Abstract
Background
Although age and sex distributions of calcified plaque (CCP) have been well described in the general population, noncalcified plaque (NCP) distributions remain unknown. This is important because NCP is a putative precursor for clinical CAD and could serve as a sentinel for aggressive primary prevention, especially in higher risk populations. We examined the distributions of NCP and CCP in healthy 30-74 year old individuals from families with early-onset coronary artery disease (CAD).
Methods and Results
Participants in the GeneSTAR family study (N=805), mean age 51.1 ± 10.8 years, 56% female, were screened for CAD risk factors and for coronary plaque using dual-source CT angiography. Plaque volumes (mm3) were quantified using a validated automated method. The prevalence of coronary plaque was 57.8% in males and 35.8% in females (p<0.0001). NCP volume increased with age (p<0.001) and was higher in males than females (p<0.001). Although NCP, as a percent of total plaque, was inversely related to age (p<0.01), NCP accounted for most of the total plaque volume at all ages, especially in males and females <55 years (>70% and >80%, respectively). Higher Framingham risk was associated with the number of affected vessels (p<0.01) but 44% of males and 20.8% of females considered intermediate risk had left main and/or 3-vessel disease involvement.
Conclusions
The majority of coronary plaque was noncalcified, particularly in younger individuals. These findings support the importance of assessing family history and suggest that early primary prevention interventions may be warranted at younger ages in families with early onset CAD.
Keywords: atherosclerosis, plaque distribution, CT angiography, family study, asymptomatic
ECG gated noncontrast computed tomography is routinely used to quantify calcified coronary plaque (CCP) to assess coronary artery disease (CAD) risk in higher risk healthy populations. CCP is associated with CAD risk factors, particularly male sex and older age, and is generally less useful in younger people 1, 2. Coronary plaque calcification is a late manifestation of atherosclerosis 3. Earlier stages of atherogenesis are represented by noncalcified or mixed composition plaques containing extracellular lipid and fibrous tissue 4, 5 and are particularly prone to plaque rupture, thrombosis, and acute CAD events 6, 7. Thus, CCP on noncontrast CT imaging is used as a marker for subclinical CAD and as a proxy for the extent of atherosclerosis. However, because this method cannot detect noncalcified plaque (NCP) 8, 9 it does not necessarily reflect the true extent of coronary artery plaque 10. The extent of subclinical NCP, a putative precursor for CAD events, may have important implications for primary prevention, especially in younger people from higher risk populations.
Familial-clustered CAD accounts for ∼60% of all CAD prior to 65 years of age 11-13. A positive family history of early-onset CAD in a parent or sibling is associated with a markedly increased risk of CAD events 11, 14. Apparently healthy adults from these families have a high prevalence of inducible ischemia by myocardial perfusion imaging 15 but the extent of total coronary plaque and NCP remains unknown. To date only coronary calcium scores have been studied, with higher levels found in persons from families with early-onset CAD16, 17. Because plaque vulnerability is so closely linked to incident CAD events and NCP is more likely to represent vulnerable plaque, we designed this study to examine the true extent of total coronary plaque, inclusive of NCP, using multidetector computed tomographic angiography (CTA) in healthy asymptomatic members of early-onset CAD families.
Methods
Sample and Recruitment
Participants (n=805) were randomly selected and then recruited (92% of those invited participated) from the larger ongoing Genetic Study of Atherosclerosis Risk (GeneSTAR), a prospective study of 4000 individuals designed to characterize genetic and biological factors associated with cardiovascular disease phenotypes in 883 families with early-onset coronary heart disease. Probands <60 years of age with documented acute myocardial infarction, unstable angina with coronary revascularization, or acute angina with angiographic evidence of a flow-limiting stenosis of >50% diameter in at least one coronary artery were identified during hospitalization and excluded. Apparently healthy siblings and the offspring of the probands and siblings were eligible if they were 30 to 75 years of age and had no known personal history of CAD. Siblings and offspring were excluded if they had systemic autoimmune disease, known allergy to iodinated contrast media, or chronic kidney disease. The study was approved by the Johns Hopkins Medicine Institutional Review Board and all participants gave informed consent.
Participant Screening
Participants underwent a comprehensive risk factor screening following a 12-hour overnight fast. Medical history, pedigree and family history information, and current medication use were elicited. A physical exam was performed by a study physician. Height was determined using a fixed stadiometer and weight was measured on a balance scale with the subject wearing light clothing and no shoes. Body mass index (BMI) was calculated as kg/m2. Current cigarette smoking behavior was assessed by self-report and verified by expired carbon monoxide (CO) levels of ≥8 ppm. Blood pressure was measured according to the American Heart Association guidelines three times over the course of the day. Hypertension was defined as an average blood pressure ≥140 mmHg systolic, or ≥90 mmHg diastolic, and/or use of an antihypertensive drug. Blood was obtained and total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using the United States Centers for Disease Control standardized methods 18. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula 19 for persons with triglyceride levels up to 400 mg/dl. Direct measurement of LDL cholesterol using ultracentrifugation was used for persons with triglyceride levels ≥4.52 mmol/l (400 mg/dL) (n=5). Glucose concentration was measured using the glucose oxidase method; 20 type 2 diabetes was defined as a physician diagnosed history, a fasting glucose level ≥6.99 mmol/l (126 mg/dl), and/or use of prescribed hypoglycemic medications. We calculated the 10-year Framingham Risk Score (FRS) to categorize siblings as low risk (<10%), intermediate risk (10-20%), or high risk (≥20%) for total CAD events based on their risk factor levels 21.
Assessment of Subclinical Coronary Plaque
All participants underwent coronary CTA using a newest generation dual-source multi-detector scanner (SOMATOM Definition Flash, Siemens Medical Solutions, Forchheim, Germany). Because of the high temporal resolution and excellent image quality of the scanner, beta-blockade was not necessary for reducing the heart rate 22. A noncontrast scan was first performed to determine the coronary artery calcium volume as well as the traditional Agatston score. Subsequently coronary CTA was performed to examine the presence, location, composition, and severity of any coronary plaque. Approximately 80 mL of isosmolar contrast agent (320 mg iodine/mL) was injected at 6 mL/s. Prospective ECG-gating was used in patients with low, steady heart rates (<65 bpm) and little heart rate variability. For patients with variable heart rates or heart rates >65 bpm, retrospective gating with dose modulation was used. Tube potential was selected on a per patient basis by the performing technologist assessment of patient size; 100 kV was used for patients that were not overweight or obese, otherwise 120 kV was used. We reconstructed 0.75-mm thick axial slices at 0.4-mm intervals with a B26 kernel; 10 reconstructions were done at 10% increments in the R-R interval. All scans were evaluated with the CT radiologist blinded to the participants' risk factor profiles. The coronary arterial tree was segmented according to the standard American Heart Association classification,23 and the segments were investigated for plaque and luminal narrowing. Any focal stenoses >50% in severity were identified with the use of quantitative software (COR Analyzer System, Rcadia Medical Imaging, Haifa, Israel) 24, 25 and verified by the expert reader.
Quantitative Plaque Volumes
The volume of CCP was measured on a workstation (Leonardo Multimodality Workstation, Syngo, Siemens Medical Solutions, Malvern, PA) using noncontrast images. Regions of interest were placed over each of the coronary arteries and a threshold of >130 HU was used for determining per vessel volumes of CCP (mm3) using standard validated methods 26, 27. Vessel CCP volumes were summed for a total CCP volume. A total Agatston score was also determined using standard methods 28.
For each affected coronary segment, noncalcified plaque volumes (mm3) were quantified using AUTOPLAQ (Cedars-Sinai Medical Center, Los Angeles, CA), as previously described 29. This automated method of NCP measurement has high interobserver correlation (r=0.97) 30, and has been previously validated against intravascular ultrasound (IVUS)29. For the present study, we also found excellent reproducibility for measured NCP volumes (intraobserver r=0.99, mean percent error 3.6%) based on two blinded reads performed 6-12 months apart on a random sample (N=30). To quantify each affected segment, CTA images were examined in multiplanar format and proximal and distal limits of the plaque were manually marked. Control points defining the lumen center-line were placed. Subsequent NCP plaque quantification was then fully automated using adaptive algorithms that are scan specific per individual 29. Segmental NCP volumes were summed for a total NCP volume per vessel, including the left main (LM), left anterior descending (LAD), left circumflex (LCX), and right coronary arteries (RCA). The vessel specific volumes were summed for a total NCP volume. Total coronary plaque (TCP) was calculated as the sum of CCP + NCP. The percent of total plaque consisting of NCP and CCP was calculated by dividing by TCP for each. For stenoses >50% in severity, plaques were classified as noncalcified (no calcium), calcified (>50% volume calcified plaque), or mixed (≤50% volume calcified plaque), based on the quantified volumetric measurements.
Statistical Analyses
Standard descriptive analyses were used to examine distributions of sociodemographic and CAD risk factor variables. The Kolmogorov-Smirnov statistic was used to test for normality of continuous variables. The median and interquartile range of NCP and CCP volumes, as well as the relative amount of NCP and CCP to total plaque volume, were examined by age and sex in subjects with coronary plaque. Total coronary plaque was transformed as log [TCP + 0.5(minimum)], given the non-normal distribution and presence of zero plaque in many individuals. Standard multivariable regression analyses were performed predicting plaque outcomes using the Generalized Estimating Equations (GEE) to account for nonindependence within families. The Cochran Armitage Trend test was used to examine trends across Framingham Risk categories for the prevalence of plaque, including by vessel location, and stenoses >50%. Given the variability of strength of family history within families, we used pedigree information in separate analyses in a subset of full siblings of affected probands to determine the incremental effect of strength of sibling history on subclinical coronary plaque burden. TCP volumes in participants with a greater number of siblings affected with clinically manifest CAD (strong family history, defined as ≥50% of siblings in the family) were compared to those with a lesser sibling history (only the proband or <50% of siblings affected in the family). A multivariable GEE regression model controlled for age, sex, race, current smoking, hypertension, diabetes, LDL cholesterol, statin medication use, and within family correlations. Logistic regression predicting any stenoses >50% was performed with the same dependent variables and inclusion of the transformed CCP, NCP, or TCP volumes, and the area under the curve (AUC) calculated.
Results
Population Demographics and Presence of Coronary Plaque
The study population consisted of 805 apparently healthy individuals identified from 388 families with the onset of CAD <60 years of age (one index case per family, 2.1 ± 1.5 participants per index case). Study participants were siblings (n=424) of the index patient or adult offspring of the index patient or the siblings (n=381). The sample was 56.1% female and 39.2% African American. All were healthy and without any chest pain or angina-equivalent symptoms. Overall, 45.5% of the total population had subclinical coronary plaque, including 5.5% with exclusively NCP without any CCP. Population demographics and CAD risk factors by the presence or absence of any coronary plaque are shown in Table 1. Coronary plaque was significantly associated with older age, male sex, white race, hypertension, diabetes, lower HDL cholesterol and higher triglyceride levels. There was no difference in mean LDL cholesterol levels but subjects with plaque were more likely to be taking a statin medication, suggesting that subjects with plaque had an a priori higher LDL cholesterol levels that triggered initiation of statin therapy. Subjects with plaque had a significantly higher 10-year mean Framingham Risk Score than those without plaque but overall most subjects were in the low Framingham risk group.
Table 1. Population demographics and coronary artery disease risk factors by the absence or presence of coronary plaque (N=805)*.
| Plaque Absent (N=439) |
Plaque Present (N=366) |
p-value | |
|---|---|---|---|
| Age, years | 46.4 ± 9.7 | 56.8 ± 9.0 | <0.0001 |
| Female sex, % | 64.2 | 35.8 | <0.0001 |
| African American, % | 41.5 | 34.4 | 0.04 |
| Hypertension, % | 30.6 | 57.9 | <0.0001 |
| Diabetes, % | 8.0 | 15.9 | 0.0005 |
| Current smoking, % | 18.3 | 20.5 | 0.43 |
| LDL cholesterol, mmol/l (mg/dl) | 2.92 ± 0.92 (113.7 ± 35.4) |
2.92 ± 0.98 (112.8 ± 38.0) |
0.73 |
| HDL cholesterol, mmol/l (mg/dl) | 1.51 ± 0.45 (58.2 ± 17.5) |
1.42 ± 0.43 (55.0 ± 16.6) |
0.009 |
| Triglycerides, mmol/l (mg/dl) | 1.12 ± 0.62 (105.9 ± 54.7) |
1.41 ± 0.91 (125.1 ± 80.4) |
0.0001 |
| BMI, kg/m2 | 30.0 ± 6.5 | 30.6 ± 5.5 | 0.19 |
| Statin medications, % | 14.8 | 39.3 | <0.0001 |
| Calculated 10-year Framingham Risk | 4.6 ± 3.8 | 9.6 ± 7.2 | <0.0001 |
Continuous variables presented as mean ± 1 standard deviation
Prevalence of Coronary Plaque
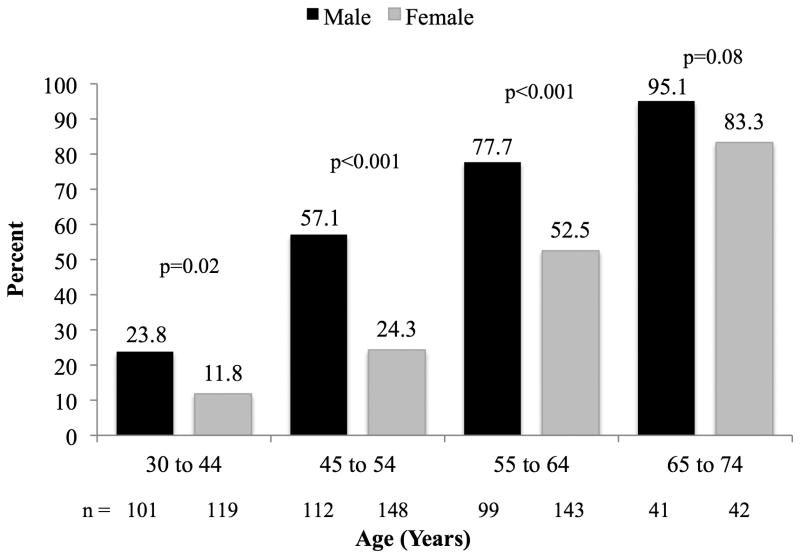
Figure 1 shows the prevalence of coronary plaque for males and females by age. The prevalence of coronary plaque increased with age in both males and females (p<0.0001 for both). The prevalence of coronary plaque was significantly higher in males at all age ranges until after age 65 years when both sexes had a prevalence >80% (p<0.0001 and p=0.08, respectively). Even in the youngest age group 30 to 45 years, nearly one quarter of males had plaque, increasing to nearly 60% in those 45 to 54 years. Using calcium scoring alone, 39.8% of all participants were identified as having plaque, whereas the addition of NCP information from the CTA identified 45.2% of the population as having plaque. The additive value of CTA varied by age, sex and race. Of the 364 persons with plaque on CTA, 87.9% had plaque on CAC screening, yielding an incremental identification of plaque by CTA of 12.1% overall.
Figure 1. Prevalence of totoal plaque by age* and sex† (N=805).
* p <0.0001 for age trend in both males and females
† p <0.0001 for overall sex differences by age group (ANOVA)
Age and Sex Distribution of Noncalcified and Calcified Plaque Volumes
Of those subjects with coronary plaque, females were twice as likely to have exclusively NCP compared to males, (16.8% vs 8.3%, p= 0.01). The volumes of NCP and CCP by age and sex in subjects with plaque are shown in Table 2. NCP volume was strongly associated with older age and male sex, p<0.0001 for both. CCP volume was also associated with older age and male sex, <0.0001 and p=0.0002, respectively. The total Agatston score is shown for comparison with calculated CCP volumes. Agatston scores were highly correlated with CCP volumes (r=0.98) with similar distributions by age and sex.
Table 2. Sex-specific noncalcified and calcified coronary plaque volumes by age group in subjects with any coronary plaque* (N=366).
| Ages 30-44 (N=38) |
Ages 45-54 (N=100) |
Ages 55-64 (N=154) |
Ages 65-74 (N=74) |
Age p-value |
||
|---|---|---|---|---|---|---|
| Noncalcified plaque volume, mm3 | Male | 94 [31-161] |
115 [51-241] |
212 [106-444] |
415 [236-653] |
<0.0001 |
| Female | 60 [30-227] |
93 [54-164] |
106 [38-205] |
170 [89-282] |
0.02 | |
| p-value | 0.51 | 0.35 | <0.0001 | <0.0001 | --- | |
|
| ||||||
| Calcified plaque volume, mm3 | Male | 8 [1-38] |
19 [4-88] |
77 [10-296] |
386 [101-776] |
<0.0001 |
| Female | 3 [1-22] |
14 [1-44] |
34 [7-108] |
48 [10-303] |
0.003 | |
| p-value | 0.51 | 0.12 | 0.005 | 0.004 | --- | |
|
| ||||||
| Agatston Score | Male | 6 [1-46] |
16 [3-81] |
86 [10-354] |
454 [100-931] |
<0.0001 |
| Female | 4 [1-24] |
9 [0-45] |
37 [7-104] |
58 [7-351] |
0.002 | |
| p-value | 0.87 | 0.13 | 0.004 | 0.005 | --- | |
Volumes as median [IQR]
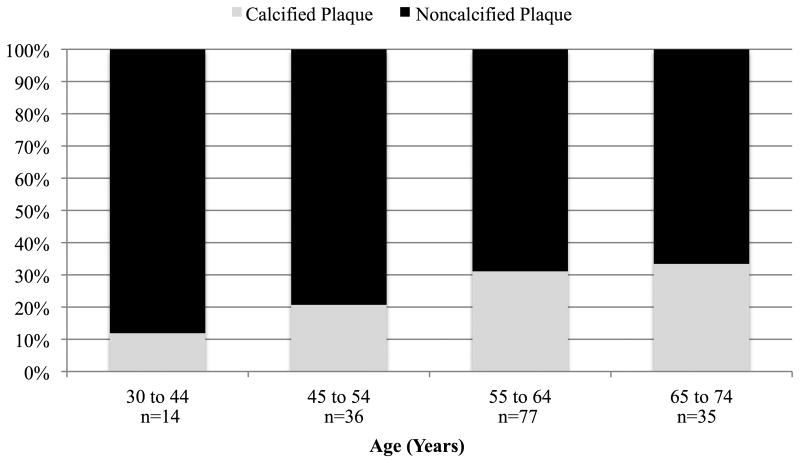
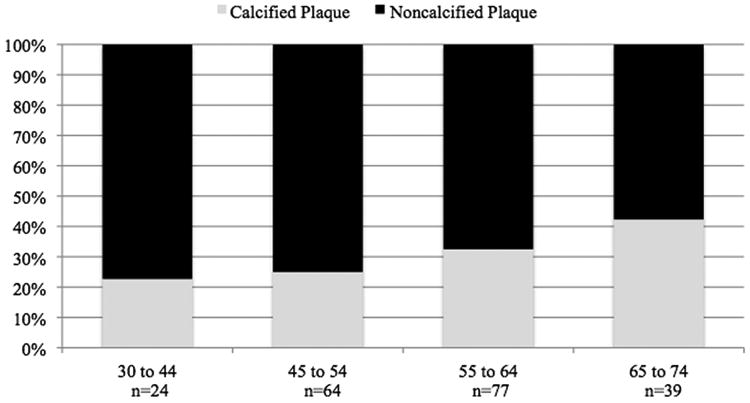
Figure 2 shows the composition of total plaque volume by age and sex in subjects with plaque. NCP accounted for more than half of TCP in both sexes at all ages and represented more than 75% of TCP in men, and 80% in women <55 years of age. NCP, as a percent of TCP, was significantly and inversely related to increasing age in both men and women (p<0.01). Adjusting for age, the percent of TCP that was non-calcified tended to be higher in females (p=0.06).
Figure 2. Percent Coronary Plaque Volumes by Composition, Age, and Sex in Subjects with Plaque.
A. Male (N=204)
*p<0.0001 for percent calcified plaque with increasing age group
B. Female (N=162)
*p=0.007 for percent calcified plaque with increasing age group
Multivariable regression analysis controlling for age, sex, race, statin medication use, and intrafamilial correlation was performed for the transformed volumes of TCP. Covariates in the model included LDL cholesterol level, body mass index, and the presence of hypertension, diabetes, and current smoking behavior. All modifiable risk factors were independently associated with TCP, including the presence of hypertension (p=0.005), diabetes (p=0.05), and current smoking behavior (p=0.0004), as well as higher LDL cholesterol levels (p=0.02). Higher body-mass-index showed a near significant association (p=0.08).
Using the family design of this study to examine the significance of family history to TCP, we examined TCP volumes in a subset of 338 full siblings of affected probands with a strong sibling history (n=49) compared to those with a lesser sibling history (n=289). In this subset, subjects were 58.9±7.4 years of age (range 36 to 74); 55% female; 36% African American. Subjects with a strong sibling history (n=49) were 3.0 times (95% CI 1.3–6.6) more likely to have any coronary plaque and had markedly greater volumes of all forms of plaque than those with a lesser sibling history (n=289) (Table 3). A strong sibling history remained a significant independent predictor of TCP volume when adjusted for all other variables in multivariate GEE regression analysis.
Table 3. Distribution of coronary plaque by strength of sibling history (N=338).
| Strong Sib History (n=49) Median [IQR] |
Lesser Sib History (n=289) Median [IQR] |
Unadjusted p-value | Adjusted p-value* | |
|---|---|---|---|---|
| Any plaque, % | 83.7 | 63.3 | 0.007 | 0.04 |
| Total plaque (mm3) | 278.2 [653.6] | 85.3 [314.9] | <0.001 | 0.05 |
A multivariate GEE regression model controlled for age, sex, race, current smoking, hypertension, diabetes, LDL cholesterol, statin medication use, and within family correlations.
Distributions of Plaque Characteristics by Framingham Risk
The sex-specific distributions of plaque characteristics by 10-year FRS categories are shown in Table 4. The majority of men and women were low FRS (72% and 88%, respectively), or intermediate-risk (20% and 11%, respectively). Although higher FRS was associated with a higher prevalence of plaque and more vessels involved, there was a high prevalence of plaque even in low or intermediate FRS men, with approximately 50% and 75% affected, respectively. Similarly over 30% of low-risk and 50% of intermediate risk women had plaque. Three vessel and/or left main involvement occurred in nearly 15% of low risk men and over 40% of intermediate risk men. Plaque was most prevalent in the LAD in both sexes. In intermediate risk men and women, LAD plaque was present in 70% and 50%, respectively. Nearly one quarter of intermediate risk males had measurable disease in the LM coronary artery. The overall prevalence of at least one stenosis >50% in severity was approximately 8% and 21% in low and intermediate risk males, respectively, and was composed primarily of mixed plaques containing both NCP and CCP. Given that 39% of subjects with coronary plaque and 15% of subjects without coronary plaque were taking statin medications, we performed the same analyses in 254 males (n=124 with plaque) and 342 females (n=98 with plaque) who were not taking statin medications and found similar sex-specific distributions of FRS categories and the same significant trends for the prevalence of plaque phenotypes across FRS categories. Although this was a cross-sectional study and the incremental value of CTA over coronary calcium alone for clinical outcomes is yet to be determined in this population, noncalcified plaque volume was significantly associated with the presence of at least one stenosis >50% (p<0.0001), independent of all traditional risk factors. Total plaque volume improved the AUC from 0.87 to 0.90 compared to the Agatston Score alone (p=0.04).
Table 4. Sex-specific plaque prevalence and characteristics by Framingham risk group (n=805).
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Framingham Risk | Low N=254 |
Intermediate N=72 |
High N=27 |
p-value for trend | Low N=396 |
Intermediate N=48 |
High N=8 |
p-value for trend |
| Any plaque | 49.2 | 73.6 | 96.3 | <0.0001 | 31.6 | 58.3 | 87.5 | <0.0001 |
| #Affected vessels | ||||||||
| 0 | 50.8 | 26.4 | 3.7 | <0.0001 | 68.4 | 41.7 | 12.5 | <0.0001 |
| 1 | 20.5 | 13.9 | 11.1 | 13.9 | 20.8 | 25.0 | ||
| 2 | 14.6 | 18.0 | 25.9 | 8.4 | 22.9 | 25.0 | ||
| 3 | 10.6 | 27.8 | 37.1 | 7.3 | 4.2 | 37.5 | ||
| 4 | 3.5 | 13.9 | 22.2 | 2.0 | 10.4 | 0.0 | ||
| Location* | ||||||||
| LM | 7.1 | 23.6 | 33.3 | <0.0001 | 5.6 | 16.7 | 25.0 | 0.0005 |
| LAD | 43.7 | 70.8 | 92.6 | <0.0001 | 27.5 | 50.0 | 75.0 | <0.0001 |
| LCX | 18.9 | 45.8 | 59.3 | <0.0001 | 11.9 | 29.2 | 25.0 | 0.002 |
| RCA | 26.0 | 48.6 | 77.8 | <0.0001 | 15.7 | 25.0 | 62.5 | 0.0007 |
| At least one stenoses >50%† | ||||||||
| Any plaque | 7.5 | 20.8 | 40.7 | <0.0001 | 4.6 | 14.6 | 0.0 | 0.07 |
| NCP | 1.6 | 5.6 | 7.4 | 0.02 | 0.5 | 4.2 | 0.0 | 0.07 |
| CCP | 2.4 | 2.8 | 18.5 | 0.0008 | 3.0 | 6.3 | 0.0 | 0.56 |
| Mixed | 4.3 | 15.3 | 29.6 | <0.0001 | 1.5 | 6.3 | 0.0 | 0.14 |
LM: Left main; LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery
NCP: Noncalcified plaque; CCP: Calcified Plaque
Discussion
In this first study of the age and sex distributions of noncalcified coronary plaque in healthy men and women from families with early-onset CAD, we found a strikingly high prevalence of coronary plaque, even in participants under 45 years of age. Importantly, noncalcified plaque was more prevalent than calcified plaque, particularly in those <55 years of age, in whom 75-80% of plaque was noncalcified. It is noteworthy that coronary calcium may markedly underestimate the total plaque burden in this population. In addition, we found a high prevalence of triple vessel and/or left main artery plaque. This is particularly striking considering that the majority of participants had a low or intermediate FRS. Finally, we were able to demonstrate that strength of sibling history was an independent predictor of the presence and extent of total coronary plaque. These findings suggest that conditioning aggressive primary prevention on traditional coronary calcium scoring algorithms or on the Framingham Risk Score, which does not include family history, may obviate appropriate risk reduction interventions in high risk individuals, such as young men and women with a family history of early-onset CAD.
Although a positive family history of CAD has been independently associated with higher coronary calcium scores16, 17, there is a paucity of data regarding the prevalence of NCP in asymptomatic populations with a few studies reporting on older, higher-risk individuals referred to CTA for clinical suspicion of CAD31, 32 or in Asian populations33. We did not have a control population for a direct comparison of NCP, but the degree of age, sex, and race specific coronary calcification using the Agatston Score was notably higher than that observed in the Multi-Ethnic Study of Atherosclerosis (MESA)34. Importantly, given the excess risk of incident CAD already demonstrated in the GeneSTAR family population35 and their concomitant higher rates of silent myocardial ischemia on stress myocardial perfusion imaging15, it is most likely that the prevalence and amount of noncalcified and total coronary plaque are also greater than would be seen in the general population. Moreover, strength of sibling history of early-onset CAD was independently associated with TCP volumes, highlighting the fact that family history is an important determinant of our findings. The classification by sibling history into lesser vs. greater family risk was in part based on previous studies showing that ≥2 affected first-degree relatives conveys the highest risk36, 37. Further studies using continuous comprehensive family history scores are needed to support heritability of NCP.
Multiple studies have shown that the presence of CCP in asymptomatic individuals from the general population is associated with incident CAD events 38-40. However, calcium scores reflect the stage of plaque progression and maturity 3, and do not necessarily reflect the true atherosclerotic burden 10 or the degree and location of coronary stenoses 41, especially in younger individuals. Vulnerable coronary plaques have been thought to be predominantly noncalcified and nonstenotic 6, 7 and the presence of such noncalcified plaques on CTA are correlated with acute coronary syndromes 42. The observed high prevalence of NCP in our study in subjects at young ages could reflect a more aggressive process of early-onset vascular aging; this may at least in part explain the higher CAD event rates observed at younger ages in populations with a strong family history of early-onset CAD 35, 43.
CTA tends to overestimate calcified plaque and underestimate noncalcified plaque when compared to IVUS 44. Most studies report the burden of coronary calcium using the Agatston scoring system which is calculated by multiplying the lesion area by a weighted attenuation coefficient, derived to better reflect overall plaque burden and disease severity 28. We used the calcium volume score representing actual CCP volumes (mm3) for comparison with NCP (mm3). This method has improved reproducibility with significantly less interscan variablility than the Agatston scoring method, especially with lower levels of CCP 45, 46. However, calculated CCP volumes tend to overestimate true CCP volumes at higher plaque densities and thus our results likely underestimate the relative extent of NCP to total plaque46.
Our findings highlight the importance of family history in the development of aggressive coronary artery disease at young ages. However, current primary prevention guidelines still do not provide specific recommendations for individuals with a family history of early-onset coronary disease. Although most subjects in the current study were low or intermediate risk using the FRS, traditional modifiable risk factors were significantly associated with higher total coronary plaque volumes independent of age and sex, suggesting a role for earlier aggressive risk factor modification in apparently healthy persons from high-risk families. However, longitudinal studies are needed to further elucidate the significance of NCP volumes and characteristics, in vulnerable asymptomatic populations. Thus we would not recommend expensive screening for NCP using CTA at this time. However, CTA is a promising modality to improve upon CCP screening, especially now that low-radiation dose CTA allows for screening of both NCP and CCP at near equivalent radiation doses as CAC screening alone.47
Conclusion
Apparently healthy men and women from families with early onset CAD have a high prevalence of subclinical CAD, composed primarily of noncalcified plaque. These findings highlight the importance of screening for family history and the implementation of primary preventive interventions at younger ages in both men and women with a family history of early-onset CAD.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by grants from the National Heart, Lung, and Blood Institute (Grants RC1HL099747, K23HL094747, R01HL59684, and R01HL071025), The Johns Hopkins General Clinical Research Center (Grant M01-RR000052 from the National Center for Research Resources, National Institutes of Health), and the National Institute of Nursing Research (Grant R01NR08153).
Footnotes
Disclosures: None.
References
- 1.Wong ND, Kouwabunpat D, Vo AN, Detrano RC, Eisenberg H, Goel M, Tobis JM. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: Relation to age and risk factors. Am Heart J. 1994;127:422–430. doi: 10.1016/0002-8703(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 2.Fallavollita JA, Brody AS, Bunnell IL, Kumar K, Canty JM., Jr Fast computed tomography detection of coronary calcification in the diagnosis of coronary artery disease. Comparison with angiography in patients < 50 years old. Circulation. 1994;89:285–290. doi: 10.1161/01.cir.89.1.285. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 5.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Schuijf JD, Beck T, Burgstahler C, Jukema JW, Dirksen MS, de Roos A, van der Wall EE, Schroeder S, Wijns W, Bax JJ. Differences in plaque composition and distribution in stable coronary artery disease versus acute coronary syndromes; non-invasive evaluation with multi-slice computed tomography. Acute Card Care. 2007;9:48–53. doi: 10.1080/17482940601052648. [DOI] [PubMed] [Google Scholar]
- 8.Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol. 2001;21:1618–1622. doi: 10.1161/hq0901.095554. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Hsiai T, Wang S, Puentes G, Fallavollita J, Shields P, Stanford W, Wolfkiel C, Georgiou D, Budoff M, Reed J. Prognostic value of coronary calcification and angiographic stenoses in patients undergoing coronary angiography. J Am Coll Cardiol. 1996;27:285–290. doi: 10.1016/0735-1097(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 11.Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The newcastle family history study ii Am J Epidemiol. 1998;147:1133–1139. doi: 10.1093/oxfordjournals.aje.a009411. [DOI] [PubMed] [Google Scholar]
- 12.Bertuzzi M, Negri E, Tavani A, La Vecchia C. Family history of ischemic heart disease and risk of acute myocardial infarction. Prev Med. 2003;37:183–187. doi: 10.1016/s0091-7435(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 13.Williams RR. Understanding genetic and environmental risk factors in susceptible persons. West J Med. 1984;141:799–806. [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 15.Kral BG, Becker LC, Vaidya D, Yanek LR, Becker DM. Silent myocardial ischaemia and long-term coronary artery disease outcomes in apparently healthy people from families with early-onset ischaemic heart disease. Eur Heart J. 2011;32:2766–2772. doi: 10.1093/eurheartj/ehr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasir K, Budoff MJ, Wong ND, Scheuner M, Herrington D, Arnett DK, Szklo M, Greenland P, Blumenthal RS. Family history of premature coronary heart disease and coronary artery calcification: Multi-ethnic study of atherosclerosis (mesa) Circulation. 2007;116:619–626. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 17.Parikh NI, Hwang SJ, Larson MG, Cupples LA, Fox CS, Manders ES, Murabito JM, Massaro JM, Hoffmann U, O'Donnell CJ. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the framingham offspring and third generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 18.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: A model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46:1762–1772. [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Pesce AJ, Kaplan LA. Methods in clinical chemistry. St. Louis: Mosby; 1987. [Google Scholar]
- 21.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.Alkadhi H, Scheffel H, Desbiolles L, Gaemperli O, Stolzmann P, Plass A, Goerres GW, Luescher TF, Genoni M, Marincek B, Kaufmann PA, Leschka S. Dual-source computed tomography coronary angiography: Influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur Heart J. 2008;29:766–776. doi: 10.1093/eurheartj/ehn044. [DOI] [PubMed] [Google Scholar]
- 23.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, american heart association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 24.Kelm BM, Mittal S, Zheng Y, Tsymbal A, Bernhardt D, Vega-Higuera F, Zhou SK, Meer P, Comaniciu D. Detection, grading and classification of coronary stenoses in computed tomography angiography. Med Image Comput Comput Assist Interv. 2011;14:25–32. doi: 10.1007/978-3-642-23626-6_4. [DOI] [PubMed] [Google Scholar]
- 25.Halpern EJ, Halpern DJ. Diagnosis of coronary stenosis with ct angiography comparison of automated computer diagnosis with expert readings. Acad Radiol. 2011;18:324–333. doi: 10.1016/j.acra.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Ohnesorge B, Flohr T, Fischbach R, Kopp AF, Knez A, Schroder S, Schopf UJ, Crispin A, Klotz E, Reiser MF, Becker CR. Reproducibility of coronary calcium quantification in repeat examinations with retrospectively ecg-gated multisection spiral ct. Eur Radiol. 2002;12:1532–1540. doi: 10.1007/s00330-002-1394-2. [DOI] [PubMed] [Google Scholar]
- 27.McCollough CH, Ulzheimer S, Halliburton SS, Shanneik K, White RD, Kalender WA. Coronary artery calcium: A multi-institutional, multimanufacturer international standard for quantification at cardiac ct. Radiology. 2007;243:527–538. doi: 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- 28.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 29.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary ct angiography: Comparison with intravascular us. Radiology. 2010;257:516–522. doi: 10.1148/radiol.10100681. [DOI] [PubMed] [Google Scholar]
- 30.Dey D, Cheng VY, Slomka PJ, Nakazato R, Ramesh A, Gurudevan S, Germano G, Berman DS. Automated 3-dimensional quantification of noncalcified and calcified coronary plaque from coronary ct angiography. J Cardiovasc Comput Tomogr. 2009;3:372–382. doi: 10.1016/j.jcct.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Hadamitzky M, Meyer T, Hein F, Bischoff B, Martinoff S, Schomig A, Hausleiter J. Prognostic value of coronary computed tomographic angiography in asymptomatic patients. Am J Cardiol. 2010;105:1746–1751. doi: 10.1016/j.amjcard.2010.01.354. [DOI] [PubMed] [Google Scholar]
- 32.Cademartiri F, Maffei E, Palumbo A, Seitun S, Martini C, Tedeschi C, La Grutta L, Midiri M, Weustink AC, Mollet NR, Krestin GP. Coronary calcium score and computed tomography coronary angiography in high-risk asymptomatic subjects: Assessment of diagnostic accuracy and prevalence of non-obstructive coronary artery disease. Eur Radiol. 2010;20:846–854. doi: 10.1007/s00330-009-1612-2. [DOI] [PubMed] [Google Scholar]
- 33.Choi EK, Choi SI, Rivera JJ, Nasir K, Chang SA, Chun EJ, Kim HK, Choi DJ, Blumenthal RS, Chang HJ. Coronary computed tomography angiography as a screening tool for the detection of occult coronary artery disease in asymptomatic individuals. J Am Coll Cardiol. 2008;52:357–365. doi: 10.1016/j.jacc.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 34.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the multi-ethnic study of atherosclerosis (mesa) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya D, Yanek LR, Moy TF, Pearson TA, Becker LC, Becker DM. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol. 2007;100:1410–1415. doi: 10.1016/j.amjcard.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Staes CJ, Adams TD, Hunt SC. Evaluation of risk scores derived from the health family tree program. AMIA Annu Symp Proc. 2009;2009:286–290. [PMC free article] [PubMed] [Google Scholar]
- 37.Leander K, Hallqvist J, Reuterwall C, Ahlbom A, de Faire U. Family history of coronary heart disease, a strong risk factor for myocardial infarction interacting with other cardiovascular risk factors: Results from the stockholm heart epidemiology program (sheep) Epidemiology. 2001;12:215–221. doi: 10.1097/00001648-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Raggi P, Callister TQ, Cooil B, He ZX, Lippolis NJ, Russo DJ, Zelinger A, Mahmarian JJ. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 39.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: A 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 40.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 41.Mautner GC, Mautner SL, Froehlich J, Feuerstein IM, Proschan MA, Roberts WC, Doppman JL. Coronary artery calcification: Assessment with electron beam ct and histomorphometric correlation. Radiology. 1994;192:619–623. doi: 10.1148/radiology.192.3.8058924. [DOI] [PubMed] [Google Scholar]
- 42.Feuchtner G, Postel T, Weidinger F, Frick M, Alber H, Dichtl W, Jodocy D, Mallouhi A, Pachinger O, Zur Nedden D, Friedrich GJ. Is there a relation between non-calcifying coronary plaques and acute coronary syndromes? A retrospective study using multislice computed tomography. Cardiology. 2008;110:241–248. doi: 10.1159/000112407. [DOI] [PubMed] [Google Scholar]
- 43.Murabito JM, Pencina MJ, Nam BH, D'Agostino RB, Sr, Wang TJ, Lloyd-Jones D, Wilson PW, O'Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 44.Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, Ohnesorge B, Fayad ZA, Becker CR, Reiser M, Steinbeck G, Boekstegers P. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: A comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–677. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 45.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: Improved reproducibility of calcium scoring with an electron-beam ct volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 46.Hong C, Bae KT, Pilgram TK. Coronary artery calcium: Accuracy and reproducibility of measurements with multi-detector row ct--assessment of effects of different thresholds and quantification methods. Radiology. 2003;227:795–801. doi: 10.1148/radiol.2273020369. [DOI] [PubMed] [Google Scholar]
- 47.Fink C, Krissak R, Henzler T, Lechel U, Brix G, Takx RA, Nance JW, Abro JA, Schoenberg SO, Schoepf UJ. Radiation dose at coronary ct angiography: Second-generation dual-source ct versus single-source 64-mdct and first-generation dual-source ct. AJR Am J Roentgenol. 2011;196:W550–557. doi: 10.2214/AJR.10.5153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.