The Canadian Nosocomial Infection Surveillance Program has been performing surveillance of antibiotic-resistant organisms in Canada since 1994. The authors of this study compared two point-prevalence surveys of antimicrobial use that were conducted in hospitals that were participating in the program in 2002 and 2009. The authors compared the use of antimicrobials between these two surveys. The changes in antimicrobial use over time are presented, in addition to potential reasons for and consequences of these changes.
Keywords: Antimicrobial use, Hospital, Prevalence
Abstract
BACKGROUND:
Increasing antimicrobial resistance has been identified as an important global health threat. Antimicrobial use is a major driver of resistance, especially in the hospital sector. Understanding the extent and type of antimicrobial use in Canadian hospitals will aid in developing national antimicrobial stewardship priorities.
METHODS:
In 2002 and 2009, as part of one-day prevalence surveys to quantify hospital-acquired infections in Canadian Nosocomial Infection Surveillance Program hospitals, data were collected on the use of systemic antimicrobial agents in all patients in participating hospitals. Specific agents in use (other than antiviral and antiparasitic agents) on the survey day and patient demographic information were collected.
RESULTS:
In 2002, 2460 of 6747 patients (36.5%) in 28 hospitals were receiving antimicrobial therapy. In 2009, 3989 of 9953 (40.1%) patients in 44 hospitals were receiving antimicrobial therapy (P<0.001). Significantly increased use was observed in central Canada (37.4% to 40.8%) and western Canada (36.9% to 41.1%) but not in eastern Canada (32.9% to 34.1%). In 2009, antimicrobial use was most common on solid organ transplant units (71.0% of patients), intensive care units (68.3%) and hematology/oncology units (65.9%). Compared with 2002, there was a significant decrease in use of first-and second-generation cephalosporins, and significant increases in use of carbapenems, antifungal agents and vancomycin in 2009. Piperacillin-tazobactam, as a proportion of all penicillins, increased from 20% in 2002 to 42.8% in 2009 (P<0.001). There was a significant increase in simultaneous use of >1 agent, from 12.0% of patients in 2002 to 37.7% in 2009.
CONCLUSION:
From 2002 to 2009, the prevalence of antimicrobial agent use in Canadian Nosocomial Infection Surveillance Program hospitals significantly increased; additionally, increased use of broad-spectrum agents and a marked increase in simultaneous use of multiple agents were observed.
Abstract
HISTORIQUE :
La résistance antimicrobienne croissante est une menace importante pour la santé dans le monde. L’utilisation d’antimicrobiens est un moteur de résistance majeur, particulièrement dans le milieu hospitalier. Il faut comprendre la portée et le type d’utilisation des antimicrobiens dans les hôpitaux canadiens pour établir les priorités nationales en matière de gouvernance antimicrobienne.
MÉTHODOLOGIE :
En 2002 et 2009, dans le cadre de sondages de prévalence d’une journée visant à quantifier les infections nosocomiales dans les hôpitaux du Programme canadien de surveillance des infections nosocomiales, les chercheurs ont colligé des données sur l’utilisation des antimicrobiens systémiques par tous les patients des hôpitaux participants. Le jour du sondage, ils ont recueilli les agents précis utilisés (à part les antiviraux et les antiparasitaires) et l’information démographique relative aux patients.
RÉSULTATS :
En 2002, 2 460 des 6 747 patients (36,5 %) de 28 hôpitaux recevaient un traitement antimicrobien. En 2009, 3 989 des 9 953 patients (40,1 %) de 44 hôpitaux recevaient un tel traitement (P<0,001). L’utilisation avait beaucoup augmenté au centre du Canada (37,4 % à 40,8 %) et dans l’Ouest canadien (36,9 % à 41,1 %), mais pas dans l’Est canadien (32,9 % à 34,1 %). En 2009, l’utilisation d’antimicrobiens était plus courante dans les unités de transplantation d’organes pleins (71,0 % des patients), les unités de soins intensifs (68,3 %) et les unités d’hématologie-oncologie (65,9 %). Par rapport à 2002, on constatait en 2009 une diminution importante des céphalosporines de première et seconde générations et des augmentations marquées de carbapénèmes, d’antifongiques et de vancomycine. L’utilisation de piperacilline-tazobactam, en proportion de toutes les pénicillines, est passée de 20 % en 2002 à 42,8 % en 2009 (P<0,001). L’utilisation simultanée de plus d’un agent a également connu une hausse importante, passant de 12,0 % des patients en 2002 à 37,7 % en 2009.
CONCLUSION :
De 2002 à 2009, la prévalence d’utilisation d’antimicrobiens dans les hôpitaux du Programme canadien de surveillance des infections nosocomiales a considérablement augmenté. De plus, les chercheurs ont constaté une augmentation marquée d’agents à large spectre et d’utilisation simultanée de multiples agents.
Antimicrobial resistance (AMR) in human bacterial pathogens has been identified as a public health problem of global significance (1–3). Hospitalized patients are at particular risk from antimicrobial-resistant pathogens due to the evolution of AMR after antimicrobial exposure and to the new acquisition of antimicrobial-resistant bacterial strains, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci and Enterobacteriaceae such as those with extended-spectrum beta-lactamases or carbapenemases. Clostridium difficile infection (CDI) is usually considered to be part of the same public health problem because it shares epidemiological characteristics with antimicrobial-resistant pathogens (ie, increasing incidence, association with antimicrobial therapy and primarily affecting hospitalized patients). In the United States, the Centers for Disease Control and Prevention (Georgia) estimates that a minimum of two million infections and 23,000 deaths annually are attributable to AMR, an additional 250,000 illnesses and 14,000 deaths result from CDI, and that most such infections occur in health care settings such as hospitals (2).
Antimicrobial use (AMU) is a major factor driving the occurrence of AMR and CDI. Consequently, antimicrobial stewardship (AS) has recently been established as a new form of clinical quality improvement. It has, as a core part of its mandate, the objective of improving the quality of antimicrobial therapy and, thereby, reducing selection pressures in bacteria favouring the development of AMR (4). AS activity can function at a local, regional/provincial, national or global level.
As in other aspects of public health, surveillance for AMR and AMU provides the information necessary for appropriate action. The Canadian Nosocomial Infection Surveillance Program (CNISP) has performed surveillance for selected antimicrobial-resistant organisms in hospitalized patients since 1994, and has documented the extent and trends over time of MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamases, carbapenemase-producing microorganisms and CDI (5–13). In Canada, there are data regarding AMU in ambulatory settings (14–16) but there are little data regarding AMU in Canadian hospitals; this is an important deficiency of information because a considerable proportion of the AMR burden occurs within the hospital sector. In 2002, and again in 2009, CNISP performed oneday prevalence surveys to quantify hospital-acquired infections (HAI) among inpatients in network hospitals on that day (17,18). As part of these surveys, data were also collected on AMU in hospitalized patients. These data represent an important snapshot of information on AMU in hospitals in Canada that may reveal important evolving trends and inform national approaches to AS.
METHODS
Surveillance network
CNISP, a network of 54 acute care hospitals from 10 provinces, is a partnership between the Public Health Agency of Canada (PHAC) and the Canadian Hospital Epidemiology Committee, a group of hospital-based physician infection prevention specialists. All CNISP hospitals have a university affiliation and provide primary, secondary and tertiary care to adult and/or pediatric patients. Seven hospitals were stand-alone pediatric centres. Surveillance for HAI and AMU in participating hospitals is considered to be within the mandate of hospital infection prevention and control programs and, therefore, does not constitute human research. In most participating hospitals, this surveillance activity does not require institutional review board review.
AMU point prevalence
A point-prevalence survey of all adult and pediatric inpatients was conducted in CNISP hospitals in February 2002 and February 2009, excluding patients on psychiatric units or long-stay units associated with acute care hospitals. Data pertaining to one 24 h period were collected, entered manually into patient data-extraction forms and forwarded to PHAC for data entry and analysis. A unique identifier linked to the patient name was used only to identify patients at the participating hospital and was not transmitted to PHAC. Data elements collected included demographic information, age and sex, information on HAIs, microorganisms isolated, antimicrobials prescribed and use of additional (transmission-based) precautions. Systemic (intravenous or oral) antimicrobial agents in use on the survey day to treat or prevent bacterial, mycobacterial or fungal infections were identified by chart review and classified according to antimicrobial drug class.
Data analysis
The CNISP hospitals were grouped according to region: western (British Columbia, Alberta, Saskatchewan and Manitoba); central (Ontario and Quebec); and eastern (Newfoundland and Labrador, New Brunswick, Prince Edward Island and Nova Scotia).
Each reported antimicrobial agent was grouped into its antimicrobial class using the Anatomical Therapeutic Chemical coding (http://www.whocc.no/atc_ddd_index/). To assess differences among patient populations, continuous variables were expressed using means and medians, and were compared using Student’s t tests and Wilcoxon rank-sum tests as appropriate. All tests were two-tailed, and P values of 0.05 were considered to be statistically significant. All analyses were performed using SAS version 9.2 (SAS Inc, USA).
RESULTS
In 2002, 6747 patients in 28 CNISP hospitals were surveyed; 2460 patients (36.5%) were receiving antimicrobial therapy. In 2009, 9953 patients in 44 hospitals were surveyed and 3989 (40.1%; P<0.001) were receiving therapy. According to region, antimicrobial prevalence significantly increased in central Canada (from 37.4% to 40.8%; P<0.01) and in western Canada (from 36.9% to 41.1%; P<0.001), but not in eastern Canada (32.9% to 34.1%; P=0.53). Table 1 describes the characteristics of surveyed patients in 2009, comparing those receiving antimicrobial therapy with those who were not receiving therapy. According to ward type, in 2009, antimicrobial therapy prevalence was highest on solid organ transplant wards (71.0%), hematology/oncology wards (65.9%, an increase from 53.6% in 2002; P<0.001) and intensive care units (68.3%). The prevalence of antimicrobial therapy was lowest on coronary care units (17.6%) and neonatal intensive care units (25.2%). From 2002 to 2009, prevalence increased significantly on surgery wards (34.6% to 40.4%) (Table 2). Table 3 compares the antimicrobial agents in use in 2002 with those in use in 2009 as a proportion of all agents used. Of the agents within the penicillin class, the proportion accounted for by pipercillin-tazobactam increased from 20% in 2002 to 42.8% in 2009 (P<0.001). Figure 1 shows the proportion of patients receiving >1 antimicrobial agent, which significantly increased between 2002 (12.0%) and 2009 (37.7%) (P<0.001). In 2009, among individuals on >1 agent (n=1505), there were 103 cases (7%) in which ≥2 of the same class of antimicrobial agent were in use.
TABLE 1.
Antimicrobial use in Canadian Nosocomial Infection Surveillance Program hospitals in 2009: Patient characteristics
| Characteristic | Receiving antimicrobial therapy | P | |
|---|---|---|---|
|
| |||
| Yes (n=3989) | No (n=5964) | ||
| Age, years, median (IQR) | 62 (43–77) | 68 (48–81) | <0.001 |
| Male sex* | 2145 (53.8) | 2956 (49.9) | <0.001 |
| Infant | 213 (5.4) | 518 (8.7) | <0.001 |
| Child | 329 (8.3) | 293 (4.9) | <0.001 |
| Adult | 3435 (86.4) | 5130 (86.3) | 0.89 |
| Type of ward† | |||
| Medicine | 1409 (35.3) | 2500 (41.9) | <0.001 |
| Surgery | 1229 (30.8) | 1810 (30.4) | 0.62 |
| Intensive care | 411 (10.3) | 191 (3.2) | <0.001 |
| Neonatal intensive care | 131 (3.3) | 388 (6.5) | <0.001 |
| Obstetrics/gynecology | 49 (1.2) | 118 (3.0) | 0.004 |
| Hematology/oncology | 280 (7.0) | 145 (2.4) | <0.001 |
| Solid organ transplant | 149 (3.7) | 61 (1.0) | <0.001 |
| Trauma/burn | 44 (1.1) | 48 (0.8) | 0.13 |
| Coronary care | 41 (1.0) | 192 (3.2) | <0.001 |
| Neurosciences/neurosurgery | 72 (1.8) | 210 (3.5) | <0.001 |
Data presented as n (%) unless otherwise indicated.
Data missing from 56 records;
Data missing from 53 records. IQR Interquartile range
TABLE 2.
Antimicrobial prevalence in 2002 and 2009 according to hospital ward type
| Ward type | 2002 | 2009 | P |
|---|---|---|---|
| Medicine/pediatric medicine | 1010 (34.7) | 1409 (36.0) | 0.044 |
| Surgery | 781 (34.6) | 1229 (40.4) | <0.001 |
| Intensive care | 233 (65.6) | 411 (68.3) | 0.44 |
| Neonatal intensive care | 87 (24.3) | 131 (25.2) | 0.75 |
| Obstetrics/gynecology | 28 (22.8) | 49 (29.3) | 0.21 |
| Hematology/oncology | 158 (53.6) | 280 (65.9) | <0.001 |
| Solid organ transplant | 71 (68.3) | 149 (71.0) | 0.62 |
| Trauma/burn | 39 (37.5) | 44 (47.8) | 0.14 |
| Coronary care | 50 (29.6) | 41 (17.6) | 0.53 |
| Other | 1 (10.0) | 153 (36.3) | 0.15 |
| Neurosciences/neurosurgery | 72 (25.5) | n/a |
Data presented as n (%) unless otherwise indicated
TABLE 3.
Comparison of antimicrobial class use in Canadian Nosocomial Infection Surveillance Program hospitals: 2002 and 2009
| Antimicrobial class | 2002 (2864 patients) | 2009 (6048 patients) | P |
|---|---|---|---|
| Penicillins | 441 (15.4) | 1023 (16.9) | 0.07 |
| Cephalosporins | |||
| First generation | 347 (12.01) | 556 (9.2) | <0.001 |
| Second generation | 84 (2.9) | 96 (1.6) | <0.001 |
| Third generation | 252 (8.8) | 446 (7.4) | 0.02 |
| Carbapenems | 73 (2.6) | 238 (3.9) | <0.001 |
| Fluoroquinolones | 519 (18.1) | 1055 (17.4) | 0.43 |
| Aminoglycosides | 106 (3.7) | 214 (3.5) | 0.69 |
| Macrolides | 60 (2.1) | 144 (2.4) | 0.39 |
| Tetracyclines | 6 (0.2) | 29 (0.5) | 0.057 |
| Antifungal agents | 124 (4.3) | 350 (5.8) | 0.004 |
| Antituberculous agents | 52 (1.8) | 102 (1.7) | 0.66 |
| Clindamycin | 48 (1.7) | 127 (2.1) | 0.18 |
| Metronidazole | 256 (8.9) | 530 (8.8) | 0.79 |
| Nitrofurantoin | 7 (0.2) | 46 (0.8) | 0.003 |
| Trimethoprim/sulfamethoxazole | 37 (1.3) | 312 (5.2) | <0.001 |
| Vancomycin | 123 (4.3) | 549 (9.1) | <0.001 |
Data presented as n (%) unless otherwise indicated
Figure 1).
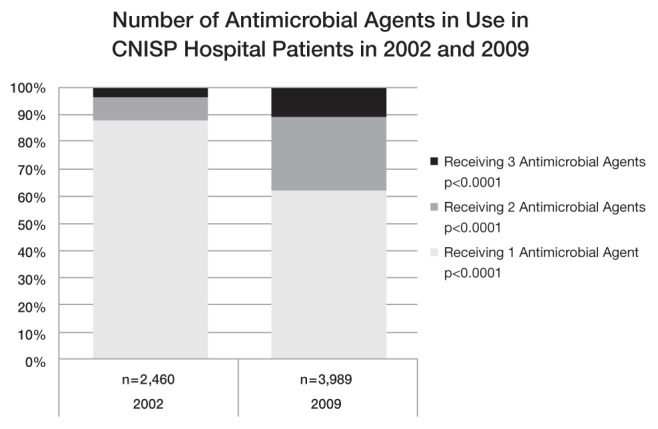
Number of antimicrobial agents in use in Canadian Nosocomial Infection Surveillance Program (CNISP) hospital patients in 2002 and 2009
DISCUSSION
Sequential national prevalence surveys have been widely used to assess secular trends in the occurrence of health care-acquired infections and AMU worldwide as a cost-effective approach to obtaining national data to assist in determining priorities for action (19–23). The prevalence data included in the present report represent a useful assessment of AMU in CNISP hospitals at two points in time separated by seven years, from which evolving trends may be recognized. The data suggest that AMU is very high in hospitalized Canadians, and is increasing over time. This rising prevalence in inpatients in CNISP hospitals is in contrast to AMU in ambulatory settings, for which decreased use has been documented over a similar time period (24). These Canadian trends imply that greater national efforts are needed to examine and address the appropriateness of AMU within hospitals in Canada because hospitalized patients are exposed to high-intensity AMU, and antimicrobial-resistant pathogens often arise in hospitals due to antibiotic exposure or are transmitted within hospitals and subsequently selected by antimicrobial exposure.
Within the group of patients receiving antimicrobial therapy, important trends may be developing. Patients surveyed in 2009 were significantly more likely to receive broad-spectrum antimicrobial agents than they were in 2002. Pipercillin-tazobactam, as a proportion of all penicillins, more than doubled in use. Use of carbapenems (imipenem, meropenem, ertapenem), often considered to be the last line of defense against invasive Gram-negative bacteria, remained low in 2009 (3.9% of all agents), but had increased from 2.6% in 2002. In contrast, use of narrower-spectrum agents such as first- and second-generation cephalosporins declined significantly. Furthermore, there was a marked increase in the prevalence of patients simultaneously receiving multiple antimicrobial agents.
Our data are silent regarding the appropriateness of AMU in CNISP hospitals. Having documented the spread of antimicrobial-resistant pathogens in CNISP hospitals, it is possible that increased AMU and increases in use of multiple agents simultaneously is, in part, a consequence as much as a cause of increasing AMR. The twofold increased use of vancomycin between the two surveys is likely a response to an increased prevalence of MRSA and CDI (6,8). In addition, increased use of broad-spectrum agents and anti-fungal therapy could reflect increasing acuity and complexity of hospital patients. Nevertheless, the data are concerning and point to a need for a better understanding of AMU in Canadian hospitals. More up-to-date information regarding AMU in CNISP hospitals would be an important first step. In addition, there is a need for information on the appropriateness of AMU in hospital clinical practice. Finally, research is required that provides a better understanding of factors driving the AMU trends we have observed, including assessment of forces that influence prescribing in hospitalized patients in Canada. This information could then be used to develop appropriate national hospital AS strategies.
Prevalence surveys in other countries have assessed AMU. Our 2009 prevalence (40.1%) appears to be higher than a survey of 172 European hospitals conducted in 2009, which found a prevalence of 29.0% (21), and 32.4% found in three Australian hospitals surveyed in 2012 (22). A report from a 2007 survey in Scotland (25) showed an overall prevalence of AMU at 32.1% of 11,608 patients in acute care hospitals, with 12.6% overall receiving >1 agent. However, direct comparison of prevalence of AMU among countries – and even among hospitals – is hampered by the need to account for differences in survey methods, patient mix, acuity and local microbial ecology. Risk adjustment of AMU is an emerging field, and methods are being developed based on the types of hospital services provided, indexes of patient severity or combinations of both (26).
Our study was subject to limitations. The data reflect only two days of AMU in the same season separated by seven years. Pediatric hospitals were not included in our survey. While large numbers of patients were surveyed, it is possible that the data over- or underestimate true AMU rates in Canadian hospitals. No data are available to fully characterize the trend between 2002 and 2009 – ie, whether a slow persistent increase or a sudden shift in utilization occurred. Similarly, we cannot comment on whether the change in AMU between 2002 and 2009 has continued. CNISP hospitals are primarily large tertiary care teaching institutions; these data may be expected to overestimate prevalence of AMU. While our data reflect some of the only publically available information on AMU in Canadian hospitals, their shortcomings prevent full description of AMU trends in the hospital sector. Ideally, AMU data from a range of Canadian hospitals should be evaluated. As reviewed by Grant et al (27), there are significant gaps in AMR surveillance (particulary in the community sector) in Canada. Only a limited range of target pathogens have been surveyed in subsets of the Canadian hospital sector – primarily performed by our group. AMU surveillance is generally absent and that which occurs is not integrated with AMR surveillance, beyond that occurring within CNISP. Grant et al (26) recommend a range of initiatives, starting with establishment of a coordinated national cross-sectoral AMR and AMU surveillance system. We agree that better data on AMU within the hospital sector are needed.
SUMMARY
Between 2002 and 2009, overall AMU prevalence in major Canadian hospitals significantly increased, and there were substantial shifts in type of agents used. These data can serve as a baseline for future prevalence studies and research into factors influencing the trends we observed.
Acknowledgments
Members of the CNISP who participated in the Point Prevalence Survey for Healthcare-Acquired Infections: Dr Elizabeth Bryce, Vancouver General Hospital, Vancouver, British Columbia; Dr Gordon Dow, The Moncton Hospital, Moncton, New Brunswick; Dr John Embil, Health Sciences Centre, Winnipeg, Manitoba; Dr Joanne Embree, Health Sciences Centre, Winnipeg, Manitoba; Dr Michael Gardam, University Health Network, Toronto, Ontario; Denise Gravel, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada; Dr Elizabeth Henderson, Peter Lougheed Centre, Calgary, Alberta; Dr James Hutchinson, Health Sciences Centre, St John’s, Newfoundland; Dr Michael John, London Health Sciences Centre, London, Ontario; Dr Lynn Johnston, Queen Elizabeth II Health Sciences Centre, Halifax, Nove Scotia; Dr Pamela Kibsey, Victoria General Hospital, Victoria, British Columbia; Dr Joanne Langley, IWK Health Science Centre, Halifax, NS; Dr Mark Loeb, Hamilton Health Sciences Corporation and St Joseph’s Healthcare, Hamilton, Ontario; Dr Anne Matlow, Hospital for Sick Children, Toronto, Ontario; Dr Allison McGeer, Mount Sinai Hospital, Toronto, Ontario; Dr Sophie Michaud, CHUS-Hôpital Fleurimont, Sherbrooke, Quebec; Dr Mark Miller, SMBD–Jewish General Hospital, Montreal, Quebec; Dr Dorothy Moore, Montreal Children’s Hospital, Montreal, Quebec; Dr Michael Mulvey, National Microbiology Laboratory, Public Health Agency of Canada; Marianna Ofner, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada; Ms Shirley Paton, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada; Dr Virginia Roth, The Ottawa Hospital, Ottawa, Ontario; Jacob Stegenga, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada; Dr Geoffrey Taylor, University of Alberta Hospital, Edmonton, Alberta; Dr Karl Weiss, Maisonneuve-Rosemont Hospital, Montreal, Quebec; Dr Alice Wong, Royal University Hospital, Saskatoon, Saskatchewan; Dr Dick Zoutman, Kingston General Hospital, Kingston, Ontario.
REFERENCES
- 1.Public Health Agency of Canada The chief public health officer’s report on the state of public health in Canada, 2013. Infectious Disease the never-ending threat. < http://publichealth.gc.ca/CPHOReport> (Accessed July 2014)
- 2.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. < www.cdc.gov/drugresistance/threat-report-2013/index.html> (Accessed July 2014) [Google Scholar]
- 3.World Health Organization Antimicrobial resistance: Global report on surveillance 2014. < http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf> (Accessed July 2014)
- 4.Dellit T, Owens R, McGowan J, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 5.Simor AE, Ofner-Agostini M, Bryce E, et al. The evolution of methicillin-resistant Staphylococcus aureus in Canadian hospitals: 5 years of national surveillance. CMAJ. 2001;165:21–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Simor AE, Ofner-Agostini M, Gravel D, et al. Surveillance for methicillin-resistant Staphylococcus aureus in Canadian hospital – A report update from the Canadian Nosocomial Infection Surveillance Program. Canada Communicable Disease Report. 2005;31:1–7. [PubMed] [Google Scholar]
- 7.Ofner-Agostini M, Johnston L, Simor A, et al. Vancomycin resistant enterococci in Canada: Results from the Canadian Nosocomial Infection Surveillance Program, 1999–2005. Infect Control Hosp Epidemiol. 2008;29:271–4. doi: 10.1086/528812. [DOI] [PubMed] [Google Scholar]
- 8.Gravel D, Miller M, Simor A, et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: A Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009;48:568–76. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 9.Ofner-Agostini M, Simor A, Mulvey M, et al. Risk factors for and outcomes associated with clinical isolates for Escherichia coli and Klebsiella species resistant to extended-spectrum cephalosporins among patients admitted to Canadian hospitals. Can J Infect Dis Med Microbiol. 2009;20:e43–e48. doi: 10.1155/2009/725872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller M, Gravel D, Mulvey M, et al. Health care associated Clostridium difficile infection in Canada: Patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Inf Dis. 2010;50:194–201. doi: 10.1086/649213. [DOI] [PubMed] [Google Scholar]
- 11.Mataseje LF, Bryce E, Roscoe D, et al. Carbapenem-resistant Gram-negative bacilli in Canada 2009–10: Results from the Canadian Nosocomial Surveillance Project (CNISP) J Antimicob Chemother. 2012;67:1359–67. doi: 10.1093/jac/dks046. [DOI] [PubMed] [Google Scholar]
- 12.McCracken M, Wong A, Mitchell R, et al. Molecular epidemiology of vancomycin-resistant enterococcal bacteraemia: Results from the Canadian Nosocomial Infection Surveillance Program, 1999–2009. J Antimicrob Chemother. 2013;68:1505–9. doi: 10.1093/jac/dkt054. [DOI] [PubMed] [Google Scholar]
- 13.Lynch T, Chong P, Zhang J, et al. Characterization of a stable, metronidazole-resistant Clostridium difficile clinical isolate. PLoS One. 2013;8:e53757. doi: 10.1371/journal.pone.0053757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass-Kaastra SK, Finley R, Hutchinson J, Patrick DM, Weiss K, Conly J. Longitudinal surveillance of outpatient beta-lactam antimicrobial use in Canada, 1995 to 2010. Can J Infect Dis Med Microbiol. 2014;25:107–12. doi: 10.1155/2014/537948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass-Kaastra SK, Finley R, Hutchinson J, Patrick DM, Weiss K, Conly J. Longitudinal surveillance of outpatient quinolone antimicrobial use in Canada. Can J Infect Dis Med Microbiol. 2014;25:99–102. doi: 10.1155/2014/291859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass-Kaastra SK, Finley R, Hutchinson J, Patrick DM, Weiss K, Conly J. Variation in outpatient oral antimicrobial use patterns among Canadian provinces, 2000 to 2010. Can J Infect Dis Med Microbiol. 2014;25:95–8. doi: 10.1155/2014/703898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravel D, Taylor G, Ofner M, et al. Point prevalence survey for health-care associated infections within Canadian adult acute care hospitals. J Hosp Infect. 2007;66:243–8. doi: 10.1016/j.jhin.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Rutledge-Taylor K, Matlow A, Gravel D, et al. A point prevalence survey of health care-associated infections in Canadian pediatric inpatients. Am J Inf Contr. 2012;40:491–6. doi: 10.1016/j.ajic.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Xie D, Fu X, Wang H, et al. Annual point-prevalence of healthcare-associated infection surveys in a university hospital in China, 2007–2011. J Infect Public Health. 2013;6:416–22. doi: 10.1016/j.jiph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Xie D, Xiang L, Li R, Hu Q, Luo Q, Xiong W. A multicenter point-prevalence survey of antibiotic use in 13 Chinese hospitals. J Infect Public Health. 2015;8:55–61. doi: 10.1016/j.jiph.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Zarb P, Goossens H. European surveillance of antimicrobial consumption (ESAC). Value of a point-prevalence survey of antimicrobial use across Europe. Drugs. 2011;71:745–56. doi: 10.2165/11591180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Cotta MO, Robertson MS, Upjohn LM, Marshall C, Liew D, Buising KL. Using periodic point-prevalence surveys to assess appropriateness of antimicrobial prescribing in Australian private hospitals. Intern Med J. 2014;44:240–6. doi: 10.1111/imj.12353. [DOI] [PubMed] [Google Scholar]
- 23.Llata E, Gaynes R, Fridkin S. Measuring the scope and magnitude of hospital-associated infection in the United States: The value of prevalence surveys. Clin Infect Dis. 2009;48:1434–40. doi: 10.1086/598328. [DOI] [PubMed] [Google Scholar]
- 24.Conly J. Antimicrobial resistance programs in Canada 1995–2010: A critical evaluation. Antimicrob Resist Infect Control. 2012;1:10. doi: 10.1186/2047-2994-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly J, Stewart S, Allardice G, et al. NHS Scotland national HAI prevalence survey. Final Report 2007, Health Protection Scotland (report) < www.documents.hps.scot.nhs.uk/hai/sshaip/publications/national-prevalence-study/report/full-report.pdf> (Accessed March 20, 2015)
- 26.Ibrahim O, Polk R. Benchmarking antimicrobial drug use in hospitals. Expert Rev Anti Infect Ther. 2012;10:445–57. doi: 10.1586/eri.12.18. [DOI] [PubMed] [Google Scholar]
- 27.Grant J, Saxinger L, Patrick D. Surveillance of antimicrobial resistance and antimicrobial utilization in Canada. National Collaborating Centre for Infectious Diseases. June2014 [Google Scholar]


