Abstract
Attachment to the caregiver, typically the biological mother, is crucial to young mammals' socio-emotional development. Although studies in nonprimate species suggest that the amygdala regulates social preference and attachment development, its role in primate filial attachment development has been little investigated and has produced mixed results. This study assessed the effects of neonatal amygdala- (Neo-A, N = 16) and sham- (Neo-C, N = 12) lesions on mother recognition and discrimination in macaques raised in species-typical social groups. Neonatal amygdalectomy did not affect social discriminative abilities and mother preference at 3 and 6 months of age, strongly suggesting that the amygdala is not involved in the cognitive processes underlying the development of filial attachment at least when the amygdala damage occurred after the third to fourth weeks of age. Nevertheless, as compared to shamoperated controls, amygdalectomized infants initiated physical contact with their mothers less frequently. The findings are discussed in relation to the known contribution of the amygdala to filial attachment in both rodents and humans.
Keywords: filial attachment, relationship, mother–infant bond, development, amygdalectomy, monkeys, primates
INTRODUCTION
Young mammals depend on their principal caregiver, typically their mother, for basic survival. Although the mother is essential for the young's emotional and social development (Harlow & Zimmermann, 1959; Hofer, 1994; Sullivan, Perry, Sloan, Kleinhaus, & Burtchen, 2011), the young mammal is an active partner in this relationship. Soon after birth, the infant learns the maternal characteristics, exhibits preferential responses towards mother-related stimuli, and develops an attachment to the mother (filial attachment) characterized in part by proximity-seeking and proximity-maintaining behaviors. Sensory recognition, hormonal mechanisms and brain-reward mechanisms seem to be involved in all bonding relationships in mammals (Curley & Keverne, 2005; Goursaud & Nowak, 2000; Nowak, Keller, & Levy, 2011) and have been widely studied in precocial and altricial species (Broad, Curley, & Keverne, 2006; Moriceau, Roth, & Sullivan, 2010; Nowak et al., 2011), but less so in more evolved species, such as primates.
Studies in sheep, voles and rats have indicated that the amygdala is involved in the development of social preferences and attachment (Keller, Perrin, Meurisse, Ferreira, & Levy, 2004; Moriceau & Sullivan, 2005; Young, Young, & Hammock, 2005). Similarly, an age-related enhanced and preferential activation of the amygdala in response to mother- versus strangers-related face stimuli has been observed in children (Todd, Evans, Morris, Lewis, & Taylor, 2011; Tottenham, Shapiro, Telzer, & Humphreys, 2012), further supporting a role of the amygdala in the development of filial attachment. Yet, only two studies so far have directly investigated the role of the amygdala in the development of filial attachment in nonhuman primates. Bauman, Lavenex, Mason, Capitanio, and Amaral (2004) reported that neonatal amygdala lesions in rhesus macaques did not impair the typical development of mother– infant interactions but led, immediately after weaning at 6 months of age, to a weaker preference for the mother as compared to an unfamiliar female in an unfamiliar environment. In contrast, Goursaud and Bachevalier (2007) demonstrated at 11 months of age that neonatal amygdala lesions did not affect the expression of a social preference for a principal human caregiver as compared to a familiar human in a familiar environment. The inconsistent findings of these two studies likely relate to several procedural differences, including specificity and extent of the amygdala lesion, nature of the object of attachment, level of familiarity with the testing environment, type of possible sensory interactions during preference testing, age at testing and complexity of the rearing environment, and thus solicit additional investigations. Especially, given that neither studies used mother–infant pairs that lived in large social environment characteristic of rhesus monkey societies, it became critical to re-assess the effects of neonatal amygdala lesion on mother–infant relationships when animals are kept in a large social group.
In a recent study investigating the effects of selective bilateral neonatal amygdala lesions on mother–infant interactions when the pairs were observed in their large social group, Raper and coworkers (see Raper, Stephens, Sanchez, Bachevalier, & Wallen, this issue) reported subtle, but significant, sex-dependent differences in mother–infant relationships, especially during infancy, from 2 to 6 months of age. However, the reasons for the emergence of these subtle changes are not clear. They could have occurred either because of alterations in the infant abilities to recognize and preferentially orient towards, and seek proximity to their mother or to differences in maternal care towards the amygdalectomized infants. Alternatively, the amygdala lesions may have reduced fearfulness of the infants, given the presence of changes in threat-processing in the same monkeys (Raper, Stephens, Goursaud, Wallen, & Bachevalier, 2008; Raper, Wallen, et al., 2013) and the essential role of the amygdala in the regulation of fear and anxious behaviors (Adolphs, 2013; Davis, Walker, Miles, & Grillon, 2010; LeDoux, 2012; Marek, Strobel, Bredy, & Sah, 2013). We directly tested the former possibility by asking whether animals with neonatal amygdala lesions exhibit a preference for their biological mother and seek proximity and comfort from the mother as compared to another familiar adult female. Thus, animals participating in the companion study (Raper et al., this issue) were given a preference test (mother vs. familiar female) at 3 and 6 months of age in an experimental, but familiar, environment allowing infants to use manual contact with the stimuli.
METHOD
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Emory University, Atlanta, GA, USA, and were conformed to the NIH Guide for the Care and Use of Laboratory Animals (HHS publication 85-23, 1985).
Subjects and Living Conditions
Twenty-eight male and female infant rhesus monkeys (Macaca mulatta) born and reared by their middle-ranking mothers in species-typical social environments at the Field Station of the Yerkes National Primate Research Center (Lawrenceville, GA) of Emory University served as subjects. They were randomly assigned to receive either bilateral neurotoxic amygdala lesion (Group Neo-A, N = 16, 9 males) or bilateral sham-operation (Group Neo-C, N = 12, 6 males). The surgical procedures were performed at an average of 24.8 ± 1.2 days of age. Detailed description of the neuroimaging and surgical procedures as well as procedures for mother–infant separations, reunions, and re-introduction to their social groups are described in detail elsewhere (Raper, Bachevalier, Wallen, & Sanchez, 2013; Raper, Wallen, et al., 2013).
Behavioral Procedures
Apparatus
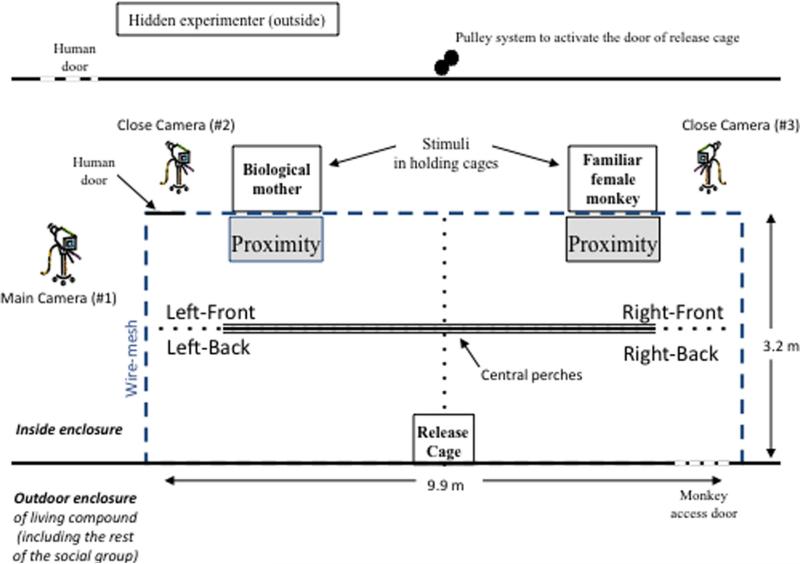
The two-choice discrimination task previously validated in juvenile monkeys (Goursaud & Bachevalier, 2007) was performed in a large enclosure divided into four quadrants of 9.9 m × 1.6 × m 2.5 m each (Left and Right Front and Left and Right Back relative to the two female stimuli; see Fig. 1). The neutral zone included the infant's release cage as well as the central perches in between the front and back zones. A proximity zone (.7 m × .3 m × .9 m) was added just in front of each of the stimuli. Visual and auditory contact were always possible between infants and stimuli, but physical contacts were permitted only when infant monkeys were in the proximity zones. The wall of the release cage facing the two stimuli was transparent allowing infant monkey to see both stimuli before the start of the trial. A pulley system permitted the experimenter (outside of the testing room) to remotely open the release cage door.
FIGURE 1.
Schematic representation of a top view of the indoor enclosure (9.9 m × 3.2 m × 2.5 m) of the living compound used for the two-choice discrimination task. Two wire-mesh cages (.80 m × .80 m × .80 m each) to hold the two female stimuli (the infant's mother and a familiar adult female) were positioned outside of the enclosure on each end of the front wire-mesh wall. The infant's release cage (.35 m × .35 m × .30 m) was located in the center of the back wall opposite the stimuli cages. The entire session was recorded with three digital video-camera recorders (Sony, DVD digital camera), one positioned behind one of the side walls to record overall subject behavior, and one on each side of both holding cages containing the adult females to record proximity interactions between subject and stimuli.
Stimuli
The mother and another familiar adult female monkey served as stimuli. The familiar adult female monkey was part of the same social group but from a different matriline (not genetically related). Additional selection criteria for the familiar female were: multiparous but not currently in gestation or caring for an infant, history of good maternal care, similar social status as that of the infant's mother and having no close social interactions with the infant. Based on these criteria, a total of 6 familiar females were used pseudo-randomly across all infants.
Discrimination Task
All subjects and stimuli were familiar with all handling procedures regularly used for regular health checks and behavioral testing (see Raper, Bachevalier, et al., 2013; Raper, Wallen, et al., 2013), and no habituation period was required. The discrimination task was given when the infants were 3 months (Mean age (days)±SEM: Neo-C: 90.42±0.36; Neo-A: 91.50±1.02; t26 = .38, NS) and 6.5 months (Mean age (days)±SEM: Neo-C: 198.67 1.12; Neo-A: 198.92±1.47; t19 = .90, NS) and lasted 10-min during which the behavior of the infant was video-recorded by three separate cameras (Fig. 1).
Before task onset, the mother–infant dyad, along with the selected familiar female stimulus, were separated from the social group and the infant was then separated from its mother and temporarily held in a “transport box.” The adult females were placed fully awake in the holding cages and were never restrained, and their left/right position was randomized across all infants at 3 months and reversed at 6 months. For each subject, the same familiar female was used at both developmental ages. The infant monkey was then introduced into the release cage, and, after 10 s had elapsed, was given 10-min to freely explore the enclosure.
Behavioral Measures
The Observer Video-Pro Software (Noldus, Netherlands; Noldus, 1991) was used to collect behavioral data on each animal (see Goursaud & Bachevalier, 2007, for details). The behavioral measures included: location within each zone (neutral, back, front, and proximity), frequency of visits and time spent within each zone, position relative to the stimuli (mother side or familiar female side) within each zone, latency to reach each proximity zone. In addition, behavioral measures within the three defined contact zones (physical/manual-contact, mesh-contact and no-contact) of each proximity zone included: latency to first entrance, frequency of visits and time spent in each contact zone as well as frequency of manual reach toward the stimulus monkey through the wire-mesh wall (see Supplementary Tab. S1). Finally, to more directly assess the preference for the mother, an index of preference [IP, defined in Goursaud and Nowak (2000)] was calculated as follow: IP = (duration of proximity with mother − duration of proximity with familiar female)/total duration of proximity with both stimuli). To evaluate the effects of amygdala lesion on overall motor and vocal activity, behavioral measures recorded also included locomotion, passivity and overall frequency of each type of vocalizations (affiliative: coo and girn, fearful: scream, aggressive: bark, and others, see Supplementary Tab. S1).
Statistical Analysis
One-, two-, or three-way parametric multivariate analyses of variance were used with Lesion Group (Neo-C and Neo-A), Sex (males and females), Stimulus (Mother and Familiar female), Location (Back, Front and Proximity), and Contact-zones (physical-contact, mesh-contact and no-contact) as factors. To compare subject's discrimination scores and behavior across the two ages, a factor Age (3 and 6 months) was added as a repeated measure with Huynh–Feldt correction. Univariate analysis of variance and/or Bonferoni corrected t-tests were used for Pairwise comparisons as appropriate. One-sample paired t-tests were used to compare the index of preference (IP) to chance level (i.e., IP value = 0, no preference) at each testing age. For data not normally distributed, nonparametric Kruskal–Wallis test, Mann–Whitney U-test or Wilcoxon signed ranks test were used. Pearson correlations test were calculated to assess the relationship between lesion extent (see Supplementary Tab. S2) and IP values. For all analyses, significance was set at p < .05 (two-tailed). Effect sizes, as indicated by Cohen's d, were calculated and reported where appropriate. Finally, due to social upheaval within one of the social groups, a total of seven animals (two males and one female from Group Neo-C, and three males and one female from Group Neo-A) were not re-tested at 6 months to avoid further social disruption of the group. Thus, only animals tested at the two ages were used in ANOVAs with Age as a factor.
Discriminant function analyses were performed separately for 3 and 6 months to assess whether the discrimination scores, index of preference and behavioral responses could accurately classify the individual monkeys within their respective lesion groups (Neo-C and Neo-A). Independent variables were selected from previous studies demonstrating significant changes after neonatal amygdala lesions (Bauman et al., 2004; Goursaud & Bachevalier, 2007; Newman & Bachevalier, 1997; Toscano, Bauman, Mason, & Amaral, 2009) and included IP, duration and frequency of proximity with the mother, latency of proximity with the mother, frequency of reaching behavior toward the mother (when in the mesh-contact zone) and total vocalizations. At each age, the Press's Q statistic (Hair, Black, Babin, & Anderson, 2009) was used to test whether the discriminant function categorized the individuals better than what could be achieved by chance. Press's Q (Chi-square with (K − 1) degrees of freedom) is defined as follow: Q = [N − (nK)]2/N(K − 1), with N total sample size, n = number of observations correctly classified and K = num ber of groups. To assess linear dependence between the predictor variables, specifically IP, duration and frequency of proximity with the mother, multicollinearity tests were performed and the Variance Inflation Factor (VIF) for each predictor variable over the other predictors was calculated.
RESULTS
Lesion Extent
The lesion extent was documented in details for each case in Raper, Bachevalier, et al. (2013) and provided on Supplementary Table S2. Briefly, the extent of bilateral damage as estimated from FLAIR images ranged from 41.2% to 98.8% (averages: males: 84.9%; females: 76.7%). In all but four cases (Neo-A-F1, -F3, -F5, -M4) the damage was substantial and bilateral. Neo-A-F3, -F5, and -M4 had moderate and asymmetrical damage (Mean: 58.5% and 70.7%, respectively), whereas Neo-A-F1 had extensive but unilateral damage in the right hemisphere only (82.3%). Extent of unintended damage to adjacent cortical areas, including perirhinal and entorhinal cortices, anterior portion of the hippocampus and tail of the putamen was overall negligible (<5%), except for slight damage to the right entorhinal cortex (<22%) in cases Neo-A-F1 and Neo-A-F4. No correlations between extent of lesion and any of the behavioral measures reported below reached significance (Pearson r, all ps > .05).
Discrimination Scores and Behaviors in the Proximity Zones
To assess whether the infant monkeys discriminated the two stimuli and displayed a recognition of and preference for their mother, we analyzed the following parameters: (1) frequency and duration spent on the side of the mother compared to the side of the familiar female, (2) the latency to reach each proximity zone and (3) the frequency and duration of behaviors within the three contact zones. We first described the effects of the neonatal lesions at 3 months of age, including all animals (N = 28), and then compared the effects of the lesions across the two testing ages, including only the animals tested at both ages (N = 21).
At 3 Months
As indicated in Supplementary Table S3 (see supplementary material), all animals entered more often and spent more time in all zones on the mother's side than on the familiar side (Stimulus: F1,24 = 10.59, p = .003 and F1,24 = 10.52, p = .003, respectively). Further, all animals entered in the proximity zone of the mother faster, more often, and for longer duration than in the proximity zone of the familiar female (Stimulus: F1,24 = 27.77, p <.001; F1,24 = 12.91, p = .001 and F1,24 = 21.35, p <.001, respectively). As illustrated in Supplementary Table S4, when in the proximity zones, all animals entered more often and spent more time in the contact zones on the mother's side than on the familiar female side (Stimulus: F1,24 = 12.91, p = .001, Cohen's d = .96, and F1,24 = 8.89, p = .006, Cohen's d = .82, for no contact; F1,24 = 21.33, p <.001, Cohen's d = 1.22 and F1,24 = 19.08, p <.001, Cohen's d = 1.17, for mesh-contact (F1,24 = 20.62, p <.001, Cohen's d = 1.12 and F1,24 = 5.25, p = .031, Cohen's d = .65, for physical-contact).
Across Testing Ages
As at 3 months, the number of visits and time spent in the zones on the mother's side was greater than on the familiar female side (Stimulus: F1,17 = 35.80, p <.001 and F1,17 = 50.92, p <.001, for frequency and duration respectively, see Fig. 2A). Further, all infants reached the proximity zone on the mother's side faster than the familiar female side (Stimulus: F1,17 = 37.99, p <.001) and entered in proximity with the mother more often (Stimulus: F1,17 = 43.60, p <.001) and for longer duration (Stimulus: F1,17 = 53.38, p <.001; Fig. 2B) than with the familiar female. In addition, all infants reached the proximity zone faster at 6 months than at 3 months (Age: F1,17 = 6.42, p = .021, Supplementary Tab. S3) and, when in the proximity zone, they spent more time on the mother's side at 6 months than at 3 months (Stimulus × Age: F1,17 = 10.37, p = .005; t20 = 3.08, p = .006; Fig. 2B), but the time spent in the proximity zone on the familiar female side did not vary with age (t20 = .59, p = .560, NS). When in the proximity zones, irrespective of group, sex and age, all animals entered more frequently and spent longer time on their mother's contact zones than on the familiar female contact zones (Stimulus effect for no-contact, mesh-contact and physical-contact zones: F1,17 = 28.15, p <.001; F1,17 = 44.51, p <.001; F1,17 = 38.70, p <.001 for frequency; F1,17 = 10.53, p = .005; F1,17 = 54.94, p <.001; F1,17 = 8.58, p = .009, for duration). Independently of their lesion and sex, all animals stayed longer in meshcontact with their mother at 6 months than at 3 months (Stimulus × Age effect: F1,17 = 15.84, p = .001; t20 = 3.56, p = .002), whereas the time spent in mesh-contact with the familiar female did not vary between testing ages (t20 = .94, p = .356, NS). As illustrated in Figure 2C, when in mesh-contact, there was a significant Group × Stimuli × Age interaction (F1,17 = 7.87, p = .012), indicating that animals from all groups manually reached for their mother through the mesh of the enclosure significantly more often than for the familiar female at both 3 months (Stimulus: F1,19 = 17.13, p = .001, Cohen's d = 1.17) and 6 months (Stimulus: F1,19 = 94.46, p <.001, Cohen's d = 2.31). Yet, at 6 months, Neo-A animals reached for their mother less frequently than did Neo-C animals (Lesion × Stimulus: F1,19 = 10.50, p = .004, t19 = 3.66, p = .002, Cohen's d = 1.62, Fig. 2C). Similarly, Neo-A animals were less often in physical-contact with their mother than Neo-C animals (Lesion × Stimulus: F1,17 = 8.48, p = .010; t19 = 2.90, p = .009, Cohen's d = 1.25; see Supplementary Tab. S4).
FIGURE 2.
Discrimination scores and behavior (mean SEM) in the proximity zones on the side of the mother (black bars) and the familiar female monkey (white bars), at 3- and 6-month-old (left and right side of each graph, respectively). (A) Frequencies of entering the back, front and proximity zones averaged across both groups. (B) Durations in seconds (s) spent in the proximity zones for males (M) and females (F) with sham-operations (Group Neo-C) or with neonatal amygdala lesions (Neo-A). (C) Frequencies of reaching behavior (defined as arm/hand passing through the mesh of the enclosure and stretched within the holding cage including the stimulus) when in the proximity (Mesh-Contact) zone for each group independently of sex. Indicates group difference p ≤ .05.
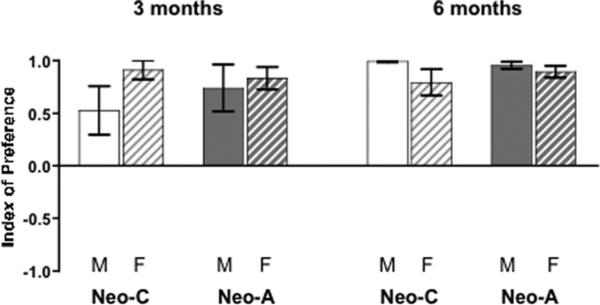
Index of Preference
Animals that did not enter in the proximity zones and thus did not display a choice between the two stimuli were excluded from this analysis. Hence, three animals (Neo-C-M1, Neo-C-F4, and Neo-A-F3) were excluded at 3 months and one animal (Neo-A-M2) was excluded at 6 months.
As illustrated in Figure 3, animals in both groups not only discriminated their mother from a familiar female but also displayed a clear preference for their mother at both ages (Lesion: F1,24 = .09, p = .769, NS; F1,19 = .29, p = .599, NS). Further, the mother preference appeared stronger at 6 months than at 3 months, although the difference did not reach statistical significance (Age: F1,13 = 3.33, p = .091, NS; with a moderate effect size: Cohen's d = .60). The mean IP values at 3 months were significantly greater than chance for Groups Neo-C females (t4 = 10.13, p = .001), Neo-A males (t8 = 3.36, p = .010) and Neo-A females (t5 = 7.93, p = .001), but only tended toward significance for Group Neo-C males (t4 = 2.26, p = .086). In this later group, the individual variability was larger than in the other groups due to two males (Neo-C-M3 and Neo-CM6) showing no preference for either stimulus (mean IP±SEM: –.17±.11; t1 = .15, p = .905, NS). Yet, when re-tested at 6 months, both Neo-C-M3 and Neo- C-M6 displayed a clear preference for their mother (both IP values = 1.00). Thus, at 6 months, the mean IP values were significantly different from chance for all Groups (Neo-C males: t3 = 120.00, p <.001; Neo-C females: t4 = 6.38, p < = .003; Neo-A males: t4 = 27.43, p <.001; Neo-A females: t5 = 16.02, p <.001).
FIGURE 3.
Average index of preference (IP, mean±SEM) for males (M) and females (F) with sham-operations (Group Neo-C) and amygdala lesions (Neo-A) at both 3 and 6 months of age. Values of IP between 0 and +1 indicate a preference for the mother, IP values betweenþ0 and −1 indicate a preference for the familiar female, and IP=0 indicates no preference for either stimulus.
General Activity and Vocalizations
At 3 Months
The exploratory activity of the proximity, front and neutral zones (for both the durations and frequencies), and of the back zone (frequency) was not affected by Neo-A lesions (all ps >.10; see Supplementary Tab. S5). Further, neonatal amygdala lesions did not significantly affect motor activity (locomotion, passivity, and other) at 3 months (all ps >.10; see Supplementary Tab. S5). Yet, independently of lesion groups, females remained passive for longer durations than males (Sex: F1,24 = 3.34; p = .080; Cohen's d = .67). Similarly, neonatal amygdala lesions did not affect the total number of vocalizations emitted (F1,24 = .10, p = .75, NS, Supplementary Tab. S5). Again, independently of lesion groups, females emitted more vocalizations than males (Sex: F1,24 = 8.11, p = .009; Cohen's d = 1.13).
Across Testing Ages
No further changes in motor activity (locomotion, passivity, and other) and exploratory activity (proximity, front, back, and neutral) in each zone were found at 6 months. Yet, all animals were more often passive at 6 months than at 3 months (Age: F1,17 = 5.83, p = .027). Further, the Sex × Age interaction showed a trend towards significance (F1,17 = 3.59, p = .075), likely because males from all groups appeared to be passive for longer duration at 6 than at 3 months (Means±SEM: 132.38±47.72 versus 308.64±51.40, respectively; t9 = 1.85, p = .097, NS; but a large effect size: Cohen's d = 1.01). The Sex × Age interactions for the frequency and duration of locomotion (F1,17 = 3.73, p = .070 and F1,17 = 4.11, p = .059, respectively) indicated that 6-month-old females were in locomotion more often than males (Means±SEM: 40.18±4.92 vs. 23.80±4.86; t19 = 2.36, p = .029; Cohen's d = 1.03). Similarly, all females were in locomotion for longer duration than all males at 6 months (Means±SEM: 176.91±27.30 vs. 109.24±32.29). Although the contrast did not reach statistical significance (t19 = 1.61, p = .124), the effect size was moderately large (Cohen's d = .70).
Amygdala lesions did not affect the total number of vocalizations emitted across testing ages (Lesion × Age: F1,17 = .22, p = .643, NS, see Supplementary Tab. S5). Irrespective of group and age, all females vocalized more than all males (Means±SEM: 319.83±35.99 vs. 198.75±38.36; Sex: F1,17 = 5.30, p = .034; Cohen's d = 1.01) and emitted more infant affiliative vocalization (girns) than males (Means±SEM: 83.10±14.19 vs. 38.69±15.13; Sex: F1,17 = 4.59, p = .047; Cohen's d = .94). Further, girns were emitted more at 6 than 3 months by all animals (Means±SEM: 75.65±13.12 vs. 46.14±10.24; Age: F1,17 = 7.03, p = .017; Cohen's d = .55). Yet, aggressive vocalizations (barks) displayed at 6 months was influenced by lesion and sex (Lesion × Sex: F1,17 = 5.35; p = .034), indicating that Neo-A females emitted more barks than Neo-A males (p = .050; Cohen's d = 1.50, see Supplementary Tab. S5 for Means) and Neo-C females (p = .034; Cohen's d = 1.63, see Supplementary Tab. S5 for Means), but did not differ from Neo-A males (p = .111, see Supplementary Tab. S5).
Discriminant Function Analyses
The overall Wilk's Lambda was significant at both (l (3 months)=.50, χ2 (7, N=25)=13.73, p=.033, and l (6 months)=.44, χ2 (6, N=20)=12.16, p= .058), indicating a good discrimination between lesion groups and accounting for 49.7% and 55.5% of the total variance at 3 and 6 months, respectively. Table 1 displays that the discriminant functions accurately classified 84% (3 months) and 85% (6 months) of the monkeys into their actual groups and this value differed significantly from chance as assessed by the Press's Q tests (Q=11.56, df=1, p<.001, and Q = 9.8, df=1, p<.001, respectively).
Table 1.
Discriminant Function Analyses
| Predicted Classification, 3 Months |
Predicted Classification, 6 Months |
||||
|---|---|---|---|---|---|
| Neo-C | Neo-A | Neo-C | Neo-A | ||
| Actual classification | |||||
| Nec-C (N = 10) | 8 (80%) | 2 (20%) | Neo-C (N = 9) | 7 (77.8%) | 2 (22.2%) |
| Nec-A (N = 15) | 2 (13.3%) | 13 (86.3%) | Neo-A (N = 11) | 1 (9.1%) | 10 (90.9%) |
Correct classification of 84% (at 3 months) and 85% (at 6 months) of original grouped cases based on duration, frequency and latency of proximity with the mother, IP, number of attempt to reach-mother and total vocalizations.
Closer analysis of the structure matrices revealed that frequency of proximity to the mother (r=.36) was the best predictor of group classification at 3 months, whereas frequency of manual reach towards mother (r=.72) was the best predictor of group classification at 6 months. None of the other variables accounted for a significant amount of variance and were not good predictors of group classification. Thus, both the number of times entering the mother's proximity zone at 3 months, and the number of times infants attempted to manually reach the mother at 6 months accurately discriminate Neo-A animals from Neo-C animals.
Low level of multicollinearity was found between the IP, the duration and frequency of proximity with the mother at both 3 months (VIF=1.95 for IP, 1.07 for duration of proximity and 1.08 for frequency of proximity) and 6 months (VIF=1.19 for IP, 1.01 for duration of proximity and 1.18 for frequency of proximity). At both ages the IP was the first variable entered, followed by duration of proximity and then frequency of proximity. These low levels of multicollinearity between these predictors strengthened the discriminant function analyses results.
Correlations With Lesion Extent
At both testing ages, none of the variables measured significantly correlated with the extent of the amygdala damage (average % damage of left and right).
DISCUSSION
Selective neonatal amygdala lesions performed during the third to fourth week after birth did not affect infants’ social discriminative abilities and preference for their biological mother at 3 and 6 months of age; a finding consistent with our previous study (Goursaud & Bachevalier, 2007), despite the different rearing conditions between the two studies (e.g., large social groups in this study vs. nursery peer-rearing in the previous one). The data suggest that the amygdala is not involved in the cognitive processes underlying discrimination and preference of the mother, at least when the amygdala damage occurred after the third to fourth week of age.
The sparing of social discrimination and strong expression of mother preference at both 3 and 6 months of age in amygdalectomized infants in the present study did differ from the weaker mother preference previously reported at 6 months in mother-reared amygdalectomized infants (Bauman et al., 2004). One factor could relate to the familiarity of the testing conditions. As already alluded to by Bauman et al. (2004), the weaker mother preference in their study may have resulted from testing the amygdalectomized infants in an unfamiliar environment. Both neonatal and adult-onset amygdala lesions increase exploratory behavior to novel stimuli (Izquierdo, Suda, & Murray, 2005; Kalin, Shelton, Davidson, & Kelley, 2001; Machado, Kazama, & Bachevalier, 2009; Meunier, Bachevalier, Murray, Málková, & Mishkin, 1999; Prather et al., 2001) and reduced emotional reactivity towards threatening environment (Bliss-Moreau, Bauman, & Amaral, 2011; Raper, Wallen, et al., 2013). Thus, the unfamiliar environment and stimulus (Bauman et al., 2004) might have increased the propensity for exploration resulting in reduced proximity to the mother in the amygdala-operated animals. An additional explanation is associated with the infant's ability to physically contact with the mother during the discrimination task. These physical contacts were absent in the earlier study (Bauman et al., 2004), given the presence of a solid, yet transparent, barrier between the infant and the mother, whereas in the present study, the infants could manually contact their mother through the mesh, though complete bodily contact was impossible. Interestingly, despite the presence of this restricted physical contact with the mother, the amygdalectomized infants reached significantly less for their mother than did controls at 6 months and this reaching behavior most accurately discriminated monkeys with amygdala lesions from controls at 6 months. Thus, the amygdala-operated infants might have been frustrated by their inability to enter in ventro-ventral contact with their mothers, resulting in less attempts to reach for her. Finally, it cannot be excluded that behavioral differences in maternal (and familiar female) responses towards the amygdala-operated animals as compared to the sham-operated animals during the discrimination tasks may have also affected the reaching behavior of the infants. Indeed, when in the large social group, mothers of amygdalectomized infants cradled their infants for shorter durations than did the mothers of control infants at 2 months of age (Raper et al., this issue). The results from these studies suggest that the specific dynamics of the expression of an infant's preference for its mother are markedly influenced by the specific testing context.
Whatever the reasons underlying the differences between the studies, all three studies (Bauman et al., 2004; Goursaud & Bachevalier, 2007; current one) are consistent with the fact that the amygdala is either not involved in the development of filial attachment in rhesus monkeys, or its contribution is required in the first month of life when the social bonding with the mother is first established, but not thereafter. Alternatively, permanent early damage to a neural structure can lead to important brain reorganization, resulting in significant functional compensatory mechanisms (Caminiti & Innocenti, 1981; Webster, Ungerleider, & Bachevalier, 1991). The early establishment of such compensatory mechanisms would be clearly warranted given the importance of primate filial attachment for the physical, emotional and social development of the infant primate. Additional anatomical, functional and behavioral studies are required to assess which other brain areas, such as the orbital frontal cortex (Bachevalier, Machado, & Kazama, 2011; Goursaud & Bachevalier, 2007), could compensate in the absence of a functional amygdala.
Although the amygdala might not be involved early in life in the typical development of primate filial attachment, its neonatal damage resulted in subtle sex-dependent changes in infant-mother interactions at about 4–5 months of age (Raper et al., this issue) reminiscent to the subtle changes observed in the present study (reduced physical contacts and proximity with their mother and increased aggressive vocalizations overall in females only). These behavioral changes could indicate decreased social motivation resulting from changes in social-reward processes. The amygdala critically contributes to reward processing in both humans and monkeys (Adolphs, 2010; Baxter & Murray, 2002; Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Murray, 2007, for reviews). In addition, amygdala lesions affect perception of personal space in humans (Kennedy, Gläscher, Tyszka, & Adolphs, 2009) and decrease interest towards infants typical of juvenile females (Toscano et al., 2009). Evidence for a failure to assess the reward value of stimuli has also been shown in monkeys with neonatal amygdala lesions while performing a discrimination devaluation task in which they demonstrate an inability to inhibit selection of objects associated with a devalued food (Kazama & Bachevalier, 2013).
Finally, the existence of alternative neural pathways, others than the amygdala, to support the development of filial attachment in monkeys needs further investigation considering recent developmental studies in rodents and humans. Indeed, albeit the differences in the level of sensory-motor development and brain maturity of the young at birth and the different ecological conditions in early life between rodents and primates, recent research in rodents indicates that the rat amygdala may not be involved in the development of infant attachment (Sullivan et al., 2011). During the brief sensitive period immediately after birth (postnatal day, PN, 1-9), rat pups display an enhanced ability for preference learning, which overlaps with a period of stress hyporesponsiveness (PN 4-14) facilitated by the attenuation of amygdala plasticity (Landers & Sullivan, 2012; Moriceau et al., 2010). Thus, at an early age, structures others than the amygdala are involved in mother preference learning (Moriceau, Wilson, Levine, & Sullivan, 2006; Sullivan & Wilson, 1993). However, this period is followed by an increased involvement of the amygdala for fear learning as the pup is ready to leave the nest. Likewise, two recent fMRI studies in humans showed a gradual increment in amygdala activation in response to mother- versus strangers-related stimuli (faces) emerging in mid-childhood (Todd et al., 2011; Tottenham et al., 2012; Tottenham & Sheridan, 2010). Accordingly, the present data in monkeys showing the presence of discriminatory behaviors and preferential responses toward the mother at both 3 and 6 months of age in the absence of a functional amygdala suggest that the learning of maternal characteristics and the development of the filial attachment per se, can most likely be supported by amygdala-independent pathways. At the same time, the subtle behavioral changes observed in the same animals (Raper et al., this issue; current study) suggest that the primate amygdala, like the rodent amygdala, most likely plays a protracted role in socio-emotional behaviors later in life in concert with other structures (Bauman et al., 2004; Bauman, Toscano, Mason, & Amaral, 2007; Raper et al., 2008; Stone, Baron-Cohen, Calder, Keane, & Young, 2003; Toscano et al., 2009; Wallen, Raper, Stephens, Goursaud, & Bachevalier, 2006).
Yet, a role of the amygdala in the very early stage of the bonding period cannot be totally excluded given that, in both rodents and primates, atypical maternal care, such as neglect, abuse, or maternal separation, during the postnatal period has been associated with socio-emotional abnormalities and dysfunctions, including atypical filial attachment (Sanchez, Ladd, & Plotsky, 2001), and with structural and functional changes in the amygdala (Gee et al., 2013; Ono et al., 2008; Lupien et al., 2011; Kikusui & Mori, 2009; Mehta et al., 2009; Sabatini et al., 2007; Tottenham et al., 2010). Thus, the precise role of the amygdala and associated brain structures in the development of typical filial attachment requires further investigations and is essential to further our understanding of the neural basis associated with dysfunctional relationships in developmental neuropsychiatric disorders in humans (Goursaud & Bachevalier, 2008; Sullivan et al., 2011).
Supplementary Material
Acknowledgments
This research was founded by grants from the National Institute for Mental Health (R01MH050268; R03MH076031), the National Center for research Resources to the Yerkes National Research Center (P51 RR00165; YNRC Base Grant; currently supported by the Office of Research Infrastructure Programs/OD P51OD11132) and the Center for Behavioral Neuroscience (NSF IBN 9876754; CBN 069-2005Y). The authors are grateful to Amy Henry and Trina Villarreal who assisted in performing the discrimination tests as well as to all members of the Bachevalier laboratory who participated in the surgical procedures. The authors also wish to thank the Veterinarian and Animal Care teams at both the main station and field station of the Yerkes National Primate Research Center (Emory University, Atlanta, GA). The Yerkes NPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Care (AAALAC) International.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher's web-site.
REFERENCES
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The biology of fear. Current Biology. 2013;23(2):R79–R93. doi: 10.1016/j.cub.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Machado CJ, Kazama A. Behavioral outcomes of late-onset or early-onset orbital frontal cortex (areas 11/13) lesions in rhesus monkeys. Annals of the New York Academy of Science. 2011;1239:71–86. doi: 10.1111/j.1749-6632.2011.06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother– infant interactions after neonatal amygdala lesions in rhesus monkeys. The Journal of Neuroscience. 2004;24(3):711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Amaral DG. Continued analysis of social development in rhesus monkeys with neonatal amygdala lesions. Society for Neuroscience. 2007 Abstracts, 748.2. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Review Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behavioral Neuroscience. 2011;125(6):848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother– infant bonding and the evolution of mammalian social relationships. Philosophical Transactions of the Royal Society London B: Biological Sciences. 2006;361(1476):2199–2214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Innocenti GM. The postnatal development of somatosensory callosal connections after partial lesions of somatosensory areas. Experimental Brain Research. 1981;42(1):53–62. doi: 10.1007/BF00235729. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Keverne EB. Genes, brains and mammalian social bonds. Trends in Ecology and Evolution. 2005;20(10):561–567. doi: 10.1016/j.tree.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic versus sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsycho-pharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Nowak R. 2-NAP, a peripheral CCK-A receptor antagonist, modulates the development of a preference for the mother by the newborn lamb. Pharmacology, Biochemistry, and Behavior. 2000;67(3):603–611. doi: 10.1016/s0091-3057(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Goursaud A-PS, Bachevalier J. Preference of infant monkeys with neonatal bilateral amygdala, orbital frontal cortex or hippocampal lesions for their human adoptive mother. Behavior Brain Research. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Goursaud A-PS, Bachevalier J. The origin of developmental psychopathologies: Insights from non-human primate studies. In: Burbacher TM, Sackett GP, Grant KS, editors. Primate models of children's health and developmental disabilities. Elsevier, Inc.; 2008. pp. 11–44. (Chap. 2. [Google Scholar]
- Hair JF, Black WC, Babin BJ, Anderson RE. Multivariate data analysis. 7th ed. Prentice Hall; New Jersey: 2009. p. 263. [Google Scholar]
- Harlow HF, Zimmermann RR. Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science. 1959;130(3373):421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Pediatrica Supplement. 1994;s397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. The Journal of Neuroscience. 2005;25(37):8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. The Journal of Neuroscience. 2001;21(6):2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Bachevalier J. Effects of neonatal amygdala damage on concurrent discrimination learning and reinforce devaluation in monkeys. Journal of Psychology and Psychotherapy. 2013 doi: 10.4172/2161-0487.S7-005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Perrin G, Meurisse M, Ferreira G, Levy F. Cortical and medial amygdala are both involved in the formation of olfactory offspring memory in sheep. European Journal of Neuroscience. 2004;20(12):3433–3441. doi: 10.1111/j.1460-9568.2004.03812.x. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Gläscher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nature Neuroscience. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. Journal of Neuroendocrinology. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34(2–3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Séguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9(2):147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: Partners in the fear circuit. Journal of Physiology. 2013;591(Pt10):2381–2391. doi: 10.1113/jphysiol.2012.248575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11(12):4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Developmental Psychobiology. 2005;47(3):230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. The Journal of Neuroscience. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Newman JD, Bachevalier J. Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Research. 1997;758(1–2):180–186. doi: 10.1016/s0006-8993(97)00212-6. [DOI] [PubMed] [Google Scholar]
- Nowak R, Keller M, Lévy F. Mother-young relationships in sheep: A model for a multidisciplinary approach of the study of attachment in mammals. Journal of Neuroendocrinology. 2011;23(11):1042–1053. doi: 10.1111/j.1365-2826.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuro-science. 2008;156:1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106(4):653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Raper J, Bachevalier J, Wallen K, Sanchez M. Neonatal amygdala lesions alter basal cortisol levels in infant rhesus monkeys. Psychoneuroendocrinology. 2013;38:818–829. doi: 10.1016/j.psyneuen.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens S, Goursaud A-PS, Wallen K, Bachevalier J. Neonatal lesions of the amygdala result in reduced fear behavior in male rhesus monkeys (Macaca mulatta) reared in a semi-naturalistic environment. Society for Neuroscience Abstracts. 2008;791.6 [Google Scholar]
- Raper J, Stephens S, Sanchez M, Bachevalier J, Wallen K. Neonatal amygdala lesions alter infant-mother interactions in infant rhesus monkeys living in species-typical social groups. Developmental Psychobiology. 9999:1–12. doi: 10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez M, Stephens SB, Henry A, Villareal T, Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Hormone and Behavior. 2013;63:646–658. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Developmental Psychopathology. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder A, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia. 2003;41(2):209–220. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Perry R, Sloan A, Kleinhaus K, Burtchen N. Infant bonding and attachment to the caregiver: Insights from basic and clinical science. Annals of the New York Academy of Sciences. 2011;1239:71–86. doi: 10.1016/j.clp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Role of the amygdala complex in early olfactory associative learning. Behavioral Neuroscience. 1993;107(2):254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ. The changing face of emotion: Age-related patterns of amygdala activation to salient faces. Social Cognitive and Affective Neuroscience. 2011;6(1):12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano JE, Bauman MD, Mason WA, Amaral DG. Interest in infants by female rhesus monkeys with neonatal lesions of the amygdala or hippocampus. Neuroscience. 2009;162:881–891. doi: 10.1016/j.neuroscience.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Developmental Science. 2012;15(3):307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K, Raper J, Stephens S, Goursaud A-P, Bachevalier J. Behavioral effects of neonatal amygdala lesions in male monkeys living in a semi-naturalistic environment. Society for Neuroscience Abstracts. 2006;578.20 [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. The Journal of Neuroscience. 1991;11(4):1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Young AZM, Hammock EA. Anatomy and neurochemistry of the pair bond. Journal of Comparative Neurology. 2005;493(1):51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.