).
We further examined the subset of 25 patients who were positive for both serum GMI and BAL GMI. When compared to IPA cases with a single positive GMI (e.g., only serum or BAL GMI positive, but not both), we found that the mean values of BAL and serum GMI in IPA cases where both GMI were positive were statistically significantly higher (Table 2).
Table 2.
Comparison of mean serum and BAL GMI in single positive versus dual positive invasive pulmonary aspergillosis cases
| Serum GMI+ only N = 28 |
BAL GMI+ only N = 43 |
Serum and BAL GMI+ N = 25 |
P-value* | |
|---|---|---|---|---|
| Serum GMI mean (range) | 1.06 (0.50–2.31) | N/A | 1.76 (0.61–6.92) | 0.016 |
| BAL GMI: mean (range) | N/A | 1.67 (0.50–5.01) | 3.81 (0.52–9.52) | <0.005 |
Unpaired t-test
BAL, bronchoalvolar lavage; GMI, galactomannan index; N, number; N/A, not applicable.
Our final analysis examined the relationship between MAT and BAL GMI sensitivity. Out of the 210 patients, 86 received ≥5 days of MAT and 116 received ≤1 day of MAT before their diagnostic workup. The remaining 8 patients received between 2 and 4 days of MAT and were excluded from this part of the analysis. To examine the BAL GMI sensitivity, we could only use the serum GMI or BAL culture as the microbiologic criteria, which resulted in 67 probable cases in the cohort of 210 patients. With this definition, we calculated the sensitivity of the BAL GMI for all included patients (52.2%; 95% confidence interval [CI]: 39.7%–64.6%), for patients who had received ≥5 days of MAT (59.1%; 95% CI: 36.4%–79.2%), and for patients who had received 0 or 1 day of MAT (50.0%; 95% CI: 34.2%–65.8%) before their pulmonary diagnostic workup (Table 3).
Table 3.
Sensitivity of BAL GM in all patients and by receipt of mold-active therapy (MAT)
| IPA category* | BAL GM+ | BAL GM− | Total |
|---|---|---|---|
| Proven / Probable | 35 | 32 | 67 |
| Possible / No IA | 33 | 110 | 143 |
| Sensitivity (95% CI): | 52.2% (39.7– 64.6%) | ||
| Patients on MAT ≥ 5 Days | |||
| Proven/Probable | 13 | 9 | 22 |
| Possible / No IA | 16 | 48 | 64 |
| Sensitivity (95% CI): | 59.1% (36.4–79.2%) | ||
| Patients on MAT ≤ 1 Day | |||
| Proven / Probable | 21 | 21 | 42 |
| Possible / No IA | 16 | 58 | 74 |
| Sensitivity (95% CI): | 50% (34.2–65.8%) | ||
Probable based on culture and/or serum GMI positivity; BAL GMI positive only are included as possible for these calculations.
BAL, bronchoalvolar lavage; GMI, galactomannan index; IPA, invasive pulmonary aspergillosis; CI, confidence interval (exact binomial); IA, invasive aspergillosis.
Discussion
In this study, we examined the performance of serum GMI, BAL GMI, and BAL culture in IPA diagnosis in a cohort of patients within 100 days of their first allogeneic HCT. Notably, as clinical signs and symptoms triggered chest CT imaging and BAL evaluation as a diagnostic workup, this cohort had a higher pre-test probability of IPA than the general allogeneic HCT population. All patients met the host criterion for IPA and most patients met the clinical (radiographic) criterion (9) for IPA. In this enriched patient population, we found that both the BAL GMI and the serum GMI contributed substantially to the diagnosis of IPA, with overlap in less than one-third of detected cases. These data indicate that both serum and BAL GMI testing enhances the diagnostic yield using a clinical sign- and symptom-based approach to IPA workup and diagnosis.
We report higher mean serum GMI and mean BAL GMI levels in patients with 2 positive tests, as compared to patients with only a single type of positive GMI test. It is possible that patients with positive BAL GMI and serum GMI tests have advanced disease or an increased fungal burden, compared with patients with a positive GMI at a single site. These findings may indicate that GMI testing at both sites may facilitate detection of IPA when a lower fungal burden is present.
We also examined the effect of MAT on the BAL GMI by comparing the sensitivity of the test both in a subset of patients who had received no or minimal MAT and in those who had received MAT for at least 5 days before diagnostic workup. Although our conclusions are limited by the relatively small sample sizes, the sensitivities were similar in both, with overlapping confidence intervals, suggesting that the MAT did not affect the performance of the BAL GMI in our patient cohort.
Strengths of our study were the number of IPA cases in our cohort and the availability of complete IPA diagnostic tests in all patients, including the availability of serum for retrospective serum GMI testing. We also had access to complete patient data from a comprehensive database, which allowed for accurate IPA phenotypic determinations.
Similar to our findings, Racil et al. (8) reported that simultaneous GMI testing from different anatomic sites increased the sensitivity and maintained good specificity for the diagnosis of invasive aspergillosis. In that study (8), the authors reported that the sensitivity of BAL and serum GMI testing was not compromised with short-term receipt of MAT (i.e., <24 h before testing). In our study, we report a longer window (i.e., 5 days) during which receipt of MAT does not compromise the sensitivity of BAL GMI testing. Notably, amphotericin B and echinocandin drugs were the mold-active agents used most frequently in their cohort, whereas mold-active azoles were more common in our cohort, and serum GM detection at the time of BAL was not included in their case definition of probable IPA (8).
It has recently been reported that storage of serum samples can lead to false-negative serum GMI testing over time (10). We cannot exclude that decay of GM epitopes in stored samples may have influenced the results of our retrospective serum GMI testing. However, we observed a trend toward higher rates of GMI seropositivity in samples tested in a retrospective rather than in a concurrent manner with regard to the diagnostic workup. A limitation of this study is the lack of a true “gold standard” for IPA; in order to examine the performance of the BAL GMI, we used the serum GMI as the main alternate diagnostic test. However, this limitation is inherently difficult to circumvent, given the low sensitivity of culture-based methods, the decline in invasive tissue biopsies in the modern management of IPA, and the dwindling number of patient autopsies (11). Importantly, we used a cutoff of ≥ 0.5 for BAL GMI positivity, which is the standard practice at our institution, while some institutions use a cutoff of ≥ 0.8. However, our analysis of the patients who had BAL GMI levels between 0.5 and 0.8 indicated that these represented bona-fide IPA cases and did not represent false-positive test results. Because we considered all of our BAL GMI-positive cases to be true positives, based on compatible radiographic findings, clinical presentations, and lack of patient exposure to piperacillin-tazobactam, we could not calculate BAL GMI specificity nor perform Receiver Operating Characteristic analysis.
The modest overlap between serum and BAL GMI tests in the diagnosis of IPA and the low sensitivity of culture- and histopathology-based approaches illustrate the limitations of current diagnostic approaches, particularly the lack of a single “gold standard” diagnostic test. Our findings underscore the limitations in sampling a single compartment for the diagnosis of IPA; the BAL GMI may be negative if the location of the lesion is difficult to access and sample, and the serum GMI may be negative if the fungal lesion is largely confined to pulmonary parenchymal and extravascular tissues. When patients undergo bronchoscopy for the diagnosis of a pulmonary process, simultaneous GMI testing of serum and BAL fluid enhances diagnostic yield in allogeneic HCT patients with a high pre-test probability for IPA. In addition, we have shown that the serum GMI value itself can have prognostic implications (12). However, a significant point in our study is that the BAL GMI performance did not vary by antecedent MAT in our cohort. These findings are relevant for the design and implementation of IA diagnostic strategies in HCT patients and in other high-risk groups.
Acknowledgments
Support: This work was supported by the Robert A. Sinskey Foundation (to TMH) and by the National Institutes of Health (CA 18029, CA 15704, and HL 093294 to MB; T32 AI007044-37 to CEF).
Thanks: We would like to thank Zach Stednick for his help with data retrieval for this study.
Footnotes
Presentation at meetings: Parts of the data within this manuscript were presented as an oral abstract at the IDWeek 2012 conference (October 2012, San Diego, CA; Abstract #37796).
References
- 1.Baddley JW, Andes DR, Marr KA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50:1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen MH, Leather H, Clancy CJ, et al. Galactomannan testing in bronchoalveolar lavage fluid facilitates the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies and stem cell transplant recipients. Biol Blood Marrow Transplant. 2011;17:1043–1050. doi: 10.1016/j.bbmt.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Maertens J, Verhaegen J, Lagrou K, Van Eldere J, Boogaerts M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: A prospective validation. Blood. 2001;97:1604–1610. doi: 10.1182/blood.v97.6.1604. [DOI] [PubMed] [Google Scholar]
- 4.Maertens J, Maertens V, Theunissen K, et al. Bronchoalveolar lavage fluid galactomannan for the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Clin Infect Dis. 2009;49:1688–1693. doi: 10.1086/647935. [DOI] [PubMed] [Google Scholar]
- 5.Hsu LY, Ding Y, Phua J, et al. Galactomannan testing of bronchoalveolar lavage fluid is useful for diagnosis of invasive pulmonary aspergillosis in hematology patients. BMC Infect Dis. 2010;10:44. doi: 10.1186/1471-2334-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron A, Belle A, Sulahian A, et al. Contribution of galactomannan antigen detection in BAL to the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies. Chest. 2010;137:410–415. doi: 10.1378/chest.09-0701. [DOI] [PubMed] [Google Scholar]
- 7.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: Variables that affect performance. J Infect Dis. 2004;190:641–649. doi: 10.1086/422009. [DOI] [PubMed] [Google Scholar]
- 8.Racil Z, Kocmanova I, Toskova M, et al. Galactomannan detection in bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in patients with hematological diseases – the role of factors affecting assay performance. Int J Infect Dis. 2011;15:e874–e881. doi: 10.1016/j.ijid.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 9.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson GL, Sarker SJ, Hill K, et al. Significant decline in galactomannan signal during storage of clinical serum samples. Int J Mol Sci. 2013;14:12970–12977. doi: 10.3390/ijms140712970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng GS, Stednick Z, Madtes DK, McDonald GB, Boeckh MJ, Pergam SA. Changing trends in the use of surgical biopsy for diagnosis of pulmonary disease in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2013;19:S260–S261. doi: 10.1016/j.bbmt.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher CE, Stevens AM, Boeckh M, Leisenring W, Pergam S, Hohl T. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin Infect Dis. 2013;57:1001–1004. doi: 10.1093/cid/cit393. [DOI] [PMC free article] [PubMed] [Google Scholar]