Abstract
We interviewed 70 healthy volunteers to understand their choices about how the information in their health record should be shared for research. Twenty-eight survey questions captured individual preferences of healthy volunteers. The results showed that respondents felt comfortable participating in research if they were given choices about which portions of their medical data would be shared, and with whom those data would be shared. Respondents indicated a strong preference towards controlling access to specific data (83%), and a large proportion (68%) indicated concern about the possibility of their data being used by for-profit entities. The results suggest that transparency in the process of sharing is an important factor in the decision to share clinical data for research.
Introduction
In parallel with the rapid accumulation of electronic health-related data, ethical considerations and public concern related to clinical data sharing for biomedical and behavioral research have been raised1. The debate on how ethical it is to use data and biospecimens—collected primarily for clinical care—for research purposes spans scientific, legal/ethical/regulatory, and patient circles. Many patients may not know how their data are being used for research. Some may receive a re-consent form, but this requirement can be waived by IRBs (institutional review boards) in certain circumstances. Consent is typically broad and binary (i.e., patients either consent or not), and tiered approaches to informed consent are seldom utilized. Innovative systems that inform patients about the current use of their data and allow them to select tiered opt-out options could offer an alternative to current practices. However, institutions may be hesitant to adopt such systems, since this might decrease participation in data sharing for research and potentially bias research results. The financial and political costs of implementing such systems, as well as their efficacy in terms of patient and provider satisfaction, are currently unknown. A recent survey indicates that many patients believe the use of electronic health records will improve care2. Technical obstacles also exist, as ways of ensuring compliance to patients’ choices may be difficult to implement and upkeep. The ethical gains in implementing choices for patients, however, may justify the development towards systems of granular control should continue3.
Input from patients may potentially drive future policies for data sharing. In Great Britain, an open consultation was launched in October 2013 to give citizens an opportunity to share their views about the collection, use, and analysis of their personal medical data. The goal of this initiative was to determine peoples’ expectations towards privacy and anonymity, as well as to address the ethical concerns of the use of private information. Results are expected mid- 20145. In addition, a leaflet campaign through the NHS system distributed information to all residents about sharing data to improve quality and care for everyone, and how they could opt out of such a system6. Reactions have been mixed: 4 out of 5 surveyed patients in favor of sharing their data yet there is suspicion and distrust for the project, and indications that the methods by which patients are invited to control their own medical records may impact their feelings about data sharing7,8.
Some literature suggests that patients may want to have control over which institutions have access to their data for research3–4. There may be multiple factors influencing subjects’ attitudes towards sharing their medical data for research9:
Type of information: subjects are less willing to share information that is highly personal, such as sensitive information about drug abuse, sexual-related diseases, or mental health disorders9.
The type of recipient: subjects’ willingness to share decreases when the recipient of the information is a commercial or for-profit entity10,11. In a study by Willison et al., participants who had specific targeted health conditions or were generally healthy individuals thought that health information should not be used for marketing purposes, and that re-consent should be needed for use in the case of research by for-profit organizations10. Focus groups and interviews about different scenarios found that most participants were concerned about for-profit uses of their information12.
Level of anonymity: subjects are concerned about privacy and are more willing to share information that is de-identified13.
Health condition of the subject: if the subject has a progressive or chronic illness, the individual tends to be more willing to share10,14.
Perceived value: recent emergence of many active Internet communities for patients experiencing similar conditions shows that subjects may value peer-based sharing models and the possibility of hastening treatment and cure of their medical conditions15.
These studies indicate that using a broad consent for personal data may not be what people desire. If a tiered consent model is used, it can provide subjects with more options and opportunity for involvement in the information sharing process. The U.S. Office of the National Coordinator supports granular control models for Health Information Technology, and suggests that patients should have a “greater degree of choice to determine, at a granular level, which personal health information should be shared with whom, and for what purpose”16.
Technological difficulties make it challenging to track and ensure compliance with patients’ choices even if they are given options for sharing medical data. Currently, most health providers do not have a system for patients to see who is accessing their data for research purposes, and also do not allow patients to select certain categories of information for sharing. The literature suggests that patients may want to have options for keeping their information private4,12. A study by Caine et al., investigated tiered sharing for patients through a system of cards and questionnaires4, while Meslin et al. gave a series of guidelines to consider when creating electronic health records18, and these studies formed the basis of our investigation into different categories and sharing options for patients. However, we are aware that theoretical surveys can be limiting4 as patients may respond differently when it is not their actual medical data at stake.
We surveyed 70 healthy volunteers to establish a baseline on patient preferences who responded to an advertisement posted at several different locations on the UCSD campus for a period of 4 months. The surveys and interviews were conducted between 7/15/2013 and 10/30/2013. The survey was designed at as preliminary study of a larger project that will implement tiered patient preferences for use with a clinical data warehouse for research (CDWR). iCONCUR (informed CONsent for Clinical record and sample Use in Research) is a project of iDASH (integrating Data for Analysis, anonymization and SHaring), an NIH-funded National Center for Biomedical Computing (NCBC)21. iCONCUR will record the patient’s choices for sharing medical information and this information will be transmitted to the CDWR where permissions will disallow sharing of corresponding data about subjects who register their preferences in iCONCUR. iCONCUR will begin in late summer 2014, and will enroll 400 patients from 2 sites – a general Internal Medicine Clinic and a specialty clinic that exclusively treats HIV positive patients. By recruiting a diversified group of patients, we hope to discover trends among patient sharing choices that could be generalized to a wider population.
Materials and Methods
Participant choices were implemented in a graphical user interface (GUI). From the GUI, participants proceed through three taxonomies where choices about sharing data can be made: (1) What am I sharing? (2) Who am I sharing it with? and (3) Which type of funding do I allow? Participants had both the GUI and the survey open in web browser windows so they could refer to both as needed. The study took place at UCSD, and participants met with the researcher (EAB) in person for 45–60 minutes to discuss their choices. During the process of using the GUI and filling out the survey, participants were encouraged to ask questions or give verbal feedback in addition to the feedback that was collected in the survey. No connection of these choices was done with the individual’s CDWR data, as the intent was only to test the appropriateness of available choices.
Educational Materials
An introduction section provided an overview of research, legal requirements for disclosure of medical information for research purposes, and information about how data sharing could contribute to research. All the material was written at an 8th grade reading level. As an example of how medical records could lead to important research discoveries, a link was provided to a recent article that explored data mining of electronic health records17. The idea to include this example came from preliminary interviews. Additional links led to websites about data anonymization, IRB policies, and NIH pages detailing HIPAA.
What am I sharing?
The first taxonomy of choices addressed the content of the person’s health records. As depicted in Figure 1, options included “demographics”, “test and lab results”, and “diagnostic information”. The diagnostic information was classified as “sensitive information” or “non-sensitive information”. The sensitive information was classified in four categories: “mental health”, “sexual and reproductive health”, “alcohol and substance abuse”, and “genetic information”.
Figure 1.
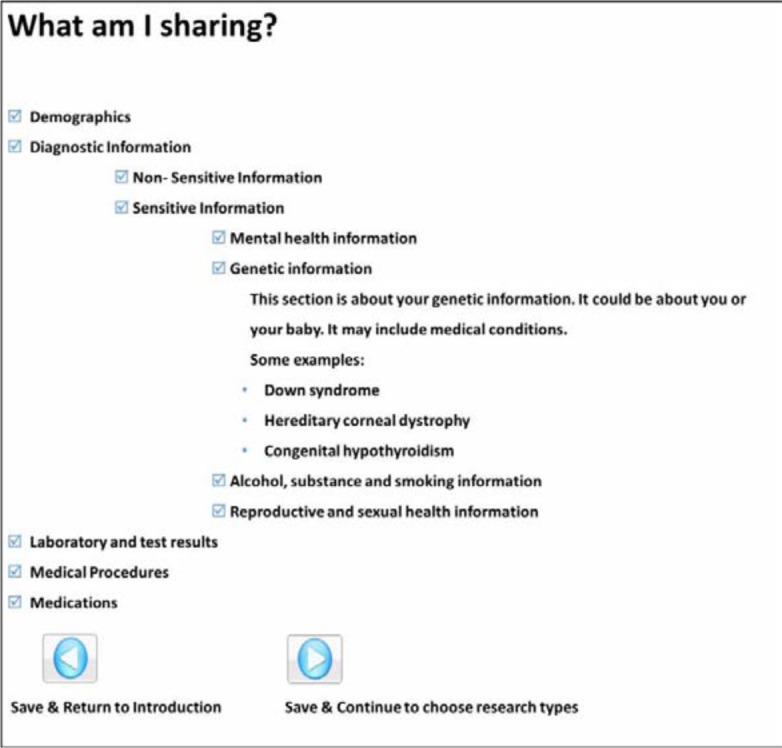
Screenshot of GUI with taxonomy of data sharing choices
Links were provided to the diagnoses for each category of sensitive information, which were selected by two clinicians. Each category expanded to give further descriptions, as depicted in Figure 1 with genetic information. A taxonomy of sensitive categories of information was chosen because previous investigations had shown that sharing sensitive medical data could be a concern to patients4. In previously published work when patients were given the option of choosing how to share their data, many chose to only share less sensitive data4. The definition of sensitive data is described by the National Committee on Vital and Health Statistics and includes five categories: domestic violence, genetic information, mental health information, reproductive and sexual health, and substance abuse19. For the development of the taxonomy, a group of UCSD clinicians used the definition of sensitive information from Points to consider in ethically constructing patient-controlled electronic health records18 to categorize diagnosis codes from CDWR. This way, when a patient decides to share or not share a category of sensitive information, we can precisely determine the set of diagnosis codes available in the CDWR that the patient was selecting and comply with his/her choices. These categorizations were arbitrary, and we acknowledge that different clinicians could have selected the diagnosis codes differently. For the classification of sensitive information we decided to focus only on diagnostic codes. There may be sensitive information contained in other parts of a patient’s medical record, but we have not addressed it in the current survey.
Who am I sharing it with?
In the second taxonomy, subjects had the opportunity to determine whether they wanted to share data with a research team that is: (1) entirely formed by UCSD or San Diego Veteran’s Administration (VA) hospital researchers, or (2) led by UCSD or San Diego VA hospital researchers but involving members outside UCSD and San Diego VA hospital. These options were included because UCSD IRB protocols require that all the research plans that request access to clinical data from the CDWR should be led by UCSD or San Diego VA, and to indicate whether there are research members outside these institutions.
Which type of funding do I allow?
In the last taxonomy subjects had the option of deciding what types of institutions should have access to their medical data. The options included no restriction on type of institution. If this option was not chosen subjects could select some or all of (1) commercial, (2) mixed, (3) non-commercial, and (4) unfunded research.
Evaluation Study
Recruitment was conducted using flyers on the UCSD campus, the UCSD medical center, and the San Diego VA hospital. The inclusion criteria used were: (1) English speaker, and (2) Age 18 or older. Participants began by using the GUI to make their own personal choices about sharing preferences. All choices were saved for the evaluation. After participants completed their choices, a link led to a 28-question survey.
Eight questions collected demographic information, in order to detect possible trends in sharing choices based on gender, income, and educational level. Three questions evaluated the study to check reading comprehension, twelve questions captured the participant’s opinions towards the sharing options and their motivations for sharing or not sharing medical data, and five questions related to the online GUI itself. In total, participants spent 45–60 minutes using the tool and taking the survey.
Results
Choices about sharing were recorded in a database and are shown in Table 1. The numbers indicate the number and percentage of participants who would choose not to share data in each category. Not sharing with researchers from for-profit institutions was the most common choice. For all of the survey questions of interest, a chi-square test was applied to test for association of attributes and the outcome of interest. Representative comments for six of the survey questions that captured the participant’s opinions towards sharing options and motivations for sharing are in shown in Table 2. Results that showed statistical significance at a level of of p <0.05 are indicated.
Table 1.
Number and percentage of participants who selected ‘do not share’ for each data category.
| Participants choices for not sharing | N (%) |
|---|---|
| Sensitive Information | |
| Genetic information | 9 (12.9) |
| Sexual and reproductive health information | 12 (17.1) |
| Mental health information | 10 (14.3) |
| Alcohol and drug use information | 6 (8.6) |
| Types of Sponsorship | |
| For-profit | 16 (22.9) |
| Mixed (for-profit and non-profit) | 7 (10) |
| Non-profit | 1 (1.4) |
| Unfunded | 5 (7.1) |
| Researcher Types | |
| Teams composed of UCSD and VA researchers | 0 |
| Teams that included external researchers | 8 (11.4) |
| Other Categories of Medical Information | |
| Demographics | 3 (4.3) |
| Non-sensitive diagnostics | 1 (1.4) |
| Laboratory and test results | 0 |
| Medical procedures | 3 (4.3) |
| Medications | 3 (4.3) |
Table 2.
Selected survey question and responses.
| Questions | Number of Participants | Some comments from participants |
|---|---|---|
| Are you more or less willing to share your data now that you had these choices? | ||
| More | 54a | “No change. I’m for research and would like to help either way. But, being explicit and providing this added information, makes me feel more at ease doing so.” |
| Less | 9 | |
| Other | 7 | |
| Is it important to you to know whether your data are being shared with for-profit or non-profit institutions? | ||
| Yes | 48b* | “Slightly important because I would only want to share this information if I knew researchers were going to use it for well meaning purposes.” |
| No | 7 | |
| I am indifferent | 15 | |
| If it were possible for you to know who is accessing your data, would you like to know this? | ||
| Yes | 62a* | “Yes, but more because of curiosity on what is going on with the research community, rather than actually feeling my privacy is being “invaded”” |
| No | 1 | |
| I am indifferent | 7 | |
| Would you feel more comfortable sharing your information if you know who is accessing it? | ||
| Yes | 62a* | No comments |
| No | 3 | |
| I am indifferent | 5 | |
| If there were an option for you to control the sharing of biosamples, such as tissue, blood and urine, would you want to control this? | ||
| Yes | 34 | No comments |
| No | 10 | |
| I am indifferent | 26 | |
| Is there anything else you would like to keep private in your medical record? (multiple choice) | ||
| No | 42 | “DNA info” “potentially criminally-related illnesses or drug use” “STDs” |
| Chronic disease | 5 | |
| Acute disease | 6 | |
p <.001
p <.05.
Indicates that this is a secondary hypothesis that was subjected to post-hoc analysis without correction for Type I Errors.
The results show that participants were significantly more willing to have their health data shared for research if they were given choices about which aspects of their data they wished to share. Participants were also interested in knowing more about the researchers and indicated a significant desire to know who was accessing their data. Ninety-four percent of participants indicated that they wanted to be able to know what kind of organization the researcher belonged to, 89% wanted to know the aim of the research study, 84% were interested in being informed of the outcomes of the research, and 70% would like access to publications that resulted from using their data. Preferences for being notified of how and when their data are being used were quite varied. Forty-four percent of participants want to be informed each time a new researcher uses their data, 20% prefer once a month (even if there were no changes), 13% once a year (even if there were no changes), 17% never if they could go online to the site and find it themselves, and 6% had other suggestions. Participants correctly answered the three questions designed to check reading comprehension 91% of the time.
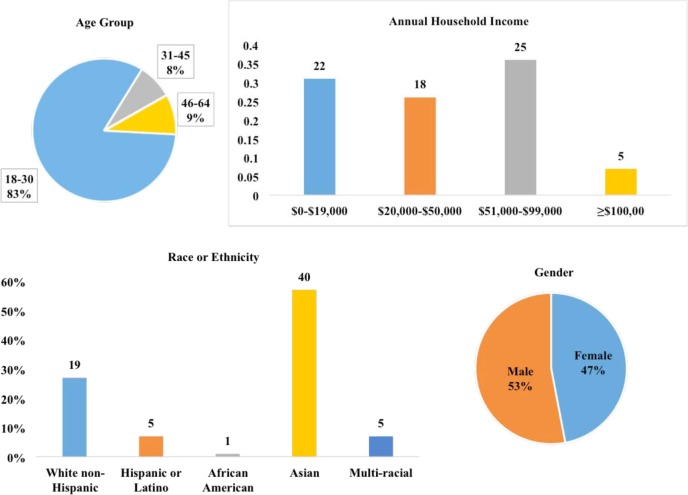
In addition to the demographics shown in Figure 2, information about education level, personal health status, weekly Internet use and health insurance status were collected. Participants were more highly educated than would be expected from a random sample of the US population20. Seventeen percent had a graduate or professional degree. When asked to describe health status, 14% said fair, 35% said good, 31% said very good and 20% said excellent. Four participants did not have health insurance. Regarding questions about the GUI and variety of choices presented, 94% of participants felt that the number of categories in What am I sharing? was adequate. However, 83% would like more granular control over sharing options, such as sharing mental health information with nonprofit sponsored researchers but not for- profit sponsored researchers. Eighty-seven percent of participants were motivated to share in order to help other patients and contribute to science and research, and 44% would do it because they trusted UCSD and believed in the importance of the research being conducted.
Figure 2.
Demographics of participants
Discussion
Patients may not be aware of how their personal health information is being used for research. By presenting information and options, we sought to discover preferences for sharing health information. We hypothesized that participants would be more likely to share their health information if they were presented with choices, specifically for controlling categories of sensitive information. Having choices available did make participants more willing to share their data, and they expressed interest in keeping specific categories of information private. These results likely do not represent the opinions of all citizens, as recruitment was limited in scope. Since recruitment occurred on the UCSD campus, where most people are 18–30 years old and of Asian ancestry, the results may not generalize to the general population. Other important limitations include the fact that most of the participants had at least some college education and mostly were in good or better health status.
Furthermore, since this study was hypothetical, participants may choose differently than if they were actually making choices for their medical record. The results of the projected iCONCUR study on actual patients and with their preferences being implemented in data sharing for research should provide insights on those differences. During this study, the news regarding the NSA (National Security Agency)22 and global security surveillance scandal was made public. Verbal comments from several participants of the study indicated that their decisions to share or not share their data were influenced by this event. Two distinct schools of thought were noted – some participants indicated that they were more willing to share because “the government has all our info anyways” and there was no longer a perceived benefit to keeping information private. Others stated they were less likely to share, as the government was potentially infringing upon their personal privacy and they would like control whenever possible.
Twenty-two percent of participants did not want to share health data with for-profit sponsored researchers. This finding was fairly consistent with the literature10–12. Although there were limitations in this study due to the small sample size, the sponsors of the research is important factor to participants. Concerns about sharing sensitive categories of information were also consistent with the literature4,9,18. Some important new findings of this study are that participants appear to be more willing to share when given granular choices over what categories of information to share, as well as when they are given information about who is accessing their information. Many participants are interested in contributing to research but would like feedback about how their data are being used, and would like to be informed of the results from the research.
Acknowledgments
This study was supported by iDASH: Integrating Data for Analysis, anonymization, and SHaring (1U54HL108460). We would also like to acknowledge Mindy Ross, MD, MBA, and Robert El-Kareh, MD, MS, MPH, for classifying CDWR data into categories of sensitive information, Jihoon Kim, MS, for the statistical analysis of the study results, Claudiu Farcas, PhD, Michele Day, PhD and Hyeoneui Kim, PhD, MPH, RN, for feedback and Mona Wong for building the GUI used in this study.
Appendix 1
Survey questions
Learning about you
- Which age group do you belong to?
- 18–30
- 31–45
- 46–64
- ≥65
- What is your gender?
- Male
- Female
- Unknown
- What is your highest education level?
- High school or less
- Beyond high school or < 4 years of college
- 4 year college graduate
- Graduate or professional school
- What race or ethnicity do you identify as?
- White non-Hispanic
- Hispanic or Latino
- African American
- Asian
- Multi-racial
- What is your annual household income?
- <$5000
- $5000–$14,999
- $15,000–$19,999
- $20,000–$49,999
- $50,000–$59,999
- $60,000–$99,999
- ≥$100,000
- How would you describe your personal health status?
- Poor
- Fair
- Good
- Very good
- Excellent
- How many hours per week do you use the Internet?
- 1–10 hours/week
- 11–15 hours/week
- >15 hours/week
-
Do you currently have health insurance?
- Yes
- No
What is this study about?
- How long will this study last?
- One month
- 6 months
- 1 year
- 2 years
- Can you change your mind about sharing your information or participating in this study?
- Yes
- No
-
When your data is shared, will you be notified?
- Yes
- No
Your Suggestions for Improvement
- Was the number of categories of information in “What am I sharing?” adequate?
- No, it needed more categories [please explain in comment box]
- Yes
- No, there were too many categories [please explain in comment box]
- Is there anything else that you would like to be able to keep private in your medical record? [multiple choice]
- No
- Chronic disease information
- Acute disease information
- Sensitive non-diagnostic information
- If there were an option for you to control the sharing of biosamples, such as tissue, blood, and urine, would you want to be able to control this?
- Yes, I would like to control the sharing of biosamples
- No, I would not like to control the sharing of biosamples
- I am indifferent
- Do you feel more or less willing to share your medical information now that you had these choices?
- More
- Less
- Other
- What is your motivation for sharing your health information? [multiple choice]
- Benefit future patients
- Contribute to science and research
- Trust in UCSD and a desire to contribute to the research they are doing
- Establish a good relationship with UCSD
- Other
- Is it important to you to know whether your data is being shared with for-profit or non-profit institutions?
- Yes
- No
- I am indifferent
- The tool (GUI) does not allow different researchers to access different information from your medical record. For instance, the tool does not allow you to choose to share your mental health information with non-profit organizations but not to share it with for-profit organization. Would you like this option?
- Yes, I would like to have the option to allow different researchers access to different information from my medical record.
- No, I would not like to have the option to allow different researchers access to different information from my medical record.
- Do you like to have control on what to share and with whom to share it?
- Yes
- No
- Yes but not enough to go through the trouble of selecting options
- Other
- If it were possible to know who is accessing your data, would you like to know this?
- Yes
- No
- I am indifferent
- Other
- Would you feel more comfortable sharing your information if you know who is accessing it?
- Yes
- No
- I am indifferent
- What would you like to know about the researchers who used your data? [multiple choice]
- What kind of organization they belong to (e.g. a profit/non-profit organization, university or healthcare system)
- What was the aim of their research?
- What papers were published using my data?
- What were the outcomes of their research?
- Other
- How often would you prefer to be notified of updates about who is using your information?
- Every time someone new uses it
- Once a month even if there are no changes
- Once a year even if there are no changes
- Never, I can go to the site whenever I want to
- Other
- For each category of sensitive information (sexual and reproductive history, substance abuse, mental health, and genetic information), there was a paragraph explaining it and offering some examples. Do you feel like these paragraphs and examples were clear enough?
- Yes, they were all clear enough
- No, none were clear enough
- No, some were unclear
- Were the categories of sensitive information (sexual and reproductive history, substance abuse, mental health and genetic information) enough to cover your own preferences?
- Yes, the categories were enough to cover my preferences
- No, the categories were not enough to cover my preferences
- Other
- In this study, you are given the option of sharing or not sharing categories of information that are not considered sensitive. These include demographics, non-sensitive diagnosis information, laboratory and test results, medical procedures, and medications. Do you feel that these categories covered all types of information you want to control, or do you wish there were more categories for you to choose from?
- Yes there were enough categories for me to control
- No, I wanted more categories of information
- Other
- Did you have trouble understanding any of the information presented in the tool?
- No, I was able to understand everything
- Yes, I had trouble understanding parts of it
- Yes, I couldn’t understand it at all
- Other
- Did you have trouble understanding how to use the tool when selecting your choices on what to share and with whom?
- No, I was able to understand how to make my choices
- Yes, I had some trouble understanding how to make my choices
- Yes, I couldn’t make any choices because I didn’t understand how to do it
- Other
References
- 1.Spriggs M, Arnold M, Pearce C, Fry C. Ethical questions must be considered for electronic health records. J Med Ethics. 38:535–9. doi: 10.1136/medethics-2011-100413. [DOI] [PubMed] [Google Scholar]
- 2.Ancker JS, Silver M, Miller MC, Kaushal R. Consumer experience and attitudes toward health information technology: a nationwide survey. J Am Med Inform Assoc. 2013;20:152–156. doi: 10.1136/amiajnl-2012-001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meslin EM, Alpert SA, Carroll AE, Odell JD, Tierney WM, Schwartz PH. Giving patients granular control of personal health information: using an ethics ‘Points to Consider’ to inform informatics system designers. Int J Med Inform. 2013;82(12):1136–1143. doi: 10.1016/j.ijmedinf.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Caine K, Hanania R. Patients want granular privacy control over health information in electronic medical records. J Am Med Inform Assoc. 2013 Jan 1;20(1):7–15. doi: 10.1136/amiajnl-2012-001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limb M. Nuffield council opens consultation on use of personal biological and health data. BMJ. 2013;347:f6315. doi: 10.1136/bmj.f6315. [DOI] [PubMed] [Google Scholar]
- 6.Dixon WG, Spencer K, Williams H, Sanders C, Lund D, Whitley EA, et al. A dynamic model of patient consent to sharing of medical record data. BMJ. 2014;348:g1294. doi: 10.1136/bmj.g1294. [DOI] [PubMed] [Google Scholar]
- 7.Sheather J, Brannan S. Patient confidentiality in a time of care.data. BMJ. 2013;347:f7042. doi: 10.1136/bmj.f7042. [DOI] [PubMed] [Google Scholar]
- 8.Ipsos Mori/Association of Medical Research Charities Public support for research in the NHS. 2001. www.ipsosmori.com/Assets/Docs/Polls/amrc-public-support-for-research-in-the-NHS-ipsos-mori-topline.pdf. Accessed February 10 2014.
- 9.Whiddett R, Hunter I, Engelbrecht J, Handy J. Patients attitudes towards sharing their health information. Int J Med Inform. 2006;75(7):530–541. doi: 10.1016/j.ijmedinf.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Willison DJ, Steeves V, Charles C, Schwartz L, Ranford J, Agarwal G, et al. Consent for use of personal information for health research: Do people with potentially stigmatizing health conditions and the general public differ in their opinions? BMC Med Ethics. 2009;10(1):10. doi: 10.1186/1472-6939-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willison DJ, Swinton M, Scwatrz L, Abelson C, Charles C, Northrup D, et al. Alternatives to project-specific consent for access to personal information for health research: insights from a public dialogue. BMC Med Ethics. 2008;9(1):18. doi: 10.1186/1472-6939-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamo LA, Browe DK, Logan HC, Kim KK. Patient informed governance of distributed research networks: results and discussion from six patient focus groups. AMIA Annu Symp Proc. 2013:920–929. [PMC free article] [PubMed] [Google Scholar]
- 13.Weitzman ER, Kaci L, Mandl KD. Sharing medical data for health research: the early personal health record experience. J Med Internet Res. 2010;12(2):e14. doi: 10.2196/jmir.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett G, Cassell JA, Peacock JL, Coleman MP. National surveys of British public’s views on use of identifiable medical data by the National Cancer Registry. BMJ. 2006;332(7549):1068–1072. doi: 10.1136/bmj.38805.473738.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye J, Curren L, Anderson N, Edwards K, Fullerton SM, Kanellopoulou N, et al. From patients to partners: participant-centric initiatives in biomedical research. Nat Rev Genet. 2012;13(5):371–376. doi: 10.1038/nrg3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health IT policy committee, privacy and security tiger team, letter to David Blumenthal, Chairman of the Office of National Coordinator for Health IT. 2010. Aug, http://www.healthit.gov/sites/default/files/hitpc_transmittal_p_s_tt_9_1_10_0.pdf Accessed Mar 5 2014.
- 17.Jaret P. Mining electronic records for revealing health data. New York Times. 2013. Jan 14, http://www.nytimes.com/2013/01/15/health/mining-electronic-records-for-revealing-health-data.html?pagewanted=all&_r=0. Accessed Oct 20 2013.
- 18.Meslin EM, Alpert S, Carroll AE, Odell JD, Scwatrz PH. Points to consider in ethically constructing patient-controlled electronic health records. 2012. http://hdl.handle.net/1805/2936. Accessed August 20 2013.
- 19.Carr J. Recommendations regarding sensitive health information. 2010. http://www.ncvhs.hhs.gov/101110lt.pdf. Accessed October 25 2013.
- 20.US Census Bureau Educational attainment in the United States. 2012. http://www.census.gov/hhes/socdemo/education/data/cps/2012/tables.html. Accessed March 11 2014.
- 21.Ohno-Machado L, Banfa V, Boxwala AA, Chapman BE, Chapman WW, Chaudhuri K, et al. iDASH: integrating data for analysis, anonymization and sharing. J Am Med Inform Assoc. 2012;19:196–201. doi: 10.1136/amiajnl-2011-000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Guardian Edward Snowden and the NSA files-timeline. 2013. Jul 5, http://www.theguardian.com/world/2013/jun/23/edward-snowden-nsa-files-timeline. Accessed March 5 2014.