Abstract
Objective
Examine how the Electronic Health Record (EHR) and its related systems support or inhibit provider collaboration.
Background
Health care systems in the US are simultaneously implementing EHRs and transitioning to more collaborative delivery systems; this study examines the interaction between these two changes.
Methods
This qualitative study of five US EHR implementations included 49 interviews and over 60 hours of provider observation. We examined the role of the EHR in building relationships, communicating, coordinating, and collaborative decision-making.
Results
The EHR plays four roles in collaboration: a repository, a messenger, an orchestrator, and a monitor. While EHR performance varied, common themes were decreased trust due to poor quality documentation, incomplete communication, potential for increased effectiveness through better coordination, and the emerging role of the EHR in identifying performance gaps.
Conclusion
Both organizational and technical innovations are needed if the EHR is to truly support collaborative behaviors.
Introduction
For over a decade, experts have agreed that more collaborative, team-based care will be required to meet the increasing burden of chronic disease.(1,2) Unlike the acute care issues that dominated medical practice in the twentieth century, treating chronic disease in the twenty-first century will require multiple visits to providers in different disciplines. Not only will increasingly specialized medical expertise be required, but chronic disease treatment also involves changing lifestyles and navigating a complex web of treatments. Different health care professionals with different skills in different locations will need to collaborate to provide a cohesive care team. This increased collaboration is likely to constitute a disruptive change in the delivery of healthcare services.
In its broadest sense, collaboration simply means “to work jointly with others or together especially in an intellectual endeavor.”(3) This definition may be too broad for use in healthcare situations; we found twenty-seven different definitions for healthcare collaboration in the literature. These definitions vary from a willingness to work with another party in any manner(4), to teamwork (defined roles and a common goal)(5), to shared decision-making.(6) For this study we adopted a taxonomy of collaboration behaviors based on the works of William Clancey(7,8) and Eduardo Salas(9) who suggest that collaboration includes the behaviors of trust and respect, communications, coordination, and adaptive collaboration. In table 1, we list the four behaviors and identify how the behavior can support a more effective healthcare process.
Table 1:
the collaborative behaviors and their benefits
| Collaborative Behaviors | Benefits |
|---|---|
| Trust and respect: willingness to rely on work of others | Less need to repeat diagnostics and procedures, more willingness to hand off or delegate |
| Communication: information flow, contextual background, understanding | Increased awareness and understanding, less mistakes due to missed data or context |
| Coordination: managing the timing and order of activities | More effective processes and increased efficiency in workflows |
| Adaptive Collaboration: changing the actual work content, tailoring solutions | Increased understanding across disciplines, when needed provides ability to tailor plans to meet patient circumstances |
Team-based models of care based on collaboration have been implemented with improved results in primary care(10, 11), intensive care units(6), and operating rooms.(12) In the primary care setting, one prominent move towards collaboration and team-based care is the implementation of the Patient Centered Medical Home (PCMH). The configuration of the PCMH varies from system to system(13, 14), but it consistently includes increased teamwork and collaboration within the clinic. In many cases it also includes improved collaboration with providers outside the clinic with whom they have working relationships (within the “Medical Neighborhood”). This is a disruptive change with significant effects on how care will be provided in the United States.
Another disruptive but potentially positive change is the introduction of the electronic health record (EHR) and its internal and related functions – computerized provider order entry (CPOE), clinical decision support (CDS) and health information exchanges (HIE). The EHR was originally designed as an electronic implementation of the paper chart.(15) Supporting collaboration (other than improved legibility) was not one of the original functional goals of this tool.(16) As the EHR has been deployed, the implementation has resulted in many examples of outcomes that are different from those anticipated when the systems were first designed. These are referred to as “unintended consequences.”(17) Unintended consequences are not necessarily negative. For example, the EHR is being used to support care coordination, a process not included in the original vision of a documentation process to support medical decision-making.(16) The use of the EHR to support collaboration is, in many ways, a collection of these unintended consequences.
The relationship between the EHR and collaboration behaviors is evolving. This has been understudied. This paper presents the first multi-system qualitative study to delve deeply into the nature of this relationship and the roles the EHR plays.
Methods
Purpose
This qualitative study examines how the EHR and related systems affect the collaboration behaviors among providers in five systems that are implementing Patient Centered Medical Home delivery models.
Methodology
We used a modified Rapid Assessment Process(18,19) for data collection, and a grounded theory approach(20) for analysis. At the end of each site visit we summarized our initial themes and reviewed them with the site sponsor to verify our interpretations. Each aspect of the method is described below.
Sample
We studied five leading-edge multi-site organizations with EHR installations in the United States that were in the process of implementing their PCMH. These organizations were participating in SAFER project (Safety Assurance Factors for EHR Resilience(21)), which focused on developing guidelines for the safe implementation and use of EHRs and related systems. Sites were purposively selected to provide a cross section of successful EHR implementations in medium to large healthcare delivery organizations. As such they enabled a broad view of the potential advantages and challenges for collaboration in state of the art systems using an EHR. The characteristics of the sites are summarized in table 2. The visits occurred between May and November of 2012.
Table 2:
Sites visited
| Location (US) | Structure | Number of physicians/providers | EHR | |
|---|---|---|---|---|
| Site 1 | Southeast | Augmented family practice/for profit | 50 to 100 | Centricity |
| Site 2 | Mid Atlantic | Integrated System/not for profit | More than 1000 | EPIC |
| Site 3 | Midwest | Community Health Center | Less than 50 | Centricity through service provider |
| Site 4 | Midwest | Community Health Center | 50 to 100 | Centricity through service provider |
| Site 5 | Northeast | Academic Integrated Health System | More than 1000 | Proprietary/ “Best of Breed” |
Within each site we identified key individuals who were involved in the implementation, use, and monitoring of the EHR. These individuals included providers, clinical leaders, and informaticists. We then worked with our sponsor at the site to schedule interviews with these individuals and field observations of providers working with patients using the EHR and other technology tools. The length of our visits varied from three to five days. The deployment of a multi-disciplinary team (see below) and a rapid assessment process(18) enabled us to reach saturation within a limited time period.
The data used for this collaboration study consisted of 49 interviews and 60 hours of field observation. This was selected from the larger SAFER project by focusing on how providers collaborate and communication with each other; over 500 pages of transcripts and field notes were annotated. The primary author and another analyst independently selected the interviews and observations included in this study from the larger set of interviews and field notes (over 2000 pages) gathered for the SAFER project.
IRB approvals
The Institutional Review Boards at Oregon Health & Science University and each of the sites approved the study.
Team
A multi-disciplinary team with experience in interviewing and observation conducted each visit. At a minimum, each site visit included a combination of professional qualitative researchers, informaticists and clinicians; several visits also included an expert researcher in communications and a human factors expert. The team met daily to debrief and prepare for the next day’s encounters.
Collection Methods
During each site visit, we conducted field observation and semi-structured interviews. The semi-structured interview guides for this segment of our study focused on how the interviewees (or those working for the interviewees) collaborated with other professionals both within and without the clinic, the role the EHR played in facilitating or inhibiting the transaction, and implementation effects. All interviews were tape recorded and subsequently transcribed with the consent of the interviewees.
For the field observation, teams of two to six trained observers went to each site. We used a template for field observations which covered broad categories of foci of interest; each observer was responsible for gathering information on topics outlined on the template. Observation periods were three to eight hours, during that time we typically shadowed a provider to see how she used (or did not use) the EHR. In addition to shadowing providers, we also observed the work flow and meetings within a unit, and in one case we followed a patient. We were particularly interested in both safety issues (for the SAFER study) and how and when they collaborated with other providers (this study). Each day we documented their observations and then discussed them with the other researchers at a daily debriefing.
At the conclusion of each site visit, we prepared a summary of findings for the entire SAFER visit and met with our site sponsors and other leaders from each organization to confirm the veracity of our data.
Data Analysis
For the data analysis, we familiarized ourselves with the data, generated initial codes, then searched for and consolidated themes based on the codes. We produced reports consisting of quotations from interviews and field notes; these were translated into results based on “sense-making” sessions with key team members.
Coding
Two of the authors independently coded data for each site visit using NVivo 10. (22)Data tagged included portions of interview transcripts, field observation notes, and written artifacts collected in the site visits. After each site visit was coded, the analysts met to review codebook categories (codes) and relevant data elements. Discrepancies were analyzed and reconciled, and a new version of the codebook was prepared. This codebook was then used by both analysts for the analysis of the next site visit’s data. New codes were added and old data elements reclassified after the analysis of each visit.
Theme generation
Following the initial coding, two of the authors met several times to identify emerging preliminary findings and implications from the analysis. During this period of reading and rereading (Miller and Crabtree’s immersion phase(23)) the primary author drafted “memos” that helped her bring together key ideas.
Sense-making
Other co-authors with subject matter expertise in informatics, communications, and clinical delivery systems reviewed the themes during “sense-making” sessions. In these sessions, the team reviewed and refined findings, working to organize the findings, and sought to make connections between what we were seeing in these data and the current literature. Miller and Crabtree(23) call these steps in the analysis process the organizing and connecting phases. During these sessions, it became apparent that the collaboration models of Clancey and Salas(8,9) would provide a useful practice lens for organizing the emerging findings.
The data analysis resulted in 49 initial codes related to collaboration, and these categories were subsequently combined into 17 themes. These themes, in turn, were related to four roles played by the EHR discussed below.
Results
The four roles
We found the EHR and related systems play four distinct roles in collaboration: Repository, Messenger, Orchestrator, and Monitor. These roles are summarized in Table 2 below. The repository role was the most established at all sites; in all of the systems there was also a robust role for the messenger role. The degree of sophistication in the orchestrator role varied significantly from system to system. All of the sites we visited were actively working to improve and implement the EHR’s performance in the monitor role.
Table 2:
Four identified collaboration roles for the HER
| Role | Purpose | Examples |
|---|---|---|
| Repository | Contain all of the quality, accessible data needed for use by healthcare providers | Encounter and phone notes, lab and imaging results, and information from providers outside the system. Input may be by providers, by scanned documents, and increasingly, by vendor specific EHR to EHR communications (e.g. CareEverywhere) and Heath Information Exchange (HIE) mediated communications. |
| Messenger | Enable information transfer and communication between providers also between providers and other members of the healthcare team | Transmitted copies of encounter notes, pinned notes and flags sent to other providers, secure email, pop-ups, broadcast messages, clinical paging. |
| Orchestrator | Ensure that the right person is doing the right thing at the right time for the patient | Input templates that drive workflows, the use of standardized order sets, bundles and smart ordersets that implement best practice algorithms. Tickler messages generated by the system. |
| Monitor | Identify care gaps for patients and populations; provide a benchmark for measuring the performance of providers and teams | Registries, data warehouses and analytic tools, reporting tools for use by the clinic teams, dashboards, incentive performance displays. |
In our interviews and observations, we found that the EHR can have varied, sometimes conflicting effects on the four collaboration behaviors (trust and respect, communication, coordination, and adaptive collaboration).
The EHR and collaboration behaviors
Each of the four roles had a primary effect on a single collaboration behavior (figure 2, below). They often had broader effects. One aspect of the EHR can and did affect several different behaviors at once (e.g. a lack of trust due to poor data quality cascaded throughout all four behaviors), but we have tried to avoid repetition.
Figure 2:
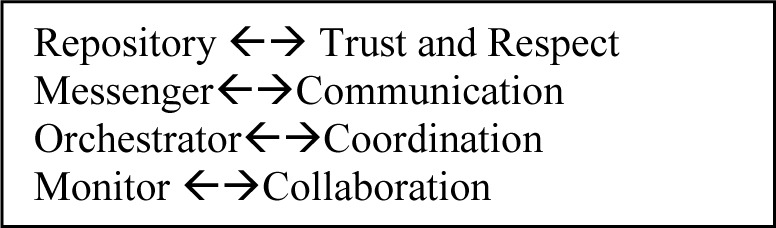
Each roles of the EHR had a primary effect on one collaboration behavior
Repository
The EHR, in its repository role had mixed effects – primarily on trust and respect. As provider told us, the major advantage to the EHR was that “it’s all there” Because it is all there, the EHR can provide proof that one discipline can trust another: “And our physicians saw [the data from the EHR] and realized ‘wow, that really works… I can reliably delegate to my nurses.” But other times poor data quality would reduce their trust in other providers: “This note that’s 15 pages and it looks like they spent two weeks with the patient when the reality is they spent five minutes … and everything in there is either a fabrication and or was correct two years ago but not today.” Providers also complained about lack of context –the patient’s “story” could not be adequately told with a series of check boxes.
A lack of quality in the notes can result from many factors. Time pressures are often a contributing factor: “there is a trade-off between patient safety… and efficiency.” “If you are a busy PCP (primary care provider) with …one minute to prepare… you don’t do it as thoroughly.” It is not clear, however, how much this decrease in perceived note quality is simply an artifact of increased note visibility: “people who wrote good notes on paper would write good notes in the electronic world, and people who wrote lousy notes …would write lousy electronic [notes].”
As a repository, however, the function is not just to store data, but to make it available, When a provider could not find a specialist’s note, she became frustrated and began to blame the other provider for poor communications. We later found the note, but finding the note involved paging through three screens of poorly indexed lists of chart notes, consultant’s reports, and laboratory data (from our field notes). The ability to find necessary information is a key element in data accessibility.
The repository role also has an effect on the other collaboration behaviors. Although it did not guarantee good communications, it provided a common dataset that could speed and simplify communications. This was true, however, only when both parties had access to the same EHR. Commonly, we found that a copy of a chart note would be sent within a referral or other inter-provider communication; this provided the basis for other communications, but this often this transfer was not enough by itself, more contextual data was needed. For example, we were told that the radiologists in one system told us that the primary reason for poor implementation was the poor quality of the contextual data provided. The common data set also facilitated collaboration in multidisciplinary team meetings: “As she describes the case the psychiatrist is displaying parts of the patient’s history and progress notes from the EHR on the screen.” [from our field notes]
The repository function can improve coordination through increasing awareness: “you can see a list of items that you need to complete for the patient. The nurse can see it, the physician can see it.” This increased awareness is, however, a two edged sword – “now we are unearthing scope of practice issues … that we didn’t have to deal with before.” At one organization a pharmacist could no longer complete a medication order with default parameters if they couldn’t reach a provider, at another site the transition to an EHR unearthed the existence of hitherto unknown “personal order sets.” These variations from standards were much more visible with the EHR than with paper records.
Messenger
The EHR and related clinical systems have significantly expanded the number of communication channels available to providers. Now, in addition to in-person, analog written, and phone/voicemail communications, providers can transmit information and communicate with other providers using secure email, clinical messages using a paging system, messages within the EHR (which can be attached to patient records), and pop-ups and general “broadcast notices.” This multiplicity of channels can result in the choice of channels that are easier to use but providers considered less effective. In our visits, we were consistently told that sending a copy of the encounter note was the most frequent means of communication. And it is easy to do. As the one provider saved his encounter note, a routing box popped up and he clicked the name of the primary care provider to automatically send a copy: “I just write a brief note and click.” This was not uniform, there were also variations in practice between providers within the same healthcare settings. Some were more inclined to pick up the phone; some used paging or email.
In its role of messenger the EHR and related systems affected all of the collaboration behaviors, but its primary effect was on communication. While it could speed information transfer, it could reduce communication by inhibiting feedback. Asynchronicity facilitates information transfer by allowing EHR-related communications to bridge time and space; to facilitate providers messaging others on different shifts or in different offices or clinics. It also can increase awareness: we know he’s checking the vitals, he’ll come when he needs to.”
But there are downsides to the default asynchronicity of information transfer. The ability to bridge time also came with a draw back – the lack of simultaneity made it difficult to give feedback and clarify, which could take more, rather than less time. Across all of the sites we heard: “we call it brain freeze where with the technology implementation, they think that they can no longer talk to one another.” We also heard about the “communication illusion”, when providers thought they were communicating, but weren’t. Often a provider would send another a copy of their note, and assume the second would continue the plan. But the second provider might not find the plan or might misunderstand it due to a lack of contextual data. Delivery failure – particularly for lab results (“I’ve seem systems where 30% of the lab results weren’t delivered to the right person”) continues to be a problem. This was a problem that all of the sites we visited were trying to correct: “You need to have that person communicate with the primary doctor …. and then have [the primary] them assume that responsibility and acknowledge it.”
Orchestrator
The use of templates, smart order sets, and bundles has the potential to increase trust and respect, facilitate information transfer, and significantly improve coordination. The primary effect of the orchestrator role was, understandably, on coordination behaviors. Leaders at all of the sites were trying to use the EHR to improve quality by encouraging or ‘orchestrating’ “the right person doing the right thing at the right time.” Often this mean having someone work at “the top of their license.” The degree of sophistication shown varied from site to site; for some it was templates that encouraged a given workflow, for others it was sophisticated smart order sets or bundles that created prompts and initiated workflows based on patient circumstances.
These smart order sets/bundles existed at more advanced sites only for the most prevalent ten to twenty-five conditions. “We have lots of initiatives … most of them boil down to sophisticated checklists.” At one site they used “automation to manage the preventative care needs of 220,000 patients … delegation of tasks [to non-physicians] as well as protocol.” As one of our key informants there told us, this does not happen well right after EHR implementation: “We figure it takes about three months for the dust to settle…and [then we say]: What can we improve about the process? About the EHR? …The most valuable thing that happened … was that throughout the organization there are people who, when we say process redesign, say ‘Okay, when and where?’”
With smart order sets and bundles, there can be automatic dissemination and notification of results and follow-up. “We look at [who]… can perform the task. The alerts fires to them and only that person. … We make it actionable so … they can address the alert at that time. … The nurse can see it. The physician can see it. And then, further in the work flow …, if they did not address the alert, it will display again.” The ability to generate tickler (reminder) notes based on a pre-agreed plan of care is a powerful communication tool within the orchestrator role.
Unfortunately, even the best thought out and planned bundles won’t work if the providers don’t accept the process. “If we ever got any of ours 50 percent [of the new processes] accepted, I don’t know what we’d do. We would be so excited.” One of the challenges for implementing EHR mediated coordinated care is getting physicians to trust in others: “our physicians have been trained that really the buck stops with you. … [to offset this] we pilot everything…and then we show them it works. Time is also an issue. All of the providers we met with felt time and productivity pressures, making them resistant to additional tasks: “One the statements that can lead to failure is ‘let’s make the doctor do it …. Regardless of topic, I’ll give you about a 30% chance … [of getting it to work when assigned to the doctors].”, and “you can have a great tool for asthma, but if it takes ten minutes to do it’s not gonna happen.”
For many of the providers delegation helped relieve the time pressure. Care plans have long been a means of facilitating collaboration and team work. A more subtle point is the orchestrator role requires consolidating fragmented care plans, about which one participant noted: “A human being should only have one care plan. This is a legitimate IT role.”
The EHR can only do so much to support collaboration, however; at every site we were told that when the situation was complex, they needed to meet with or “pick up the phone and call” another provider. One clinical leader told us that, as far as collaboration was concerned, “the computer is giant hinder[ance]” When collaboration was important, so was the person to person contact – either a warm handoff, a face to face meeting, or a phone call.
Monitor
These systems of care were all striving to improve quality. With an EHR in place, data can be collected and analyzed to reveal care gaps. Several of the systems we visited were using these data to evaluate the performance of teams and individual providers; these data were also used to determine incentives and guide compensation decisions. By providing dashboard performance summaries to teams, and individual results to providers, systems were able to give feedback that could, in turn, facilitate performance improvement. It was in this role that we found the widest variation in system capabilities. None of the sites were completely satisfied with their EHR and the associated data warehouses that they were building to facilitate analysis. Given that, however, the capabilities ranged from automating data collection for mandatory reporting (e.g. HEDIS and meaningful use data) to enabling teams to use custom data extracts as they tried to improve their performance on quality metrics.
As a monitor, the EHR makes it possible to do ongoing reviews of process and outcomes. Perhaps because this is the least developed role, we found the most variation in effects. Uniformly organizations found that setting appropriate goals was difficult: “If the A1c target is 6.9 and we are at 7.0, I’m not sure that is a fail.” And getting good data could be difficult “Our chlamydia and gonorrhea screening rates were three percent, and we said ‘no way’…. It was a problem with the coding.” But once the goals were accepted, they could be used as an impetus for change: “We roll out the measures…. [showed] there is a disconnect there… and so that allow us to change the workflow.”
When the monitoring through EHR data showed improvement, this willingness to change was reinforced. The use of dashboards, graphical indicators of provider and team performance, provided a sense of progress. The EHR as monitor could also trigger action: “every month we pull a report [of diabetics] that includes nine different measures…and we then use this report to trigger telephone outreach…, vaccinations …and screenings.” Sometimes, however, the goals between providers would differ, and this would lead to conflict – for example when the endocrinologists were treating to an LDL goal of 100, but the primary care providers were only targeting a decrease to 120 ng/dl.
Overall
We found four roles for the EHR – repository, messenger, orchestrator, and monitor; and that these four supported or inhibited collaboration behaviors and processes differently. Although they conceptualized it more in terms of the specific systems (“data warehouse”, “clinical messaging,” etc.), the leadership groups at every clinic we visited were actively working to improve the performance of their electronic health record in each of its roles. Table 3 summarizes the principle effects discusses above. In each role, we found evidence that the EHR affects all of the collaboration behaviors – both supporting and inhibiting collaboration. The inhibiting actions, however, appear to be more known, while the supporting factors are potential changes.
Table 3:
The key issues for each collaboration behavior by role
| Repository | Messenger | Orchestrator | Monitor | |
|---|---|---|---|---|
| Trust and respect: Enhancing positive relationships between providers | Increased awareness, but cut and paste and other quality issues decrease trust. | Asynchrony helps, but lack of richness in channel can result in misinterpretation | Particularly strong in establishing clear expectations | Key appears to be common goals and measurement |
| Communication: Providing the information and mutual understanding needed to care for patients | Facilitates information transfer “It’s all there” (potentially), but “it’s hard to find” | But doesn’t guarantee communication Multiple channels can speed message delivery, but issues with “closing the loop” | Some successes, but clinical information is often not accessed/ignored by provider | When implemented can communicate gaps where practice improvement needed |
| Coordination: Having the right person do the right thing at the right time | A record of what actions and plans were, but each document frozen in time. | Issues due to variations in communications practice between providers | Bundles and “smart” worksheets particularly effective, but not implemented for enough conditions | Can facilitate team-based actions; if one member slips, another can fill in for them. |
| Collaboration: Facilitating collaborative decisions | Lack of interaction, one document per provider | No real time discussions, everything is lagged | Creates new boundaries, but doesn’t encourage adaptation | Using dashboard and incentives to focus on common goals promotes dialogue |
Limitations
Like any qualitative study, our results may not be generalizable. These sites were purposively selected to provide a cross section of successful EHR implementations in the United States. Readers will need to make their own judgments about the transferability of the results.
Discussion
Recent Research
Our results suggest that the EHR has evolved from its original role as a paper chart replacement (a repository) to also serving as messenger, an orchestrator, and a monitor. Issues with the performance of the EHR threaten its ability to support collaboration. Of particular concern were data quality and accessibility problems, which threaten the foundational behaviors of trust and respect. We also are concerned about the communication illusion, and issues with the multiplicity of communication channels. Other researchers have found similar issues at other locations using different EHRs and different qualitative perspectives.
Other studies with similar findings
One of our principal findings was that issues with the quality and accessibility of data threaten the EHR’s utility for collaborative use. Weir, Hammond, Embi et al(24) used a lens based on Clark’s theory of communication, joint action and common ground to examine the effects of computerized documentation on coordination and collaboration. Data were collected from focus groups at four different VA sites. Like our study, they found that the EHR could create a shared awareness and common database from which to act. They also found that “cut and paste” and failure to close the communications loop could create unintended consequences. Their work also discusses the value of narrative (richer contextual data) in building and maintaining shared mental models. This data were expanded to five sites and re-analyzed by Embi, Weir, Ehthiminiadis et al(25) using a grounded theory approach. Our work provides further evidence for the emergent themes from this expanded analysis. These included the inadequacy of the EHR as a sole communication channel, difficulties in finding relevant information, a need for better support for coordinated care, and disruptions in both trust and workflow due to problems with the EHR.
Our concerns about the communication illusions created by the EHR were foreshadowed by Lanham, Leykum, and McDaniel.(26) They used a complex systems approach to examine the effects of communication patterns on practice relationships. These relationships included trust and respect as well the appropriate use of communication channels. In a sample of six family medicine and specialty practices within a single system using the same EHR, they found that increased heterogeneity in communication patterns within each practice appeared to be related to increased practice fragmentation. We discuss a similar finding – the multiplicity of channels can result in communication patterns that inhibit collaboration.
Further research directions
EHR-related barriers to collaboration could be classified as predictable unintended consequences. The EHR was originally intended to replace the paper chart as a repository of data that would support medical reasoning and communication. But as healthcare is changing, so are the demands placed on the EHR and its related systems. It is unreasonable to expect an EHR to meet undefined needs. To meet these new demands we need a clear vision of what is needed. Both technological and organizational changes are needed. Processes need to be redesigned as well as technologies changed. We also need to be able to measure the effects of the changes once they are implemented.
Improved technology can help. Our study sites were developing better data warehousing and improved data exchange which would improve monitoring. We saw technology that could identify when a process step was missed and notify the proper person to get the gap filled to improve coordination. There is much innovation – and it is not confined to the sites we visited. The authors’ informal discussion at both the AMIA student design competition and with EHR developers have shown possibilities for improving the EHR’s ability to support collaboration. Better interface and data input technologies can provide time for better communications(27). Curation can be improved by using plagiarism tools to identify inappropriate cut and paste(28). Bundles that allow for more effective pathways and delegation can also free up time for providers(29).
More interestingly, the paradigm can be revised to fit a coordinated and collaborative process, rather than an individual practitioner. Models from other fields offer some possibilities. Ratings (“Amazon”) for quality and usefulness might increase the quality of notes. Better identification of team members and their capabilities (“Facebook”) could increase visibility and trust. With the advent of care managers, several “add on systems” have been developed to create a common care plan. What is needed is a reconfiguration of the basic structure of the EHR. The ideal EHR for chronic disease management would allow for the integration of multiple care plans from different providers. This integrated care plan could be implemented as a wiki with the primary care provider or designee as the curator, or it could incorporate “column” care planning. It would be one care plan for one person.
Implementation and training are also key to improving collaboration through technology. As we saw in our visits, it is important to train users so they remember to keep talking, and not rely exclusively on leaner electronic media. Designing implementation processes that build buy-in from providers significantly increased adherence to the new protocols. And tying incentives to the data in the EHR – especially the problem lists – increased the value of the EHR data. There are other possibilities. Interventions to be tested include evaluating providers on the clarity and comprehensiveness of their notes, rather than on the number of billing factors; training providers on when to use what communication channels, and creating a “curators” who annotate and index of the documents for future use, accessibility would improve. Process innovations to gain acceptance are also understudied.
Finally, we need a tool to measure collaboration. The extant tools (cite) focus on supporting teams – collocated groups with a common goal. But collaboration between providers crosses organizational, geographic, professional and time boundaries. A more generalized tool would help us measure and manage the effects of the interventions discussed above.
Conclusion
In this study, we have identified four roles the EHR can and should play in the collaborative process. The organizations we visited demonstrated the potential for the EHR to support increased collaboration. These same systems also demonstrated barriers to collaboration presented by the implementations of current systems. Only by explicitly considering the different collaboration roles played by the EHR can designers and implementers develop the informatics tools needed for the twenty-first century. With these tools, the EHR can fill its role in the collaborative process.
Acknowledgments
In addition to my co-authors, the primary author would like to thank the POET/SAFER team (especially Adam Wright PhD, Dean Sittig PhD, and Hardeep Singh MD); Paul Gorman MD, Rachel Dresbeck PhD, and the Department of Medical Informatics and Clinical Epidemiology at OHSU; and the National Library of Medicine (training grant 5 T15-LM007088-22) for their contributions and support.
References
- 1.Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000 Feb 26;320(7234):569–572. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic disease. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 3.Merriam-Webster Merriam-Webster Online Dictionary. Available at: http://www.merriam-webster.com/dictionary/collaborate. Accessed August 4, 2013.
- 4.Zillich AJ, Doucette WR, Carter BL, Kreiter CD. Development and initial validation of an instrument to measure physician-pharmacist collaboration from the physician perspective. Value in Health. 2005 Jan-Feb;8(1):59–66. doi: 10.1111/j.1524-4733.2005.03093.x. [DOI] [PubMed] [Google Scholar]
- 5.Stock R. Developing Team Based Care. 2013. Available at: http://www.pcpci.org/resources/webinars/developing-team-based-care-in-patient-centered-primary-care-home. Accessed March 11, 2014.
- 6.Baggs JG. Nurse-physician collaboration in intensive care units. Crit Care Med. 2007 Feb;35(2):641–642. doi: 10.1097/01.CCM.0000254039.89589.99. [DOI] [PubMed] [Google Scholar]
- 7.Sierhouse M, Clancey W. Modeling and simulating practices, a work method for work systems design. Intelligent Systems, IEEE. 2002;17(5):31. [Google Scholar]
- 8.Clancey WJ, Sierhuis M, Damer B, Brodsky B. Cognitive modeling of social behaviors. In: Sun R, editor. Cognition and multi-agent interaction. New York, New York: Cambridge University Press; 2005. pp. 151–185. [Google Scholar]
- 9.Salas E, Wilson KA, Murphy CE, King H, Salisbury M. Communicating, coordinating, and cooperating when lives depend on it: tips for teamwork. Joint Commission Journal on Quality & Patient Safety. 2008 Jun;34(6):333–341. doi: 10.1016/s1553-7250(08)34042-2. [DOI] [PubMed] [Google Scholar]
- 10.Fields D, Leshen E, Patel K. Analysis & commentary. Driving quality gains and cost savings through adoption of medical homes. Health Aff. 2010 May;29(5):819–826. doi: 10.1377/hlthaff.2010.0009. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal TC. The medical home: growing evidence to support a new approach to primary care. Journal of the American Board of Family Medicine. JABFM. 2008 Sep-Oct;21(5):427–440. doi: 10.3122/jabfm.2008.05.070287. [DOI] [PubMed] [Google Scholar]
- 12.Gittell JH, Fairfield KM, Bierbaum B, Head W, Jackson R, Kelly M, et al. Impact of relational coordination on quality of care, postoperative pain and functioning, and length of stay: a nine-hospital study of surgical patients. Med Care. 2000 Aug;38(8):807–819. doi: 10.1097/00005650-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Quality Assurance Patient-centered medical home recognition. Available at: http://www.ncqa.org/Programs/Recognition/PatientCenteredMedicalHomePCMH.aspx. Accessed March 11, 2014.
- 14.Oregon Health Authority Patient Centered Primary Care Home. 2013. Available at: http://www.oregon.gov/oha/pcpch/Pages/index.aspx. Accessed March 11, 2014.
- 15.Shortliffe EH. The evolution of electronic medical records. Academic Medicine. 1999 Apr;74(4):414–419. doi: 10.1097/00001888-199904000-00038. [DOI] [PubMed] [Google Scholar]
- 16.Tang PC, McDonald CJ. Computer-based patient-record systems. Medical Informatics: Springer. 2001:327–358. [Google Scholar]
- 17.Sittig DF, Ash J, editors. Clinical information systems: Ovecoming adverse consequences. Sudbury, Mass: Jones and Bartlett; 2011. [Google Scholar]
- 18.Beebe J. Rapid Assessment Process, An Introduction. Walnut Creek, CA: Altamira Press; 2001. [Google Scholar]
- 19.McMullen CK, Ash JS, Sittig DF, Bunce A, Guappone K, Dykstra R, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299–307. doi: 10.3414/ME10-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauss A, Corbin J. Basics of Qualitative Research, Grounded Theory Procedures and Techniques. Thousand Oaks, CA: Sage Publications; 1990. [Google Scholar]
- 21.Office of the National Coordinator for Health Information Technology The SAFER Guides. Available at: http://www.healthit.gov/policy-researchers-implementers/safer. Accessed March 10, 2013.
- 22.QSR International . NVivo. 2011. p. 9. [Google Scholar]
- 23.Crabtree BF, Miller WL. Doing Qualitative Research. 2nd. Thousand Oaks, CA: Sage Publishing; 1999. [Google Scholar]
- 24.Weir CR, Hammond KW, Embi PJ, Efthimiadis EN, Thielke SM, Hedeen AN. An exploration of the impact of computerized patient documentation on clinical collaboration. Int J Med Inf. 2011 Aug;80(8):e62–71. doi: 10.1016/j.ijmedinf.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Embi PJ, Weir C, Efthimiadis EN, Thielke SM, Hedeen AN, Hammond KW. Computerized provider documentation: findings and implications of a multisite study of clinicians and administrators. Journal of the American Medical Informatics Association. 2013 Jul-Aug;20(4):718–726. doi: 10.1136/amiajnl-2012-000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanham HJ, Leykum LK, McDaniel RR., Jr Same organization, same electronic health records (EHRs) system, different use: exploring the linkage between practice member communication patterns and EHR use patterns in an ambulatory care setting. Journal of the American Medical Informatics Association. 2012 May-Jun;19(3):382–391. doi: 10.1136/amiajnl-2011-000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyt R, Yoshihashi A. Lessons learned from implementation of voice recognition software in the military electronic health record. Perspect Health Inf Manag. 2010;7(1e):1. [PMC free article] [PubMed] [Google Scholar]
- 28.Wrenn JO, Stein DM, Bakken S, Stetson PD. Quantifying clinical narrative redundancy in an electronic health record. J Am Med Inform Assoc. 2010 Jan-Feb;17(1):49–53. doi: 10.1197/jamia.M3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloom F, Graf T, Anderer T, Stewart W. Redesign of a Diabetes System of Care Using an All-or- None Diabetes Bundle to Build Teamwork and Improve Intermediate Outcomes. Diabetes Spectrum. 2010;20(3):165. [Google Scholar]


