Abstract
We analyzed audio recordings of telephone calls between emergency departments (EDs) and poison control centers (PCCs) in order to describe the information requirements for health information exchange. Analysis included a random sample of 120 poison exposure cases involving ED-PCC communication that occurred during 2009. We identified 52 information types characterized as patient or provider information, exposure information, ED assessment and treatment/ management, or PCC consultation. These information types constitute a focused subset of information that should be shared in the context of emergency treatment for poison exposure. Up to 60% of the information types identified in the analysis of call recordings can be represented using existing clinical terminology. In order to accomplish standards-based health information exchange between EDs and PCCs using data coded according to a standard clinical terminology system, it is necessary to define appropriate terms, information models and value sets.
Introduction
Poison control centers (PCCs) routinely collaborate with emergency departments (EDs) to provide care for poison exposed patients. Of approximately 2.3 million poison exposures reported to U.S. PCCs in 2012, 27% were managed in a healthcare facility.1 The PCC and ED share information about the patient, the patient’s status, and circumstances surrounding the poison exposure throughout the ED visit. As poison exposure cases evolve, the PCC and ED are in regular communication via telephone for the purpose of collaborative care planning and on-going consultation.2 The ED care providers verbally share clinical information with a PCC specialist, including patient symptoms, general condition, and the results of certain laboratory tests; the PCC frequently updates treatment recommendations as additional information becomes available. Multiple handoffs can occur for both the PCC and ED.2 Information may be communicated to one or multiple ED care providers depending on the workload in the ED and the status of the poisoned patient or other patients in the ED. Crucial information documented during this complex process remains isolated within the respective information systems of the ED and PCC and is not shared across organizational boundaries.
In preliminary studies, we found that the current process of telephone-based communication and information sharing between emergency departments and poison control centers is vulnerable to miscommunication, data loss, and error.2 Examples of safety vulnerabilities include: difficulty establishing synchronous verbal communication via telephone, discussion of multiple patients during the same telephone conversation, and communication with nonclinical staff members.1 Additionally, any information moved among patient care settings via phone may or may not be documented for continued use by the recipient health care facility. Verbal communication, though expressive, is fraught with pitfalls that affect patient care.3,4
The current process of telephone-based communication is also vulnerable in disaster scenarios. PCCs manage calls from both public and health care providers, and routinely see increases in call volume due to infectious disease outbreaks and other non-poisoning public health concerns. A process based solely on verbal, telephone communication is fragile. Studies of poison control center operations during call surges are scarce. However, in a previous study, we found that even routinely encountered busy times tax the ability of PCC specialists to communicate.5 A 2007 article by Vassilev and colleagues describes an incident in which a poison control center experienced compromised operations due to an unexpected 148% increase in call volume.6 In the setting of mass scale poisonings due to natural disasters, accidents or terrorism, a system based entirely upon telephone communication is fragile.
Given existing technology to support the electronic exchange of data and information across organizational boundaries, there is potential to reduce medical error, reduce time to treatment, and improve continuity of care through a more efficient and structured process of ED-PCC collaboration. In order to design a more robust, structured process for ED-PCC collaboration, supported by health information exchange, it was necessary to determine and characterize the information requirements We analyzed audio recordings of current telephone-based communications between EDs and PCCs in order to describe the information requirements for health information exchange between poison control centers (PCCs) and emergency departments (EDs). Our goal was to identify a focused subset of available health information, most relevant to emergency treatment of poison exposure, in order to support generalizable process re-design. The first research assistant analyzed the transcripts, creating a formative list of information types evident in the communication.
Methods
Setting
We conducted the study at a single poison control center in the intermountain west. The poison control center has a very high utilization rate compared to national data, and so it constitutes a rich source of data describing interactions between PCCs and EDs. In 2011, the poison control center managed 42,544 human poison exposures, with approximately 13% of cases managed in the emergency department setting and entailing collaboration between an emergency department and the poison control center.
Sample
We used a saturation sampling approach, based upon an initial random sample of 500 poison exposure cases, managed in the emergency department setting and involving ED-PCC communication, during the calendar year 2009. As described in Cummins et al (2013), we sequentially analyzed the calls until we failed to identify new information types in 40 additional, randomly selected cases.2 This saturation sampling approach ensured an adequate sample size to describe the types of information routinely shared in ED-PCC communication, while conserving the resources necessary to analyze calls and minimizing the use of patient data. Ultimately, we analyzed calls corresponding to a sample of 120 poison exposure cases.
Data Preparation and Analysis
As described in Cummins et al (2013), telephone calls between the poison control center and its collaborating EDs are routinely recorded and stored on a secure server, and we previously developed a process for linking cases to analog call recordings.2,7 We applied this procedure in 20 case increments according to the saturation sampling plan, linking calls to cases as necessary until saturation was reached. For each 20 case increment used in the analysis, we linked call recordings to cases, verified the linkage, exported the call recordings, and converted them to digital format. We transcribed the call recordings and removed identifiers of personnel, patients, and health care facilities. Two research assistants, graduate students in biomedical or nursing informatics, analyzed the data. The first research assistant analyzed the transcripts, creating a formative list of information types evident in the communication. The second research assistant independently reviewed the transcripts, in order to validate the information types. Questions or difficulties in conceptualizing and categorizing information types were resolved through discussion by the research team, and in cases of disagreement, the principal investigator made final determination. Given the lack of pre-determined concepts and categories, this process was formative and iterative. This process resulted in a list of information types and the frequency of their occurrence per poison exposure case.
Representation in Clinical Terminology and Coding Systems
A research assistant manually searched for inclusion of each information type in existing clinical terminology or coding systems, with review and supervision by the principal investigator. These systems included AAPCC (American Association of Poison Control Centers) substance codes, CPT (Current Procedural Terminology), ICD-9 and ICD-10 (International Classification of Diseases, Ninth and Tenth Revisions), NDC (National Drug Code), RxNorm, Poisindex, SNOMED-CT (SNOMED Clinical Terms), LOINC (Logical Observation Identifiers Names and Codes), DEEDS (Data Elements for Emergency Department Systems) 1.0, and NPDS (National Poison Data System).8,9,10,11 Information types were insufficiently granular for precise mapping at the concept level, but the capability to represent each information type could be assessed. In these cases, the principal investigator reviewed the nature and scope of each terminology system with respect to the information type, and determined whether the system could be used to represent the information type.
Results
Identification of Information Types
We reached sampling saturation upon analysis of calls corresponding to 120 cases. Inter-coder disagreement was infrequent and resolved through team discussion and review of transcripts. The research team reviewed the information types, and where appropriate, aggregated duplicate or nested concepts. After validation and aggregation, we identified 52 information types listed and categorized in table 1. The information content of analyzed calls included essential identifying information – information identifying both health care provider (location, type of provider, name) and patient (name, age, gender). It also included essential health information about the patient, including current medications, allergies, and health history. Many information types described the poison exposure incident. Information was exchanged about the poison, its characteristics and effects, and clinical treatment. Information was also exchanged about the poisoning scenario, including important circumstances that bear upon decision making related to care of the patient.
Table 1.
Types of information shared during telephone calls between EDs and PCCs, excluding patient/ provider identifying information.*
| Exposure information | |
|---|---|
| Exposure Type | E.g., polypharmacy, accidental, overdose. |
| Certainty of Formulation | In a circumstance where the poison is a therapeutic agent, the level of certainty about the precise formulation, based on the subjective/ objective information provided. |
| Chronicity | Duration of exposure (acute vs. chronic) |
| Establishing background/certainty | Narrative information about the poison exposure scenario specifically relevant to determining the certainty of ingestion/exposure, and general information helpful in constructing a clinical picture of the patient and treatment plan. |
| Substance class | General grouping or characterization of poison (e.g., beta-blocker) |
| Substance information | General characteristics of a poison (e.g., half-life, mechanism of action, peak effect) |
| Substance name (generic) | Generic name of poison that is also a therapeutic agent |
| Substance amount | Dosage or amount of substance (e.g., 500mg metformin) |
| Substance name (brand) | Brand name of poison that is also a therapeutic agent |
| Substance description | Informal description ranging from general class of drug (e.g., antiarrhythmic) to intended purpose (e.g., a chemical used to clean carburetors) |
| Substance form | E.g., tablet, pill, powder, lozenges. |
| Substance formulation | E.g., extended release vs. Rapid release |
| Substance identification rationale | Narrative describing reasoning of PCC specialist in identifying a substance based on described characteristics of the poison (e.g., “A blue pill in that shape can be a few different things, but it is probably Viagra”) |
| Substance-nonpharmacological | Name of non-pharmacological substance |
| Substance type | E.g., solid, inhalant, liquid. |
| Time since ingestion | Amount of time elapsed since initial poison exposure (e.g., “It has been about 5 hours since she took the pills.”) |
|
| |
| Patient health history | |
|
| |
| Medical history | Information about patient’s past medical history. |
| Patient medications | Medications that the patient currently takes at home. |
|
| |
| Subjective & objective Information | |
|
| |
| Chief complaint/eason for visit | Patient’s chief complaint upon presenting to emergency department or calling poison control center. |
| Absence of clinical effects | The absence of any signs or symptoms attributable to poison exposure. |
| Mental status | Information about patient’s mental status. |
| Caller reported symptoms | Symptoms as reported by patient or caller to PCC. |
| Physical exam findings | Signs observed by ED health care providers. |
| Unrelated symptoms | Health care provider or PCC assessment that a symptom is unrelated or likely unrelated to the poison exposure. For example, a patient may exhibit tremors related to underlying Parkinson’s disease, unrelated to an acute narcotics overdose. |
| Vital signs | Information describing patient’s blood pressure, heart rate, and/or respiratory rate along with the time the vital signs were obtained. |
|
| |
| PCC recommendations &toxicology information | |
|
| |
| PCC recommendations for treatment and discharge parameters | PCC recommendations for treatment and/or duration of direct observation prior to discharge from the ED. |
| Toxic dose | Specific dose or amount of exposure, at which a substance becomes toxic. |
| Toxicity levels | Circulating blood level of a substance considered toxic. |
| Clinical effects of substance | Potential or expected clinical effects of a given poison exposure. |
| Worst case scenario | Description of the most harmful clinical effects and poorest outcome that patient might experience (a description of risk), usually PCC to ED. |
|
| |
| ED treatment / management information | |
|
| |
| Confirmation that was treatment given | ED staff member indicates whether or not a PCC recommended treatment has been administered to a patient. |
| Patient discharge medications | Medications prescribed for patient at time of discharge from ED. |
| Patient status | ED description of patient’s clinical condition, particularly whether clinical effects of exposure are observed. |
| Plan of care | Refers to ED health care provider plan of care for patient (e.g., treatments, procedures, length of stay, parameters for discharge). |
| Diagnostic test results | Inquiry about results of diagnostic tests (e.g., laboratory, ECG, imaging). |
| Time next laboratory tests will be ordered | Information about the ED’s planned or recommended timing of subsequent diagnostic testing (e.g., “We’ll get another level at 4 hours [after ingestion].”). |
| Time laboratory test was performed/drawn | Information about the timing of a treatment or a lab test, usually in relation to the time of ingestion (e.g., “That level was drawn 2 hours after ingestion”). |
| Treatment performed | Information that a treatment, directly related to the poison exposure, was administered. |
|
| |
| Information types idiosyncratic to telephone communication | |
|
| |
| Ambiguous Test Result | Characterization of the results of laboratory or non-laboratory diagnostic testing |
| Confirmation Patient Arrived at Hospital | Confirmation by an ED staff member that a given patient is under the care of that ED |
| Request for Chronicity | Inquiry about duration of exposure |
| Patient Status Request | Inquiry into patient’s status, both clinical condition and whether the patient is still under ED care |
| Request for clinical effects information | PCC inquiry as to clinical effects effects observed by ED health care provider |
| Request for Lab/Test | Inquiry as to results of laboratory diagnostic testing |
| Results | |
| Request for Test Results | Inquiry as to results of non-laboratory diagnostic testing |
PCCs collect information for entry into the National Poison Data System (NPDS) and consequently, some information types resemble NPDS data elements. However, NPDS data elements are defined differently, as described in a published coding manual.11
Narrative information, the poisoning “story”, included details important for discerning whether the poisoning was intentional (overdose or suicide attempt) or unintentional, and details that help to establish the certainty, dose, and timing of the exposure. As a fictional example, a narrative might include a description of the parent’s estimate of the number of tablets remaining in a full prescription bottle, and the time at which a child was found eating tablets from that open bottle. Both the PCC and ED collected and shared these types of information. The ED care providers, who assess the patient in person, shared information about the physical exam and appearance of the patient, clinical findings, and the results of any diagnostic testing. They also shared information about the patient’s plan of care, and treatment or management. The poison control center, acting as consultant, provided feedback on clinical findings as well as treatment and monitoring recommendations. This frequently entailed general communication about the type of poison, its characteristics, effects, and treatment in a type of communication that appears to establish common ground for the discussion of the specific patient and exposure at hand. Communication also frequently included requests for information from the other party.
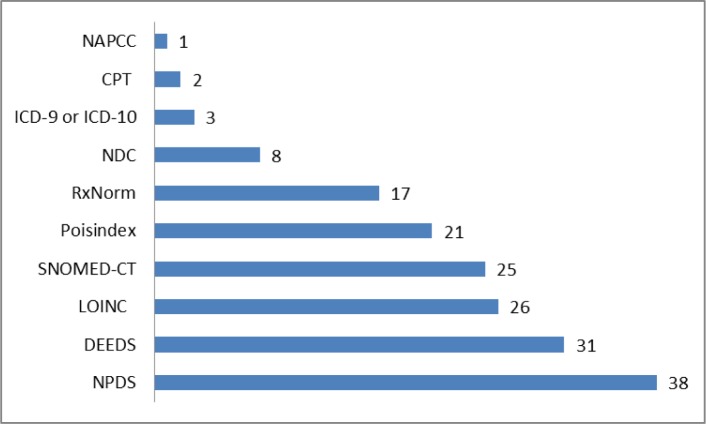
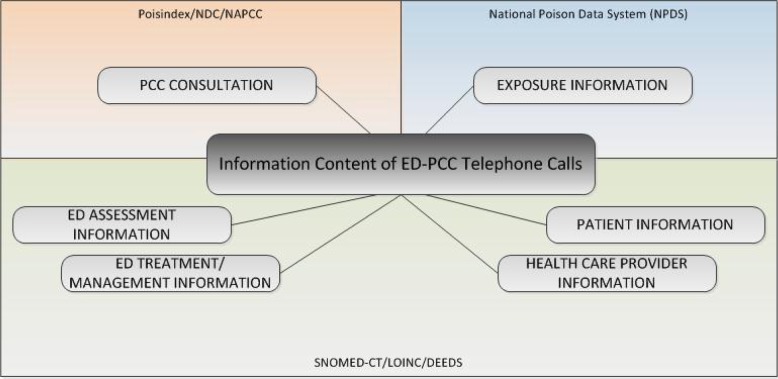
We compared these information types to commonly accepted clinical terminology and coding systems using the UMLS Metathesaurus, terminology browsers or coding manuals. In this preliminary screening, none of the terminology systems provided complete coverage of the identified information types. NPDS (National Poison Data System) represented 38/52 information types (73%). Clinical terminologies LOINC and SNOMED-CT represented approximately half of the information types. A specialized set of data elements designed for the emergency department setting (DEEDS), represented 31/52 information types (60%). See figure 1 for results. In figure 2, we show the categories of information types along with the coding or terminology systems that best represented the information types within those categories.
Figure 1.
Representation of information types by commonly accepted clinical terminology and coding systems.
Figure 2.
Terminology coverage of information types used in ED-PCC communication.
Discussion and Implications
Approximately 50–60% of the information types identified in the analysis of call recordings map to the widely used clinical terminologies LOINC and SNOMED-CT, and a specialized set of data elements designed for the emergency department setting, DEEDS. However, many of the identified information types are highly specialized to the context of poisoning scenarios and are not found in standard clinical terminology systems. ED-PCC communication about poison exposed patients involves types of information that are not commonly represented in standard clinical terminology systems. In order to accomplish standards-based health information exchange using data coded according to a standard clinical terminology system, additional terms must be proposed and adopted.
The National Poison Data System (NPDS) data elements mapped most successfully to the information types. However, NPDS is structured to support case-level data vs. patient-level data, and is not structured as a clinical terminology. NPDS data elements were designed for the purpose of surveillance and not to support clinical information systems or patient care. Although used by all U.S. poison control centers, NPDS data elements are not currently mapped to any standard clinical terminology system and preliminary attempts at mapping have yielded disappointing results, so they do not facilitate interoperability with emergency departments.12 Moving toward interoperability, it is important to create a mapping of NPDS data elements to standard clinical terminology systems or expand existing clinical terminologies. It is also necessary to identify standard information models (e.g., CDA) that would be used to instantiate the standard codes, since those codes need to be bound to slots in standard information models. Standard value sets (allowed set of codes for a given data element) should be used in the standard information models, and the NLM (National Library of Medicine) value set authority center could be used as the central resource for submitting and managing these value sets.
Representing Information Content with Existing Clinical Terminology and Coding Systems
One key challenge in designing a health information exchange supported collaboration process, particularly in the context of an emergency poisoning event, is to focus attention on information immediately relevant to the health event and decision making at hand. In the context of ED-PCC collaboration to care for poison exposed patients, we identified 52 information types that should be addressed in the design of health information exchange activities. However, it is currently not possible to represent all of these information types using standard clinical terminologies.
Patient and Health Care Provider Information
Basic information about patients and health care providers (name and gender, for example) is commonly collected in health care settings, and so it is commonly included in standard clinical terminologies and standard clinical information models. Similarly, information about health care providers can be represented using multiple clinical terminology systems and information models.
Emergency Department information
ED assessment information, including the initial history & physical examination and subsequent clinical observations, can be represented using concepts found in DEEDS (Data Elements for Emergency Department Systems)/ LOINC and SNOMED-CT. Similarly, ED treatment or management information can be described using concepts found in DEEDS/LOINC and SNOMED-CT. DEEDS is a terminology system designed to support emergency department operations, and closely fits the type of assessment and observational data exchanged in ED-PCC telephone calls. DEEDS is included in LOINC, a much larger clinical terminology system.
Exposure
Exposure information closely matches NPDS data elements, a logical circumstance given that collection of these data elements is one purpose of the telephone calls. To some extent, these information types can also be represented using DEEDS/ LOINC. However, the coverage of exposure information by DEEDS/LOINC is incomplete or insufficiently granular. This is not surprising, given the highly specialized nature of poison control center operations. PCCs collect information about poison exposure at a much more granular level of detail, a level of detail that supports surveillance and public health intervention. NPDS is case-based, collected for the purpose of surveillance, and NPDS data elements are not structured as a clinical terminology. New information models such as CDA templates would need to be created to represent PCC exposure information.
Substance
Substance information can be represented using several terminology or coding systems. However, the nature and extent of information varies. Poisindex, a proprietary, subscription-based toxicology database, best matches the depth of information shared in telephone calls between EDs and PCCs. Poisindex codes are currently used to identify a very specific type, brand, and formulation of a substance, pharmaceutical or non-pharmaceutical. NAPCC substance codes are also currently used to characterize the type of substance in a meaningful way from the perspective of poisoning epidemiology. Drugs manufactured for therapeutic purposes are often involved in poisoning and if the poison is a manufactured therapeutic agent (Vicodin, for example), it could be coded using NDC or RxNorm. However, many poisonings involve naturally occurring substances not indexed in NDC or RxNorm (snake venom or toxins from wild mushrooms, for example). Many more poisonings involve inhaled gases (e.g. carbon monoxide), illicit recreational drugs, industrial chemicals, household cleaning agents, and other non-therapeutic chemicals not indexed in NDC or RxNorm. A specific value set for substances could include a subset of RxNorm codes (e.g., ingredients and branded drugs) and a combination of codes from other non-drug terminologies. Additionally, these codes must be used in a way that clearly establishes the context as poison exposure vs. pharmacotherapy.
Limitations
The findings of this study are observational in nature, describing information that is currently shared using telephone communication. In current process re-design efforts, we are obtaining clinician input about additional, potentially helpful information types as well as prioritization of information types. As a single site study, the findings possibly include some information types that are idiosyncratic to the region (intermountain west) or poison control center and emergency departments studied. However, the communication process used by this poison control center is highly similar to the process used by other U.S. poison control centers, and the specific information types found in this study appear to reflect universal aspects of emergency care for poison exposure. Consequently, the findings provide a reasonable basis for standards development and process improvement efforts. Comparison of information content according to ED characteristics was outside the scope of this study. However, the emergency departments served by the poison control center are diverse and include both small, rural hospitals and urban academic medical centers. Additionally, information content may also have varied during surge conditions.5 The primary purpose of our study was to inventory information types, and the presence of an information type in a single transcript was sufficient for inclusion in the inventory.
Conclusion
Given evidence of inefficiencies and safety vulnerabilities in the current telephone-based process of ED-PCC communication and information sharing, new models of collaboration must be considered. We are currently developing a health information exchange supported collaboration process, designed to support dynamic, real time, bidirectional communication between EDs and PCCs to support care of poison exposed patients. This study identified the subset of health information that should be included in such a process. Moreover, the results indicate that telephone-based communication about poison exposed patients entails the sharing of information types that are not currently represented in standard clinical terminology systems. In order to accomplish standards-based health information exchange between EDs and PCCs using data coded according to a standard clinical terminology system, additional terms must be proposed and adopted. Corresponding information models, such as CDA templates, require development and specific value sets must be identified.
Acknowledgments
This study was supported by the US Department of Health and Human Services, Agency for Healthcare Research and Quality, grant 1R21HS018773-01.
References
- 1.Mowry JB, Spyker DA, Cantilena LR, Jr, Bailey JE, Ford M. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila) 2012 2013 Dec;51(10):949–1229. doi: 10.3109/15563650.2013.863906. PubMed PMID: 24359283. [DOI] [PubMed] [Google Scholar]
- 2.Cummins MR, Crouch B, Gesteland P, Wyckoff A, Allen T, Muthukutty A, et al. Inefficiencies and vulnerabilities of telephone-based communication between U. S. poison control centers and emergency departments. Clin Toxicol (Phila) 2013 Jun;51(5):435–43. doi: 10.3109/15563650.2013.801981. PubMed PMID: 23697459. [DOI] [PubMed] [Google Scholar]
- 3.Bhasale AL, Miller GC, Reid SE, Britt HC. Analysing potential harm in Australian general practice: an incident-monitoring study. Med J Aust. 1998 Jul 20;169(2):73–6. doi: 10.5694/j.1326-5377.1998.tb140186.x. PubMed PMID: 9700340. [DOI] [PubMed] [Google Scholar]
- 4.Donchin Y, Gopher D, Olin M, Badihi Y, Biesky M, Sprung CL, et al. A look into the nature and causes of human error in the intensive care unit. Qual Saf Health Care. 2003;2003(12):143–7. doi: 10.1136/qhc.12.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caravati EM, Latimer S, Reblin M, Bennett HK, Cummins MR, Crouch BI, et al. High call volume at poison control centers: identification and implications for communication. Clin Toxicol (Phila) 2012 Sep;50(8):781–7. doi: 10.3109/15563650.2012.713968. PubMed PMID: 22889059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassilev ZP, Kashani J, Ruck B, Hoffman RS, Marcus SM. Poison control center surge capacity during an unusual increase in call volume–results from a natural experiment. Prehosp Disaster Med. 2007 Jan-Feb;22(1):55–8. doi: 10.1017/s1049023x00004349. PubMed PMID: 17484364. [DOI] [PubMed] [Google Scholar]
- 7.Poynton M, Jasti S, Ellington L, Dudley W, Crouch B, Caravati M, et al. Matching waveform audio files with toxicall data: Record linkage in a poison control center. Stud Health Technol Inform. 2006;122:849. PubMed PMID: 17102421. [PubMed] [Google Scholar]
- 8.Regenstrief Institute Inc . LOINC. 2013. [Google Scholar]
- 9.International Health Standards Development Organization . SNOMED-CT. 2013. [Google Scholar]
- 10.Poisindex. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc; Updated periodically. ed. [Google Scholar]
- 11.American Association of Poison Control Centers . National Poison Data System (NPDS)© Reference ManualPart 2 - System Information Manual. 2009. [Google Scholar]
- 12.Cummins M, Doing-Harris K, Passman J, Mateos B. Automated mapping of NPDS data elements to the UMLS Metathesaurus; Proceedings of AMIA 2013: American Medical Informatics Association (AMIA) Annual Symposium; 2013. [Google Scholar]