Abstract
Organophosphorus (OP) pesticides are a diverse class of acetylcholinesterase (AChE) inhibitors that are responsible for tremendous morbidity and mortality worldwide, killing approximately 300,000 people annually. Enzymatic hydrolysis of OPs is a potential therapy for acute poisoning. OpdA, an OP hydrolase isolated from Agrobacterium radiobacter, has been shown to decrease lethality in rodent models of OP poisoning. This study investigated the effects of OpdA on AChE activity, plasma concentrations of OP, and signs of toxicity after administration of dichlorvos to nonhuman primates.
A dose of 75 mg/kg dichlorvos given orally caused apnea within 10 minutes with a progressive decrease in heart rate. Blood AChE activity decreased to zero within ten minutes. Respirations and AChE activity did not recover. The mean dichlorvos concentration rose to a peak of 0.66 μg/ml. Treated monkeys received 1.2 mg/kg OpdA iv immediately after poisoning with dichlorvos. In Opda-treated animals, heart and respiratory rates were unchanged from baseline over a 240-minute observation period. AChE activity slowly declined, but remained above 25% of baseline for the entire duration. Dichlorvos concentrations reached a mean peak of 0.19 μg/ml at 40 minutes after poisoning and decreased to a mean of 0.05 μg/ml at 240 minutes.
These results show that OpdA hydrolyzes dichlorvos in an African Green Monkey model of lethal poisoning, delays AChE inhibition, and prevents lethality.
Keywords: organophosphorus, pesticide, dichlorvos, hydrolysis, monkey
Introduction
Organophosphorus (OP) pesticide poisoning is a major global health problem. An estimated 3,000,000 people are acutely poisoned each year, with a case fatality rate in excess of 9% in Sri Lanka (Eddleston et al., 2012). In nearly all countries the deliberate oral ingestion of OP pesticides is the most common method of self-harm (Chang et al., 2012). In the developed world, however, intentional self-harm with OP pesticides is an uncommon event. The main concern is the risk of terrorist attack through the release of OP gases in public spaces or introduction of highly toxic OP pesticides into water supplies.
The acute toxicity of OPs is primarily due to inhibition of acetylcholinesterase (AChE) (Sidell, 1994; Thiermann et al., 2005). Current therapy for OP poisoning requires the use of atropine to antagonize the muscarinic effects of AChE inhibition, followed by administration of oximes to reactivate AChE, plus benzodiazepines to prevent neurocognitive sequelae (Bird et al., 2003; Eddleston et al., 2005b). However, the effectiveness of these antidotes is limited. Despite intensive care support in the best of Western hospitals, up to 40% of patients die. (Eyer et al., 2003). Although OP pesticides have been a clinical problem for 50 years, no new therapies have been introduced since the 1960s.
One promising and novel approach to treating OP pesticide poisoning is the use of therapeutic enzymes to degrade pesticides in vivo. These enzymes, called OP hydrolases, are found endogenously in bacteria and humans. The use of OP hydrolases has the potential to decrease morbidity and mortality after acute OP poisoning, and to decrease limited resource utilization in the developing world. One particularly attractive feature of OP hydrolases is that they are amendable to genetic engineering, allowing the construction of mutant enzymes capable of hydrolyzing a diverse group of OPs (Vilanova et al., 1999; Bird et al., 2010). Recombinant enzymes with enhanced catalytic activities against many OPs have been developed (Sutherland et al., 2004; Jackson et al., 2009). However, few of these enzymes have undergone efficacy testing in animals, and no catalytic enzymes have ever been tested in non-human primates (NHP).
One metal-dependent OP hydrolase called OpdA (accession number EU002557), from Agrobacterium radiobacter, that shows high activity against many OPs and military G-series nerve agents, has been characterized (Horne et al., 2002; Yang et al., 2003) (figure 1). The combination of its high catalytic efficiency, broad substrate range, and stability make it an excellent therapeutic OP hydrolase candidate.
Figure 1.
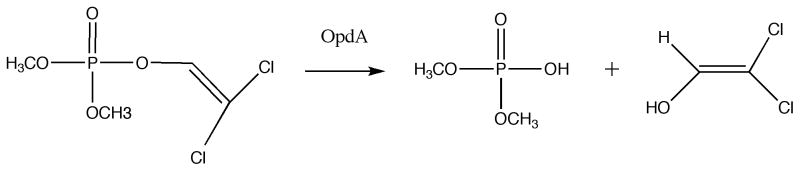
Hydrolysis of dichlorvos by OpdA
The persistent shortcomings in currently available treatment options have led us to investigate OpdA as a possible therapeutic agent for acute OP pesticide poisoning. The pharmacokinetics and efficacy of OpdA against dichlorvos, ethyl-parathion, and methyl-parathion in rats (Herkert et al., 2009; Gresham et al., 2010) have been published. Furthermore, the pharmacokinetics of OpdA nonhuman primates (Bird et al., 2008) have been published and are favorable for the further development of OpdA as a countermeasure to acute OP poisoning. This current work is an extension of previously published studies in rodents, and the purpose was to determine whether the results with OpdA in rodents can be extrapolated to NHP. The goals of this study were to: 1) determine if OpdA preserves AChE activity in an African Green Monkey model of severe dichlorvos (2,2-dichlorovinyl dimethyl phosphate) poisoning; 2) determine if OpdA hydrolyzes dichlorvos in vivo, and; 3) determine if OpdA prevents dichlorvos associated lethality in a novel nonhuman primate model.
Methods
OpdA preparation
The wild-type opdA gene was inserted between the NdeI and EcoR1 restrction sites of the pETMCSI plasmid (Neylon et al., 2000). BL21(DE3)RecA− (Invitrogen, Carlsbad, California, U.S.A) cells were transformed with pETMCSI-opdA vector heat-shock as per manufacturers instructions. Cells were grown on a Luria-Bertani broth-agar plate (containing 100 μg/mL ampicillin) at 37°C overnight. A single colony was inoculated into 50 mL Terrific broth (TB) medium supplemented with 1 mM CoCl2 (Sigma-Aldrich, St. Louis, Missouri, USA) and 100 μg/mL ampicillin (Sigma-Aldrich, St. Louis, Missouri, USA) and incubated at 37°C until mid-log phase. This start-culture was then diluted 1:50 in 2 L of the same medium and grown at 30 °C for 40 h. Cells were harvested by centrifugation at 6000 x g for 20 min at 4°C and resuspended in 50 mL buffer containing 50 mM HEPES (Sigma-Aldrich, St. Louis, Missouri, USA), pH 8.0, with 1 mM CoCl2 and 1 x Bugbuster cell lysis reagent and 1 U/mL benzonase (Novagen, EMD Chemicals, Gibbstown, New Jersey, U.S.A.). Lysis occurred at 20 °C for 30 minutes before centrifugation at 30,000 x g for 40 min at 4°C to sediment the cell debris. The supernatant was loaded onto a 60 mL DEAE Fractogel column (Merck, Frankfurt, Germany) and the unbound fraction containing OpdA was collected and dialysed against buffer containing 50 mM HEPES (Sigma-Aldrich, St. Louis, Missouri, USA), pH 7.0, overnight. This fraction was then twice loaded onto a 5 mL HiTrap SPFF column (GE Healthcare, Piscataway, New Jersey, U.S.A.) equilibrated with 50 mM HEPES, pH 7.0. Bound OpdA was eluted over a linear gradient of 0 to 0.5 M NaCl (Sigma-Aldrich, St. Louis, Missouri, USA). SDS-PAGE indicated that OpdA was >95% pure and the overall yield was in excess of 50 mg OpdA per L of growth medium. For storage, the protein was dialysed against 50 mM HEPES, 1 mM CoCl2, 150 mM NaCl, pH 7.5. For storage, the enzyme was dialyzed against 50 mM HEPES, 1 mM CoCl2, 150 mM NaCl, pH 7.5. In previous studies, there was negligible loss of activity after 8 months of storage at 4°C.(Bird et al., 2008)
Endotoxin removal and assays
After preparation, the amount of endotoxin present in the OpdA as determined by the Pyrotell Limulus Amebocyte Lysate gel-clot assay (Associates of Cape Cod, Inc., East Falmouth, Massachusetts, USA) was 51.4 EU/mg OpdA. Endotoxin was removed by running the enzyme through an endotoxin removal column (Detoxi-GeL Endotoxon Removing Column, Pierce Protein Research Products, Rockford, Illinois, USA). After one passage through the column, endotoxin concentration decreased to 2.1 EU/mg OpdA. This level of endotoxin concentration corresponds to less than 0.5 ng/ml (Petsch et al., 2000) is safe when injected iv (Xiao et al., 1998) and meets the standards of the FDA for therapeutics (FDA, 2012). Prior to administration, the OpdA was dialyzed overnight at 4°C against a solution of 150mM NaCl and 20 mM HEPES buffer to remove Co2+ metal ions present in the storage buffer.
Dichlorvos poisoning model
NHP studies were performed in 5–7 kg male African green monkeys (Chlorocebus sabaeus) that were naïve to OpdA. All African green monkeys were purchased from the New Iberia Research Center, New Iberia, Louisiana, and housed at the New England Primate Research Center (NEPRC) in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and Harvard Medical School’s Animal Care and Use Committee. The animal quarters were under a 12-hour: 12-hour light-dark cycle, with fresh water freely available in the home cage. Animals were fed a diet ad libitum of commercial primate food supplemented with fresh fruit daily. On arrival at NEPRC the animals were quarantined for 45 days during which time they underwent physical examinations, tuberculosis testing, fecal analysis for bacterial and parasitic pathogens, complete blood counts, serum chemistries and virological screening.
Animals were fasted overnight and weighed immediately before experimentation. Monkeys were sedated with intramuscular ketamine (10–15 mg/kg, Fort Dodge, IA, USA) and intubated endotracheally. The entire experiment was performed under isoflurane anesthesia with continuous end-tidal carbon dioxide monitoring. A saphenous or cephalic vein catheter was placed for serial blood sampling. A continuous three-lead electrocardiogram monitor was used to accurately measure changes in cardiac activity.
After vital signs were stabilized, dichlorvos (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 75 mg/kg (approximating 3 x the rat oral LD50) suspended in 0.5 ml/kg of peanut oil was administered through an orogastric tube, followed by a flush of 2 ml of peanut oil, after which the orogastric tube was removed. This dose of dichlorvos was based upon previous rodent models of dichlorvos poisoning (Bird et al., 2008). Development of the dichlorvos poisoning model required that dichlorvos alone be administered to 2 animals. Timing of physiologic changes was recorded and was used to guide our treatment regimen.
Due to the rapid and severe physiological changes seen after dichlorvos administration, the animals enrolled in the OpdA-treatment study were given 1.2 mg/kg intravenous OpdA immediately following oral administration of dichlorvos. This dose of OpdA was based upon known pharmacokinetics of OpdA in the African green monkey. Approximately 1 ml blood samples were taken every 5 minutes for 20 minutes, then every 10 minutes for 20 minutes, then every 20 minutes until 240 minutes post-poisoning. The blood was placed immediately on ice, then centrifuged to separate the serum, and frozen at −20 °C until analyzed.
The primary endpoint was survival to 240 minutes after poisoning. Secondary endpoints were area under the curves (AUC) of AChE activity and dichlorvos concentration. Animals were humanely euthanized with >50 mg/kg pentobarbital intravenously (Sleepaway, Fort Dodge, IA) at 240 minutes post poisoning or after 20 minutes of apnea, whichever occurred first.
All personnel working with dichlorvos or animal carcasses wore nitrile gloves and disposable gowns, according to standard procedures. Carcasses were disposed of in an incinerator equipped with afterburner and scrubber per current institutional protocol.
AChE Determination
Measurement of RBC AChE activity was performed using the point-of-care Test-mate ChE Cholinesterase Test System (EQM Research, Cincinnati, OH, USA). This device determines AChE activity using a modified Ellman method(Ellman et al., 1961) from just 10 μL of blood. The Test-mate device has been validated and used in both research and clinical applications in humans and NHP (Taylor et al., 2003; Rajapakse et al., 2011).
Dichlorvos Quantification
Quantification of serial dichlorvos concentrations was performed in triplicate on the serum samples according to published methods (Inoue et al., 2007). In summary, stock solutions of dichlorvos were prepared in methanol. The working solution for the internal standard (IS), dichlorvos-D6, (Santa Cruz Biotechnology, Dallas, Texas) was prepared by diluting an aliquot of stock solution with methanol.
Monkey serum calibration standards of dichlorvos solutions (0.001, 0.005, 0.01, and 0.05 mcg/mL) were prepared by spiking the working standard solution and internal standard (2 mcg/mL) into a sample of dichlorvos-free NHP serum (25 μL).
Serum aliquots of 50 μL were added to each 5 μL of the IS solutions prepared in methanol. Acetonitrile (50 μL) was added to the mixture. The resulting mixture was vortexed for 1 minute and then centrifuged at 3000 × g for 5 minutes. The supernatant was filtered through a C18 spin filter (Pierce) and 20 μL of the filtrate was injected and separated using an XTerra C18 3.5μ 1.0 × 50 mm (Waters, Inc) using a Surveyor (Thermo) HPLC with mobile phase solvents consisting of 10 mM ammonium formate in water (solvent A) and methanol (solvent B). The elution gradient was 15% B to 95% B (0–3 minutes), 95% B (3–9.5 minutes), 95% B to 15% B (9.5–10 minutes), and 15% B (10–20 minutes) at a flow rate of 0.3 mL/minute. The data were acquired using a LTQ (Thermo) and by monitoring the tandem mass fragment ion m/z 109 from m/z 221 (dichlorvos) and fragment ion m/z 115 from m/z 227 (D6-dichlorvos). A linear calibration curve (1/x weighting, excluding origin) was created from the ratio of the area of the standard vs. the area of the internal standard and the sample concentrations were determined against the curve.
The method was validated by establishing linearity, intra- and interassay accuracy and precision, limits of detection (LODs) and limits of quantitation (LOQs). Batches consisting of triplicate calibration standards for each concentration were analyzed two different times. Graph of the dichlorvos-to-internal standard peak-area ratios versus the theoretical concentration was constructed using linear least- squares regression analysis.
Statistical analyses
Area under the curves for AChE activity and dichlorvos concentration were calculated using GraphPad Software’s Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). In order to normalize the AUC for dichlorvos for time, the total AUC for serum dichlorvos concentration was divided by the time in minutes for both groups of animals (treated vs. un-treated).
Results
Oral Dichlorvos administration model
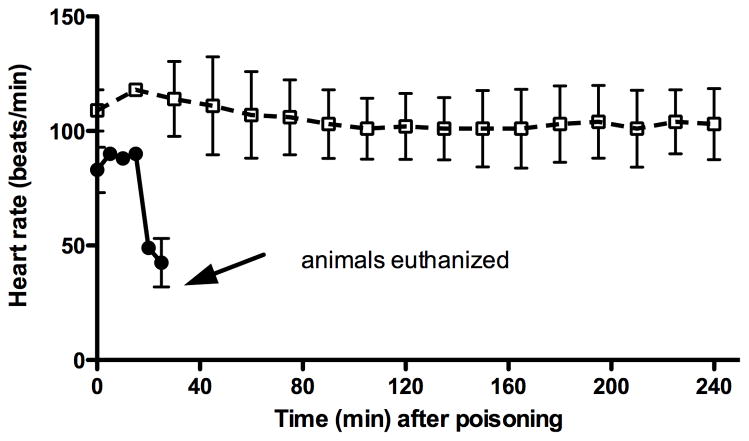
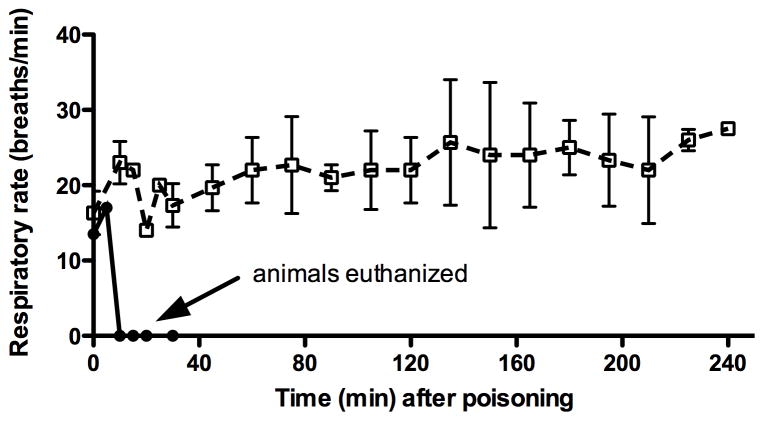
Dichlorvos was administered orally to two monkeys. Both monkeys experienced a progressive decrease in heart rate (figure 2). Both monkeys also experienced apnea within 10 minutes (figure 2). Neither respirations nor AChE activity recovered after 20 minutes, at which time the monkeys were euthanized.
Figure 2.
Heart rate (●) in animals given dichlorvos alone (n=2) and heart rate (□) in those treated with OpdA immediately after dichlorvos (dashed line, n=3). Error bars represent SD.
OpdA treatment model
Due to the rapidity with which dichlorvos exerted it toxic effects on the monkeys, OpdA at a dose of 1.2 mg/kg/IV (0.5 ml/kg) was given immediately after dichlorvos administration to a group of 3 monkeys. No other antidote was administered. All three monkeys maintained normal heart rates (figure 2) and normal respiratory rates (figure 3) for the entire 240 minutes.
Figure 3.
Respiratory rate (●) in animals given dichlorvos alone (n=2) and respiratory rate (□) in those treated with OpdA immediately after dichlorvos (dashed line, n=3). Error bars represent SD.
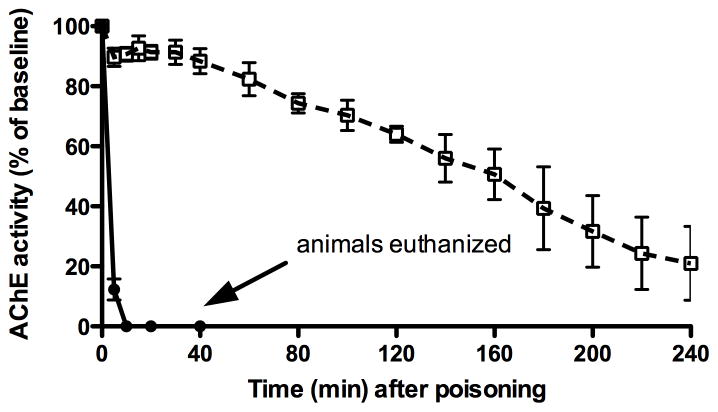
AChE activity
Blood AChE activity rapidly decreased to zero by ten minutes in both dichlorvos-only animals. In the OpdA-treated animals, AChE activity slowly declined over the 240 minutes, but remained above 25% of baseline (figure 4).
Figure 4.
Acetylcholinesterase activity as a percentage of baseline in animals given dichlorvos alone (●) and those treated with OpdA immediately after dichlorvos (□, dashed line). Error bars represent SD. Mean initial baseline AChE activity was 0.25 U/gm hemoglobin.
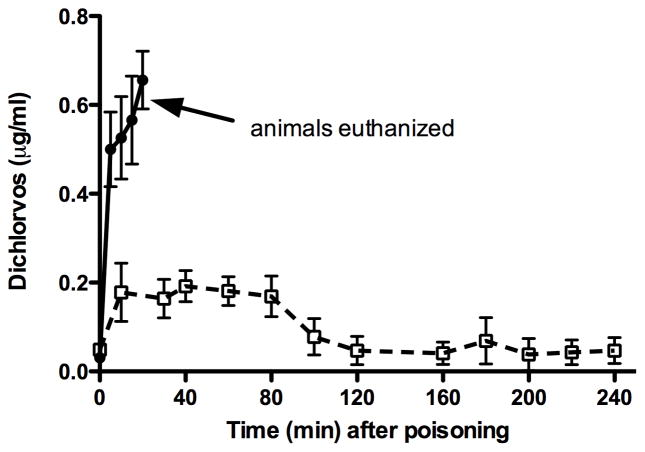
Dichlorvos concentrations
In the dichlorvos-alone monkeys, the mean dichlorvos serum concentration steadily rose to a peak of 0.66 μg/ml at the time of euthanasia (figure 5). In the OpdA-treated monkeys, the mean serum dichlorvos concentration reached a peak of 0.19 mcg/ml 40 min post-poisoning, and decreased to a mean value of 0.05 mcg/ml by 240 minutes. The AUCs for dichlorvos concentrations were 9.69 and 22.93 for the control and OpdA-treated animals, respectively (p<0.01). However, because the treated animals survived much longer than the control animals, the AUCs were normalized to time of survival (by minutes). Doing so yielded a value of 0.484 for the control animals and 0.095 for the OpdA-treated animals (p<0.01)
Figure 5.
Serum dichlorvos concentrations in animals given dichlorvos alone (●) and those treated with OpdA (□, dashed line). Error bars represent SD.
Discussion
We found that OpdA, when used alone without atropine or an oxime, prevented lethality in a NHP model of lethal OP pesticide poisoning. Furthermore, we have shown that OpdA prevents changes in respiratory rate and heart rate after OP administration, protects AChE from inhibition for up to 4 hours, and effectively hydrolyzes an OP in vivo. This is the first study demonstrating the proof-of-principal of a catalytic enzyme treating acute OP poisoning in a NHP.
Organophosphorus (OP) pesticides are commonly used in the developing world and are the most common class of chemicals used for deliberate self-harm (Van der Hoek et al., 1998; Eddleston et al., 2004b)). In the United States, the situation is quite different. Only a few thousand cases of exposure to OP pesticides were reported to the U.S. Association of Poison Control Centers in 2011, with just 2 deaths (Bronstein et al., 2012). The greatest concern from the perspective of U.S. authorities and the National Institutes of Health is the risk of terrorist attacks with OP compounds, including nerve agents. These concerns has led the NIH to develop the Countermeasures Against Chemical Threats (CounterACT) program, whose mission is the development of new and improved medical countermeasures designed to prevent, diagnose, and treat the conditions caused by potential and existing chemical agents of terrorism.
Prophylactic therapy is not feasible for either pesticide self-poisoning or for a terrorist attack on civilians. Therefore, novel and highly effective post-exposure therapy is needed. Overall mortality after OP poisoning in the developing world is unknown, but is believed to be between 4% and 30% (Karalliedde, 1999). Mortality for patients admitted to the hospital is as high as 50% (Munidasa et al., 2004). Patients exhibiting severe clinical signs associated with OP exposure are a challenge to treat, even in the most sophisticated hospitals: mortality for parathion poisoning in the ICUs of Munich, Germany, where toxicologist specialist services were available, was 40% (Eyer et al., 2003). It could be expected that in the event of a mass poisoning due to terrorist attack that the mortality would be even higher.
Dichlorvos is a commonly used OP in laboratory and animal studies (Sinha et al., 2003; Bird et al., 2008), and is the most common lethal OP poisoning in China (Phillips et al., 2002). In order to more closely mimic these clinical poisoning scenarios, we elected to administer dichlorvos via gavage. At a dose of 75 mg/kg dichlorvos very rapidly and completely inhibited AChE. Furthermore, apnea and bradycardia soon developed, necessitating the administration of OpdA immediately following dichlorvos administration in the treatment group. As the goal of this investigation was to determine the efficacy of of OpdA treatment alone, we did not treat with either atropine or an oxime.
Despite treatment only with OpdA, monkeys maintained normal heart and respiratory rates throughout the entire 4-hour experiment. In addition, while there was a steady decrease in AChE activity, the mean AChE activity 4 hours after poisoning remained above a clinically relevant 25%. Although OpdA was effective in hydrolyzing dichlorvos in vivo to a low concentration, this hydrolysis took some time (peak dichlorvos concentration occurred at 40 minutes after dosing) and was not entirely complete.
The reasons for discrepancy between the inhibitory constant of human AChE by dichlorvos (1 × 10−7 M to 1 × 10−5 M in vitro) (Nakagawa et al., 1977) and our measured serum concentration of dichlorvos and AChE are unclear, but likely multi-factorial. For instance, we were able to measure free dichlorvos in serum, but methods to quantify OpdA-, AChE-, or butyrylcholinesterase-bound dichlorvos do not exist. Perhaps even more importantly, human AChE has been shown to undergo significant spontaneous reactivation after inhibition by dichlorvos (Askar et al., 2011).
Due to IACUC requirements, animals were euthanized while under general anesthesia 4 hours after poisoning. It is important to keep in mind that this severe poisoning model was successful despite including no other antidotal therapies. Longer term survival and behavioral studies using OpdA plus conventional therapies (atropine and an oxime) in NHP will require significantly more animal subjects (as the survival rate in would be expected to be high). The current study is one of the first steps in demonstrating the efficacy of OpdA for severe acute OP poisoning.
This study demonstrates that when used as a monotherapy, OpdA protects AChE from inhibition, hydrolyzes dichlorvos in vivo, and prevents lethality in an African green monkey model of severe dichlorvos poisoning. This is the first study of a catalytic enzyme for treating OP poisoning in a non-human primate, and supports the contention that further research into expanding the catalytic properties of the enzyme and the development of OpdA as a post-exposure therapy for acute OP pesticide poisoning are warranted.
Acknowledgments
Funding information
This work was supported by the National Institute of Environmental Health Sciences (1R21ES014019 to S.B.B.) at the National Institutes of Health, by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number R21 NS070306-01 and by a Primate center base grant (RR00168) to the New England Primate Research Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences or the NIH.
We wish to thank Dr. Michael Eddleston for stimulating and informative discussions on acute OP poisoning and the potential for OP hydrolase therapy, and for reviewing drafts of the funding application.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Colin J Jackson, Email: cjackson@rsc.anu.edu.au.
Angela Carville, Email: aalcarville@gmail.com.
Jeanine Ward, Email: Jeanine.ward@umassmemorial.org.
David L. Ollis, Email: ollis@rsc.anu.edu.au.
Tejvir Khurana, Email: tsk@mail.med.upenn.edu.
References
- Askar KA, Kudi AC, Moody AJ. Spontaneous reactivation and aging kinetics of acetylcholinesterase inhibited by dichlorvos and diazinon. J Toxicol Sci. 2011;36:237–241. doi: 10.2131/jts.36.237. [DOI] [PubMed] [Google Scholar]
- Bird SB, Dawson A, Ollis D. Enzymes and bioscavengers for prophylaxis and treatment of organophosphate poisoning. Front Biosci (Schol Ed) 2010;2:209–220. doi: 10.2741/s58. [DOI] [PubMed] [Google Scholar]
- Bird SB, Gaspari RJ, Dickson EW. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med. 2003;10:295–298. doi: 10.1111/j.1553-2712.2003.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Bird SB, Sutherland TD, Gresham C, Oakeshott J, Scott C, Eddleston M. OpdA, a bacterial organophosphorus hydrolase, prevents lethality in rats after poisoning with highly toxic organophosphorus pesticides. Toxicology. 2008;247:88–92. doi: 10.1016/j.tox.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR, Jr, Rumack BH, Dart RC. 2011 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th Annual Report. Clinical toxicology. 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- Chang SS, Lu TH, Eddleston M, Konradsen F, Sterne JA, Lin JJ, Gunnell D. Factors associated with the decline in suicide by pesticide poisoning in Taiwan: a time trend analysis, 1987–2010. Clinical toxicology. 2012;50:471–480. doi: 10.3109/15563650.2012.688835. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Adhikari S, Egodage S, Ranganath H, Mohamed F, Manuweera G, Azher S, Jayamanne S, Juzczak E, Sheriff MR, Dawson AH, Buckley NA. Effects of a provincial ban of two toxic organophosphorus insecticides on pesticide poisoning hospital admissions. Clinical toxicology. 2012;50:202–209. doi: 10.3109/15563650.2012.660573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Dawson A, Karalliedde L, Dissanayake W, Hittarage A, Azher S, Buckley NA. Early management after self-poisoning with an organophosphorus or carbamate pesticide - a treatment protocol for junior doctors. Crit Care. 2004a;8:R391–397. doi: 10.1186/cc2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MH, Szinicz L, Dawson AH, Buckley NA. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005a;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Phillips MR. Self poisoning with pesticides. Bmj. 2004b;328:42–44. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Singh S, Buckley N. Organophosphorus poisoning (acute) Clin Evid. 2005b:1744–1755. [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eyer F, Meischner V, Kiderlen D, Thiermann H, Worek F, Haberkorn M, Felgenhauer N, Zilker T, Eyer P. Human parathion poisoning. A toxicokinetic analysis. Toxicol Rev. 2003;22:143–163. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- FDA; Services, U.D.O.H.A.H, editor. Pyrogen and Endotoxins Testing: Questions and Answers. Washington, D.C: 2012. Guidance for Industry. [Google Scholar]
- Gresham C, Rosenbaum C, Gaspari RJ, Jackson CJ, Bird SB. Kinetics and efficacy of an organophosphorus hydrolase in a rodent model of methyl-parathion poisoning. Acad Emerg Med. 2010;17:736–740. doi: 10.1111/j.1553-2712.2010.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkert NM, Lallement G, Clarencon D, Thiermann H, Worek F. Comparison of the oxime-induced reactivation of rhesus monkey, swine and guinea pig erythrocyte acetylcholinesterase following inhibition by sarin or paraoxon, using a perfusion model for the real-time determination of membrane-bound acetylcholinesterase activity. Toxicology. 2009;258:79–83. doi: 10.1016/j.tox.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Horne I, Sutherland TD, Harcourt RL, Russell RJ, Oakeshott JG. Identification of an opd (organophosphate degradation) gene in an Agrobacterium isolate. Appl Environ Microbiol. 2002;68:3371–3376. doi: 10.1128/AEM.68.7.3371-3376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Saito T, Mase H, Suzuki Y, Takazawa K, Yamamoto I, Inokuchi S. Rapid simultaneous determination for organophosphorus pesticides in human serum by LC-MS. J Pharm Biomed Anal. 2007;44:258–264. doi: 10.1016/j.jpba.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Jackson CJ, Weir K, Herlt A, Khurana J, Sutherland TD, Horne I, Easton C, Russell RJ, Scott C, Oakeshott JG. Structure-based rational design of a phosphotriesterase. Applied and Environmental Microbiology. 2009;75:5153–5156. doi: 10.1128/AEM.00629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalliedde L. Organophosphorus poisoning and anaesthesia. Anaesthesia. 1999;54:1073–1088. doi: 10.1046/j.1365-2044.1999.01061.x. [DOI] [PubMed] [Google Scholar]
- Munidasa UA, Gawarammana IB, Kularatne SA, Kumarasiri PV, Goonasekera CD. Survival pattern in patients with acute organophosphate poisoning receiving intensive care. J Toxicol Clin Toxicol. 2004;42:343–347. doi: 10.1081/clt-120039539. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Kobayashi H, Kojima S, Uemura A, Uchiyama M. Comparison of inhibitory actions of organophosphate pesticides on cholinesterase and lecithin-cholesterol acyltransferase in human plasma. Chem Pharm Bull (Tokyo) 1977;25:2530–2534. doi: 10.1248/cpb.25.2530. [DOI] [PubMed] [Google Scholar]
- Neylon C, Brown SE, Kralicek AV, Miles CS, Love CA, Dixon NE. Interaction of the Escherichia coli replication terminator protein (Tus) with DNA: a model derived from DNA-binding studies of mutant proteins by surface plasmon resonance. Biochemistry. 2000;39:11989–11999. doi: 10.1021/bi001174w. [DOI] [PubMed] [Google Scholar]
- Otto TC, Scott JR, Kauffman MA, Hodgins SM, Ditargiani RC, Hughes JH, Sarricks EP, Saturday GA, Hamilton TA, Cerasoli DM. Identification and characterization of novel catalytic bioscavengers of organophosphorus nerve agents. Chemico-biological interactions. 2013;203:186–190. doi: 10.1016/j.cbi.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol. 2000;76:97–119. doi: 10.1016/s0168-1656(99)00185-6. [DOI] [PubMed] [Google Scholar]
- Phillips MR, Li X, Zhang Y. Suicide rates in China, 1995–99. Lancet. 2002;359:835–840. doi: 10.1016/S0140-6736(02)07954-0. [DOI] [PubMed] [Google Scholar]
- Rajapakse BN, Thiermann H, Eyer P, Worek F, Bowe SJ, Dawson AH, Buckley NA. Evaluation of the Test-mate ChE (cholinesterase) field kit in acute organophosphorus poisoning. Ann Emerg Med. 2011;58:559–564. e556. doi: 10.1016/j.annemergmed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Sidell FR. Clinical effects of organophosphorus cholinesterase inhibitors. J Appl Toxicol. 1994;14:111–113. doi: 10.1002/jat.2550140212. [DOI] [PubMed] [Google Scholar]
- Sinha C, Shukla GS. Species variation in pesticide-induced blood-brain barrier dysfunction. Human & experimental toxicology. 2003;22:647–652. doi: 10.1191/0960327103ht405oa. [DOI] [PubMed] [Google Scholar]
- Sivilotti ML, Bird SB, Lo JC, Dickson EW. Multiple centrally acting antidotes protect against severe organophosphate toxicity. Acad Emerg Med. 2006;13:359–364. doi: 10.1197/j.aem.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Sutherland TD, Horne I, Weir KM, Coppin CW, Williams MR, Selleck M, Russell RJ, Oakeshott JG. Enzymatic bioremediation: from enzyme discovery to applications. Clin Exp Pharmacol Physiol. 2004;31:817–821. doi: 10.1111/j.1440-1681.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- Taylor PW, Lukey BJ, Clark CR, Lee RB, Roussel RR. Field verification of Test-mate ChE. Mil Med. 2003;168:314–319. [PubMed] [Google Scholar]
- Thiermann H, Szinicz L, Eyer P, Zilker T, Worek F. Correlation between red blood cell acetylcholinesterase activity and neuromuscular transmission in organophosphate poisoning. Chem Biol Interact. 2005;157–158:345–347. doi: 10.1016/j.cbi.2005.10.102. [DOI] [PubMed] [Google Scholar]
- Van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998;46:495–504. doi: 10.1016/s0277-9536(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Vilanova E, Sogorb MA. The role of phosphotriesterases in the detoxication of organophosphorus compounds. Critical reviews in toxicology. 1999;29:21–57. doi: 10.1080/10408449991349177. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Barth A, Zhu J, Ferin M. Stress and the menstrual cycle: relevance of cycle quality in the short- and long-term response to a 5-day endotoxin challenge during the follicular phase in the rhesus monkey. The Journal of clinical endocrinology and metabolism. 1998;83:2454–2460. doi: 10.1210/jcem.83.7.4926. [DOI] [PubMed] [Google Scholar]
- Yang H, Carr PD, McLoughlin SY, Liu JW, Horne I, Qiu X, Jeffries CM, Russell RJ, Oakeshott JG, Ollis DL. Evolution of an organophosphate-degrading enzyme: a comparison of natural and directed evolution. Protein Eng. 2003;16:135–145. doi: 10.1093/proeng/gzg013. [DOI] [PubMed] [Google Scholar]