Abstract
Glutamate plays an important role in the central nervous system as an excitatory neurotransmitter. However, its abundance can lead to excitotoxicity which necessitates the proper function of active glutamate transporters. The glutamate-aspartate transporter (GLAST) has been shown to exist and function within non-human cochlear specimens regulating the inner ear glutamate concentration. In this study, we examined micro-dissected human cochleas from formalin-fixed celloidin-embedded temporal bone specimens of three different types of patients (Meniere's disease, normal controls, and other otopathologic conditions) and examined the differential expression of GLAST in the spiral ligament of the basal, middle, and apical turns of the cochlea. Immunohistochemical staining was performed with polyclonal antibodies against GLAST and image analysis was carried out with the Image J analysis software. In contrast to other studies with non-human specimens, GLAST was expressed in the spiral ligament fibrocytes but was not detected in the satellite cells of the spiral ganglia or supporting cells of the organ of Corti in the human cochlea. Our data also showed that GLAST expression significantly differs in the basal and apical turns of the cochlea. Lastly, post-hoc analysis showed a difference in the GLAST immunoreactive area of patients with Meniere's disease when compared to that of patients with other otopathologic conditions—such as presbycusis or ototoxicity. These results may potentially lead to further understanding of different disease states that affect hearing.
Keywords: Glutamate-aspartate transporter, glutamate, human temporal bone, immunohistochemistry, Meniere’s disease, hearing loss
1. Introduction
The lateral wall of the cochlea houses the stria vascularis and spiral ligament, two cochlear organs that play an essential role in the homeostasis of cochlear fluids by controlling the gradient of potassium ions. Maintenance of this ion gradient is the driving force of the endocochlear potential (EP), which has been shown to be required for perception of finely tuned hearing (Wangemann, 2006). The spiral ligament contains an extracellular matrix that has five regionally and morphologically distinct types of fibrocytes, types I–V (Spicer and Schulte, 1996). Fibrocytes are also found over the perilymphatic surface of the spiral limbus, a ridge of tissue on the osseous spiral lamina that runs internal to the organ of Corti (Furness et al., 2009). These cells contain Na+/K+ ATPase and other ion pumps, allowing them to contribute to the maintenance of ion homeostasis. Fibrocyte degeneration has been shown to result in one form of hereditary deafness (DFN3) and is a major factor in mouse models of presbycusis and loss of EP (Minowa et al., 1999; Wu and Marcus, 2003).
The major glutamate transporter of the cochlea is the excitatory amino acid transporter 1 (EAAT-1) and has also been referred to as glutamate–aspartate transporter (GLAST). It has been found in the supporting cells surrounding the inner hair cells (IHCs) and satellite cells of the spiral ganglion in non-human species (Furness and Lehre, 1997). Previous studies have demonstrated the presence of a GLAST-associated current that can be monitored using electrophysiological techniques (Glowatzki et al., 2006). Also, with reverse transcriptase polymerase chain reaction (rtPCR), GLAST expression has been localized to fibrocytes (mainly type II fibrocytes) in the lateral wall and spiral limbus in mice. (Hakuba et al., 2000; Jin et al., 2003; Li et al., 1994).
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS) whose presence has also been demonstrated in synapses between the IHCs and their afferent auditory nerve fibers (Eybalin, 1993; Hakuba et al., 2000). In the CNS, glutamate uptake is thought to be of vital importance in protecting glutamatergic synapses from excitotoxicity since glutamate is cleared by highaffinity transporters present in the membranes of neurons and glia (Danbolt, 2001). Likewise, the role of the glutamate transporters around IHCs likely involves uptake of synaptically released glutamate during stimulation (Furness and Lehre, 1997; Furness and Lawton, 2003). By limiting the accumulation of extracellular glutamate, these transporters prevent tonic activation of receptors, and thus, prevent excitotoxicity (Glowatzki et al., 2006). Glutamate levels measured in mouse perilymph before, during and after ischemia increase from a baseline of 1 pmol/μl to 15 pmol/μl and then return close to baseline after the ischemia is reversed (Hyodo et al., 2001). Thus relatively high levels of glutamate may accumulate in perilymph, especially under acoustic or hypoxic stress, requiring a general homeostatic process to control them (i.e.: glutamate-aspartate transporters).
While the expression of GLAST has been proven in animal models (such as the mice, rat, and guinea-pig), its presence in the human cochlea has not yet been demonstrated. In this study, formalinfixed celloidin-embedded (FFCE) human temporal bones were examined with attention to the spiral ligament of the basal, middle, and apical turns of the cochlea using immunocytochemistry to detect the presence of GLAST. In addition, changes in GLAST expression were examined in normal control humans (“Normal”); patients with Meniere’s disease (“Meniere’s”); and patients with other otopathologic conditions (“Other”).
2. Results
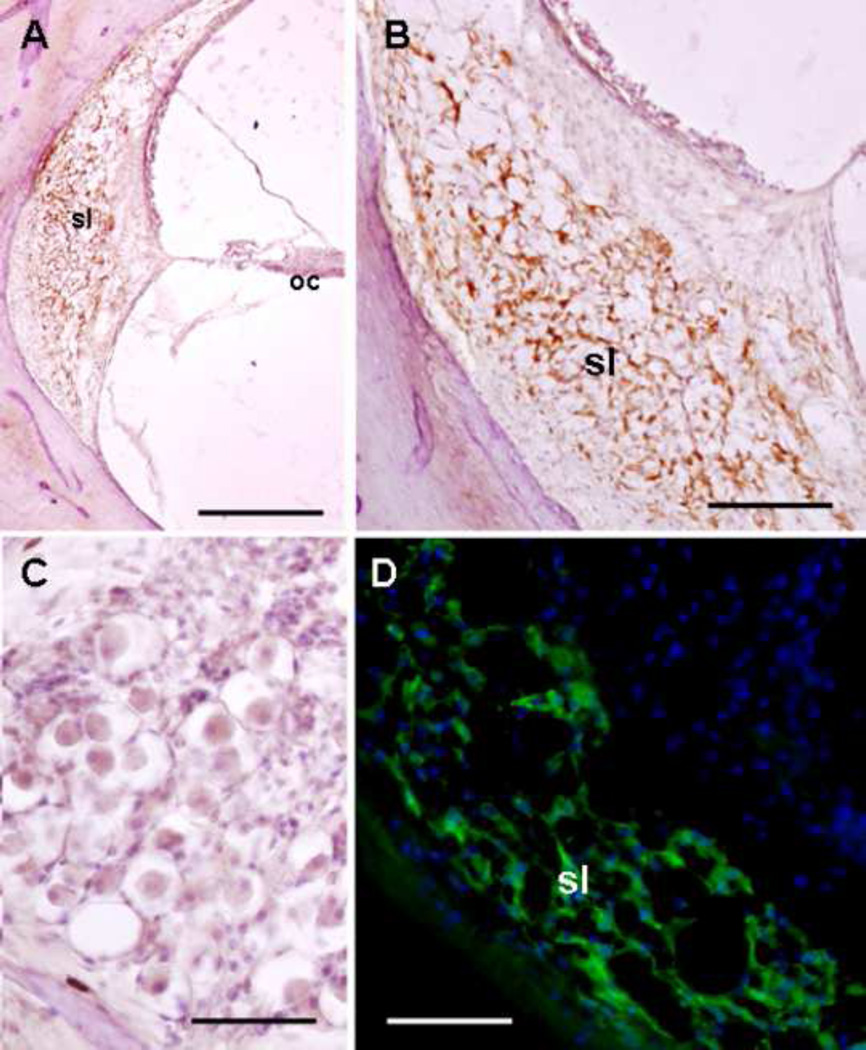
In the human cochlea, GLAST was detected in the fibrocytes of the spiral ligament of the basal, middle, and apical turns of the cochlea (Figure 1a). Upon higher magnification, GLAST was seen in the cytoplasm of the spiral ligament fibrocytes (Figure 1b). Of note, GLAST was neither seen in the Organ of Corti (Figure 1a) nor in the spiral ganglia (Figure 1c). Light microscopic findings were corroborated with fluorescent immunostaining using formalin-fixed frozen sections (not embedded in celloidin) of the human cochlea (Figure 1d). The pattern of GLAST immunoreactivity in these frozen sections was similar to the pattern using celloidin embedded sections. In the lateral wall, GLAST was localized in spiral ligament fibrocytes (types III and IV) and the stria vascularis was not immunoreactive. Additionally, GLAST was not detected in the Organ of Corti or the spiral ganglia. In contrast, the mice cochlea showed GLAST immunoreactivity in fibrocytes of the spiral ligament, satellite cells of the spiral ganglia, and supporting cells of the Organ of Corti (Figure 2).
Figure 1.
GLAST immunoreactivity in the human cochlea. A. Low magnification of the human cochlea showing GLAST in the spiral ligament (sl) but not in the Organ of Corti (oc). B. Higher magnification view showing GLAST in the spiral ligament fibrocytes (amber color). C. The spiral ganglia showed no immunostaining for GLAST. Nuclei were counterstained with hematoxylin in Figs A, B, and C. D. GLAST immunofluorescence (green) in the human spiral ligament. The cell nuclei were stained with DAPI (blue). The rabbit secondary antibody was tagged with Alexa 488 (full protocol described by Balaker et al (2013). Magnification bar in Fig. A: 500 µm; Fig. B: 200 µm; Figs. C and D: 100 µm.
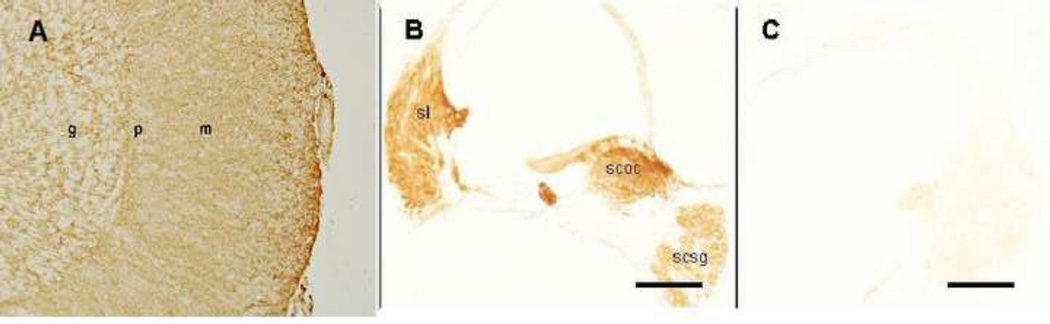
Figure 2.
Control specimens for GLAST-IR. A. A section of human cerebellum stained for GLAST serves as a human positive control. The immunoreactive staining is strongest in the molecular layer (m) of the cerebellum. B. A section of mouse cochlea stained for GLAST serves as a mouse positive control. The spiral ligament (sl), satellite cells of the spiral ganglia (scsg), and supporting cells of the Organ of Corti (scoc) all stained positive. C. Mouse negative control. The primary GLAST antibody was omitted and no immunoreactivity was seen in the mouse cochlear tissue. g: granular cell layer; p: Purkinje cell layer; m: molecular cell layer; sl: spiral ligament; scsg: satellite cells of the spiral ganglia; scoc: supporting cells of the Organ of Corti. Magnification bar is 150 µm for all figures.
Eighteen temporal bones from human patients were analyzed in this study. The GLAST immunoreactive area (GLAST-IR) was found in the spiral ligament of all the FFCE human temporal bones and is demonstrated in three sample patients in Figure 3. The differential expression of GLAST in different sections of the cochlea was reflected by its varying immunoreactive area in different patients (Tables 1 and 2). Four patients had normal hearing without any otopathologic history (“Normal” group). In three of these patients, the basal turn of the cochlea had the highest IR area; the one exception was a Down syndrome patient whose middle section of the cochlea had the greatest IR area. Eight patients were diagnosed with Meniere’s disease. In 5 out of 8 patients with Meniere's disease, the GLAST-IR was highest in the basal turn of the cochlea. Of the remaining three, one had an equivalent GLAST-IR between the apical and basal turn, and the other two demonstrated the highest GLAST-IR in the middle turn. In the “Other” group, 6 patients had the following otologic conditions that rendered some degree of hearing loss: rheumatoid arthritis, ototoxicity, otosclersosis, congenital deafness, hearing loss, and two patients with presbycusis. Of these patients, 4 out of 6 had the highest GLAST-IR in the basal turn of the cochlea. Of note, the absolute lowest area of GLAST-IR of all the sections for all the patients was found in the basal turn of the cochlea of the congenitally deaf patient.
Figure 3.
Distribution of GLAST in formalin-fixed celloidin-embedded temporal bone sections of different patients. A1–A3: GLAST-IR in the cochlea of a patient in the “Normal” group (75 year-old female without any hearing problems). GLAST-IR was localized to the spiral ligament in the apical (A1), middle (A2), and basal (A3) turns of the cochlea. B1–B3: GLAST-IR in the spiral ligament of the apical (B1), middle (B2), and basal (B3) turns of the cochlea of a patient in the “Meniere’s disease” group (83 year-old female with a 20 year history of Meniere’s disease). C1–C3: GLAST-IR in the spiral ligament of the apical (C1), middle (C2), and basal (C3) turns of the cochlea of a patient in the “Other” group (80 year-old male with a 15 year history of presbycusis). Magnification bar is 80 µm for all figures.
Table 1.
GLAST-IR Area Categorized by Cochlear Region.
| Patient # | Description | Apical Area (µm2) |
Middle Area (µm2) |
Basal Area (µm2) |
|---|---|---|---|---|
| 1 | Normal (Down Syndrome) | 9121 | 12823 | 7284 |
| 2 | Normal | 9187 | 13097 | 17312 |
| 3 | Meniere's Disease | 12556 | 28218 | 20202 |
| 4 | Meniere's Disease | 7618 | 14216 | 26938 |
| 5 | Normal | 4413 | 13253 | 19894 |
| 6 | Rheumatoid Arthritis | 16334 | 9787 | 25450 |
| 7 | Meniere's Disease | 22028 | 30369 | 39578 |
| 8 | Ototoxicity | 3904 | 6300 | 25330 |
| 9 | Otosclerosis | 15447 | 12044 | 15078 |
| 10 | Meniere's Disease | 12213 | 17829 | 16584 |
| 11 | Meniere's Disease | 14780 | 11102 | 14936 |
| 12 | Congenital Deafness | 6134 | 9878 | 1680 |
| 13 | Normal | 16892 | 35352 | 20070 |
| 14 | Presbycusis | 6633 | 4119 | 14184 |
| 15 | Meniere's Disease | 6642 | 15541 | 16634 |
| 16 | Presbycusis | 5336 | 16806 | 19739 |
| 17 | Meniere's Disease | 19022 | 11718 | 35056 |
| 18 | Meniere's Disease | 2826 | 15723 | 14531 |
Table 2.
GLAST-IR Area Categorized by Patient Characteristics.
| Patient Group | Mean Area (µm2) | Standard Deviation (µm) |
|---|---|---|
| Meniere's Disease | 17785.8 | 1663.5 |
| Normal | 14891.5 | 2352.6 |
| Other | 11899.1 | 1920.9 |
ANOVA analysis of “Cochlear Location” and “Total Area” was found to be statistically significant (F-ratio 5.9421, p=0.0048; Figure 4). Post-hoc analysis showed a step-wise increase in the area of glutamate transporters from the apical to the basal segments of the cochlea. Apical total area was 10600 ± 1800 µm2, middle total area was 15450 ±1800 µm2, and basal total area was 19470 ± 1800 µm2. Post-hoc pairwise comparisons showed statistically significant differences between apical and basal segments (p=0.0012); comparison of apical to middle (p=0.0657) and middle to basal (p=0.1246) approached significance (Table 3).
Figure 4.
ANOVA One Way Analysis of Total GLAST-IR Area by Cochlear Location.
Table 3.
Post-hoc Pairwise Comparison between Cochlear Location and GLAST-IR.
| Cochlear Location Comparison |
Difference in GLAST- IRArea (µm2) |
SE (µm) | p-Value |
|---|---|---|---|
| Basal to Apical | 8855.222 | 2572.377 | 0.0012* |
| Middle to Apical | 4838.278 | 2572.377 | 0.0657 |
| Basal to Middle | 4016.944 | 2572.377 | .1246 |
SE: Standard Error.
p ≤ 0.05 considered to be statistically significant.
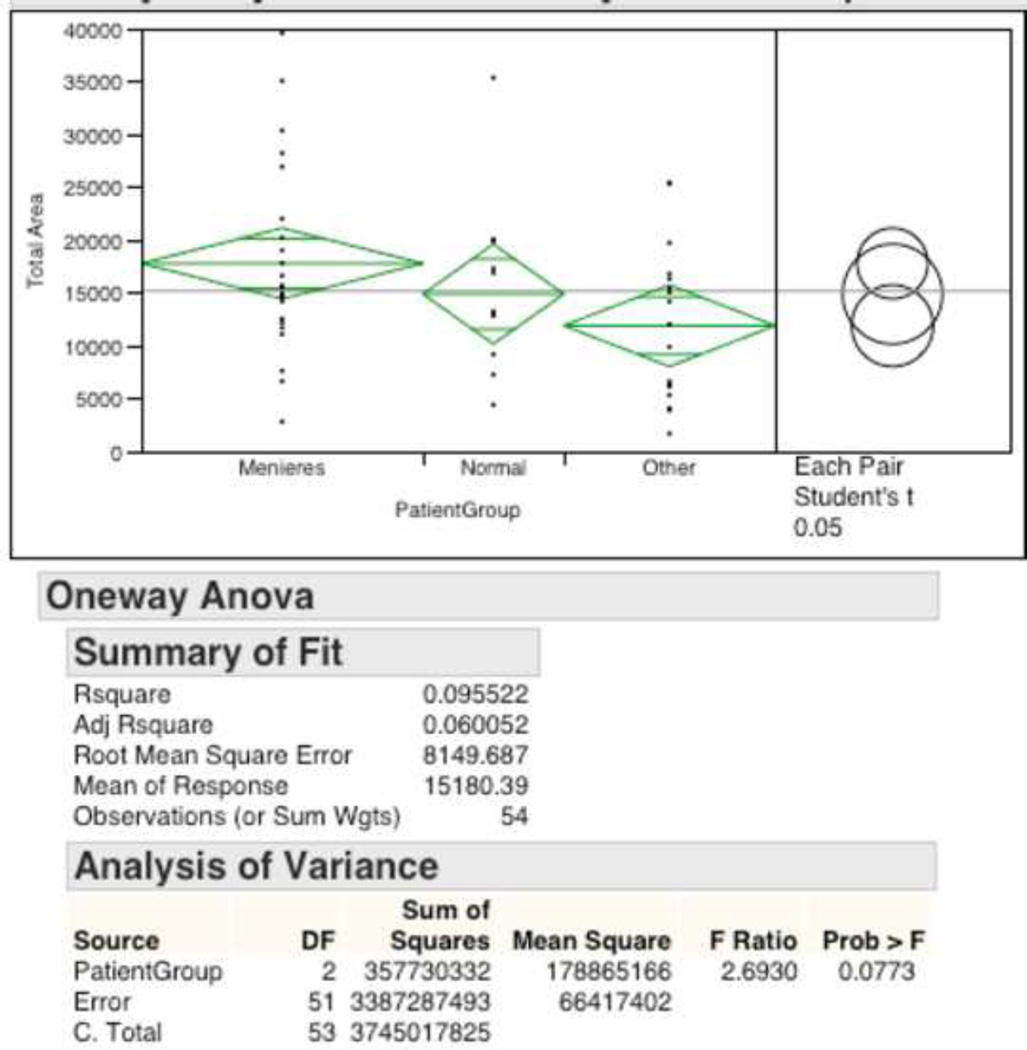
ANOVA analysis of “Patient Group” and “Total Area” was not found to be significant but approached significance with a p-value = 0.0773 (Figure 5). Exploratory post-hoc pairwise analysis using Student’s T-test suggested no statistical difference between the groups “Meniere’s disease” and “Normal” (17800 ± 1660 µm2 vs. 14890 ± 2350 µm2, p=0.3199) as well as no difference between the groups “Other” and “Normal” (11900 ± 1920 µm2 vs. 14890 ± 2350 µm2, p= 0.3291). However, exploratory analysis did show “Meniere’s disease” to be have significantly higher total area of glutamate receptors compared to “Other” (17800 ± 1660 µm2 vs. 11900 ± 1920 µm2, p=0.0246; Table 4).
Figure 5.
ANOVA One Way Analysis of Total GLAST-IR Area by Patient Grouping.
Table 4.
Exploratory Post-hoc Pairwise Comparison between Patient Grouping and GLAST-IR.
| Patient Group Comparison |
Difference in GLAST-IR Area (µm2) |
SE (µm) | p-Value |
|---|---|---|---|
| Meniere’s vs. Other | 5886.778 | 2541.111 | 0.0246* |
| Normal vs. Other | 2992.444 | 3037.209 | 0.3291 |
| Meniere’s vs. Normal | 2894.333 | 2881.350 | 0.3199 |
SE: Standard Error.
p ≤ 0.05 considered to be statistically significant.
Subsequent multivariate analysis between the variables “Cochlear Location,” “Patient Group,” and the outcome “Total Area” was found to be underpowered for meaningful statistical analysis.
3. Discussion
In our study of FFCE human temporal bone sections, the presence of GLAST in the human cochlea was demonstrated for the first time. In particular, we showed the presence of GLAST in the fibrocytes of the spiral ligament in all 18 of our patients. In contrast to previous studies in several animal models, our analysis of human cochleas did not demonstrate GLAST in the satellite cells of the spiral ganglia or supporting cells of the Organ of Corti in any of our patients. To ensure that this was not a phenomenon related to the celloidin, we used frozen sections of formalin-fixed human cochlea (no celloidin) to show a pattern of immunostaining that was similar to the pattern seen in FFCE human temporal bone sections. The difference in GLAST expression in humans and other non-human species became more evident after examining the immunostaining pattern in our mice cochlear positive controls (Figure 2). While GLAST may not be present in certain regions of the human cochlea, our findings do not preclude the possibility of other transporters playing a role in in glutamate transport in these same regions. As mentioned earlier, glutamate must be cleared in order to prevent excitotoxicity. Recent work by Dalet et al demonstrated the wider presence of non-GLAST glutamate transporters (such as EAAT4 and EAAT5) in mice cochlea (2012). Similarly, it may be possible that other transporters also play an expanded role in the human cochlea since GLAST was not expressed in the Organ of Corti or spiral ganglia in our experiments.
In this study, we mainly used human cochlear sections embedded in celloidin (a nitrocellulose compound). Celloidin embedding is a lengthy process that takes several weeks. Celloidin infiltration uses different type solvents. The thickness of these sections is usually between 20–30 µm. The advantages of celloidin embedded sections is that they can be preserved for decades in a sealed jar containing 80% ethanol. Additionally, the process of celloidin embedding occurs without utilizing heat, which can be detrimental for antigen preservation. As a result, cochlear and vestibular endorgans are very well preserved along with their structural relationships.
Our data also showed that the immunoreactive area of GLAST was different among the different regions of the cochlea; this difference was statistically significant after ANOVA testing. Interestingly, when breaking down each region of the cochlea, the two sites that had the most statistically significant difference in GLAST expression were the basal and apical turns of the cochlea (p=0.001). The majority of our patients demonstrated the highest immunoreactive area of GLAST in the basal region of the cochlea while the least immunoreactive area was found in the apical region. Similarly, Furness and Lawton demonstrated that the mid-basal turn of the cochlea had the most GLAST expression in mice cochleas (2003). Glowatzki et al noted that GLAST expression may be closely related to the glutamate load that is released into the extracellular space in that particular region of the CNS. And since the basal portion of the cochlea has more synapses, this would suggest that GLAST expression should be higher in the basal turn, as has been demonstrated in mice and now in humans (Glowatzki et al., 2006).
The variable expression of GLAST in different patients may reflect each individual's genetic predisposition, otologic disease history, or a combination of the above. Most of the patients had the highest immunoreactive area in the basal turn. In contrast, our patient who was congenitally deaf had the least amount of GLAST in the basal turn suggesting the strong likelihood of an under-developed or maldeveloped cochlea. Interestingly, our two patients with presbycusis demonstrated substantially higher GLAST-IR in the basal turn than in the apical or middle turns. The basal turn of the cochlea is classically known to be responsible for high-frequency hearing, and in presbycusis, patients present with high-frequency hearing loss. Nelson and Hinojosa showed that patients with presbycusis were found to have degeneration of the stria vascularis, outer hair cells, inner hair cells, and spiral ganglion neurons (2006). Johnsson and Hawkins showed that there was an association between degeneration of the stria vascularis and dramatic loss of capillaries in the spiral ligament (1972). Thus, one could potentially expect some element of spiral ligament degeneration in the basal turn for presbycusis patients which would theoretically decrease the GLAST expression; however, this was not the case in our two patients. Nevertheless, it is important to remember that age related atrophy is most severe in the apical and middle turns of the cochlea (Wright and Schuknecht, 1972). And since both of our presbycusis patients were elderly (76 and 80 years-old), it is possible that the decrease in GLAST in the apical and middle turns was more pronounced than the decrease in GLAST in the basal turn. In examining only the Meniere's disease patients, a consistent relationship with GLAST-IR in a particular region of the cochlea was not seen.
When patients were stratified into three groups (“Meniere's" vs. “Normal”/Controls vs. “Other”), interesting patterns were also noticed. Meniere's patients had the most GLAST-IR area, the “Normal”/Control group had the second most, while those in the “Other” group had the least. However, ANOVA analysis showed no significant relationship between “Patient Group” and “Total Area.” When delving deeper into the data, post-hoc exploratory analysis suggested an increase in GLAST for patients with Meniere’s disease patients and a decrease in GLAST for patients in the “Other” group. However, these exploratory findings between “Meniere’s” and “Other” remain to be confirmed by further analysis using a larger sample size.
Significant attention has been directed towards GLAST and other glutamate transporters. In an elegantly designed study, Glowatzki et al demonstrated that IHCs have a GLAST associated current that is detectable electrophysiologically (Glowatzki et al., 2006). Examining a different region of the cochlea, Furness et al similarly demonstrated the immunohistochemical presence and functional capability of GLAST in the spiral ligament of the cochlea (Furness et al., 2009). Both studies demonstrated the active functional role of GLAST seen in rat and mice cochlea, respectively. In addition, previous studies demonstrated that GLT-1 was likely not involved in glutamate transport within the spiral ligament, as immunohistochemical staining did not identify this particular transporter in the spiral ligament in the Furness et al study (2009). However, it is possible that other glutamate transporters (such as EAAT4 and EAAT5) play an important role in glutamate homeostasis. In our experiments examining the human spiral ligament, GLAST immunoreactivity was mainly localized in types III and IV fibrocytes. Future colocalization studies with fibrocytes immuno-markers may help to determine the extent of GLAST in the different types of fibrocytes.
Our study was unique in that it is the first reported account of GLAST in human cochlear specimens. However, our study has several limitations. First, our small sample size hurts our ability to comparatively make claims between different patients. Yet, future studies with temporal bone analysis may yield enough data to make more meaningful comparisons between patient groups. Secondly, our analysis of the immunoreactive area requires images taken from temporal bone specimens and attempted isolated analysis of the spiral ligament. It is conceivable that the background noise from the stria vascularis or surrounding bone was also included in the polygon outlining tool utilized in Image J. To mitigate this factor, the outlining of Image J was done multiple times to maximize the accuracy of our recordings. In addition, the utilization of Image J has been proven to be effective in other immunocytochemical staining experiments in other regions of the body (Rangan and Tesch, 2007). And lastly, temporal bone specimens were collected after the patient had passed away. It is possible that the amount of GLAST may be different in a patient who has passed away when compared to a patient who is alive.
In summary, we have identified the glutamate-aspartate transporter (GLAST) in fibrocytes of the spiral ligament of the human cochlea. In contrast to previous studies done on non-human specimens, GLAST was not found in the satellite cells of the spiral ganglia or in the supporting cells of the Organ of Corti. We have shown that intra-cochlear location correlates with the total immunoreactive area of GLAST and the differences in expression are statistically significant. Future studies may show increased GLAST in patients with Meniere’s disease and decreased GLAST in patients with other otologic diseases. These findings may lead to new ideas about the molecular mechanisms underlying hearing loss and Meniere’s disease.
4. Experimental Procedure
4.1 Human Specimens
The Institutional Review Board (IRB) of UCLA approved this study. Appropriate informed consent was obtained from each patient before inclusion in the study. Archival temporal bones were used in the present study. The temporal bone donors were part of a National Institute of Health (NIH)-funded Human Temporal Bone Consortium for Research Resource Enhancement through the National Institute on Deafness and Other Communication Disorders (NIDCD). The medical history for each of the patients who donated their temporal bones was maintained and preserved in a secured electronic database.
4.2 Human Tissue Processing
At autopsy, the whole brain, including the brainstem and blood vessels, were removed from the cranial cavity. The temporal bones were removed and microdissected as described before (Lopez et al., 2005). The temporal bones were harvested within 3 to 12 hours after death. Temporal bone specimens were harvested using a modified protocol of temporal bone harvesting as described by Schuknecht (1968). A temporal bone plug cutter was used to remove the petrous temporal bone en bloc. Immediately after removal from the skull base, within the autopsy room, the stapes was carefully detached from the oval window and fixative was infused with a micropipette to ensure a uniform distribution of the fixative throughout the vestibule. The temporal bones were placed in ice-cold 4% paraformaldehyde plus 0.2% glutaraldehyde in 0.1 mol/L Sorensen buffer at pH 7.4 (Gopen et al., 2003). Under the operating microscope, the middle ear was exposed and the tympanic membrane and the ossicles were carefully extracted. Bone surrounding the vestibular endorgans was removed and the individual vestibular organs with their respective nerve branches were transected from the 8th vestibular nerve. In addition, the cochlea was removed with careful surgical technique (Lopez et al., 2005).
4.3 Archival Celloidin Specimens
Celloidin sections were placed in a glass Petri dish and immersed in 100% acetone for 2 × 15 minutes. Thereafter, sections were sequentially immersed in a mixture of sodium-ethoxide-100% ethanol (1:3) for 10 minutes; 100% ethanol, 50% ethanol, and distilled water (5 minutes each) and then immersed in hydrogen peroxide 3% in methanol (10 minutes). Slides were then washed with double distilled water before the antigen retrieval step. Slides were placed horizontally in a glass Petri dish that contained antigen retrieval solution (Vector Antigen Unmasking Solution, Vector Labs, Burlingame CA diluted 1:20 with distilled water). Sections were heated in the microwave oven using intermittent heating of two 2-min cycles with an interval of 1 minute between the heating cycles. Sections were allowed to cool for 15 minutes and washed with PBS for 10 minutes.
4.4 Indirect Immunocytochemistry (Light Microscopy of Archival Temporal Bones)
For indirect immunocytochemistry, endogenous peroxidase quenching was performed by incubation for 10 minutes with 3% hydrogen peroxide (diluted in 100% in methanol). Slides were then washed with PBS for 10 minutes. Slides were then transferred to a slide carrier filled with antigen retrieval solution (Vector antigen unmasking solution, Vector Labs, Burlingame CA) and heated in the microwave for a total of 5 min. The slides were then cooled and transferred to a PBS wash for 10 min. Sections were incubated for 30 min with blocking solution containing 5% normal goat serum (Vector Labs, Burlingame, CA) and 0.5% Triton X-100 (Sigma) in PBS. Incubation with GLAST antibodies was performed for 48 hr at 4° C in a humid chamber (Lopez et al., 2007). Anti-glutamate transporter— preserved in guinea pig antiserum—has its immunogen as a synthetic peptide from the carboxyterminus. This antibody recognizes human, mouse, and rat GLAST. By western blot it gives a 67–68 kDA band in whole cell lysates (Lehre et al., 1997).
The sections were washed with PBS (3 × 5 min) and then incubated for 1 hr with biotinylated secondary antibody, goat anti-rabbit polyclonal IgG (1:1000, Vector Labs). Afterwards, sections were washed with PBS (3 × 5 min). Next, 1-hr incubation was performed with Vectastain Elite ABC reagent (Vector Labs) followed by PBS wash (5 min × 2). Immunoperoxidase staining was performed using Immpact DAB solution (Vector Labs). The reaction was stopped with PBS washes (5 min × 3) after 10–15 s. Slides were then mounted with Vectamount AQ aqueous mounting media and glass cover slips (Lopez et al., 2005; Lopez et al., 2007; Ishiyama et al., 2010).
4.5 GLAST Immunofluorescence
Tissue sections were incubated at room temperature for 60 min with a blocking solution containing 1% bovine serum albumin (BSA) fraction-V (Sigma, St. Louis, MO) and 0.5% Triton X-100 (Sigma) in PBS. At the end of the incubation, the blocking solution was removed and the primary polyclonal antibody against GLAST was applied. The primary antibody was incubated for 16 hr at 4° C in a humid chamber. Alexa 488 anti-rabbit secondary antibody (1:1,000, Molecular Probes, Carlsbad, CA) was applied for 2 hr at room temperature in the dark. Then, sections were washed with PBS (3 × 10 min) and covered with Vectashield mounting media (Vector Labs, Burlingame, CA) containing DAPI to visualize all cell nuclei (Balaker et al., 2013).
4.6 Microscopic Observation and Documentation
The human temporal bone immunohistochemical sections were viewed and imaged with an Olympus BX51 fluorescent microscope (Olympus America Inc, NY, USA) equipped with an Olympus DP70 digital camera. To provide unbiased comparisons of the immunoreactive signal between each specimen, all images were captured using strictly the same camera settings. Images were acquired using MicroSuite™ Five software (Olympus America Inc). Images were processed using the Adobe Photoshop™ software program run on a Macintosh iMAC computer.
4.7 Quantification of Immunostained Areas
The GLAST distribution in the spiral ligament was quantified at the apical, middle, and basal turns of the cochlea as per the protocol previously described by Ishiyama et al and Calzada et al (2010; 2012). To minimize bias, the first author was blinded to the identity of the tissue samples being analyzed. The senior author was not blinded to the identity and thus coded each sample. Image acquisition and quantitative analyses were made using micrographs acquired at 40×. The area that was immunostained was quantified using the computer image analysis software ImageJ, version 1.46 (available online at http://rsb.info.nih.gov/ij/index.html). The GLAST immunostained areas in the spiral ligament were then compared amongst the apical, middle, and basal turns of the cochlea.
4.8 Controls
Cryostat sections from human cerebellar tissue and from mouse cochlear tissue were incubated with the rat GLAST antibody as positive controls (Figures 2A and 2B). GLAST is known to be highly expressed in the molecular layer of cerebellar tissue (Lehre et al., 1995). In mice cochlea, GLAST was present in the spiral ligament, satellite cells of the spiral ganglia, and supporting cells of the Organ of Corti. As a negative control, the primary antibody was omitted and the immunoreaction was performed on mouse cochlear tissue as described above. No immunoreactivity was detected in any of the negative controls (Figure 2C).
4.9 Statistical Analysis
Data were entered into a Microsoft Excel Worksheet (Excel 2011; Microsoft Corp, USA) and then examined for statistical analysis using JMP 10 Statistics Software (Sas Inc., 2012). Univariate analysis of variance analysis (ANOVA) was performed to test an association between the variables “Patient Group” and “Total Area,” as well as “Cochlear Location” and “Total Area.” Post-hoc individual univariate group comparisons were made for any significant results. A p-value of 0.05 was used in all statistical tests for establishing significance.
Highlights.
The presence of GLAST in the human cochlea was demonstrated for the first time.
In every human patient, GLAST was found in the spiral ligament fibrocytes.
GLAST was not found in the spiral ganglia or around the Organ of Corti.
GLAST expression significantly varied based on intracochlear location.
Acknowledgment
The authors acknowledge Dr. Soroush Zaghi form the Department of Head and Neck Surgery at UCLA for his valuable assistance with the statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balaker AE, Ishiyama P, Lopez IA, Ishiyama G, Ishiyama A. Immunocytochemical localization of the translocase of the outer mitochondrial membrane (Tom20) in the human cochlea. The Anatomical Record. 2013 Feb;296(2):326–332. doi: 10.1002/ar.22622. [DOI] [PubMed] [Google Scholar]
- 2.Calzada A, Lopez IA, Ishiyama A, Beltran Parrazal L, Ishiyama G. The expression of cochlin and extracellular matrix proteins in vestibular endorgans obtained from Meniere’s disease patients. Cell Tissue Research. 2012 Nov;350(2):373–384. doi: 10.1007/s00441-012-1481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalet A, Bonsacquet J, Gaboyard-Niay S, Calin-Jageman I, Chidavaenzi RL, Venteo S, Desmadryl G, Goldberg JM, Lysakowski A, Chabbert C. Glutamate Transporters EAAT4 and EAAT5 Are Expressed in Vestibular Hair Cells and Calyx Endings. PLoS One. 2012;7(9):e46261. doi: 10.1371/journal.pone.0046261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 5.Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- 6.Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci. 2003;23:11296–11304. doi: 10.1523/JNEUROSCI.23-36-11296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furness DN, Lawton DM, Mahendrasingam S, Hodierne L, Jagger DJ. Quantitative analysis of the expression of the glutamate–aspartate transporter and identification of functional glutamate uptake reveal a role for cochlear fibrocytes in glutamate homeostasis. Neuroscience. 2009;162:1307–1321. doi: 10.1016/j.neuroscience.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Furness DN, Lehre KP. Immunocytochemical localization of a high-affinity glutamate-aspartate transporter, GLAST, in the rat and guinea-pig cochlea. Eur J Neurosci. 1997;9:1961–1969. doi: 10.1111/j.1460-9568.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 9.Glowatzki E, Cheng N, Hiel H, Yi E, Tanaka K, Ellis-Davies GC, Rothstein JD, Bergles DE. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J Neurosci. 2006;26:7659–7664. doi: 10.1523/JNEUROSCI.1545-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakuba N, Koga K, Gyo K, Usami SI, Tanaka K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. J Neurosci. 2000;20:8750–8753. doi: 10.1523/JNEUROSCI.20-23-08750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyodo J, Hakuba N, Koga K, Watanabe F, Shudou M, Taniguchi M, Gyo K. Hypothermia reduces glutamate efflux in perilymph following transient cochlear ischemia. Neuroreport. 2001;12:1983–1987. doi: 10.1097/00001756-200107030-00041. [DOI] [PubMed] [Google Scholar]
- 12.Inage YW, Itoh M, Wada K, Takashima S. Expression of two glutamate transporters, GLAST and EAAT4, in the human cerebellum: their correlation in development and neonatal hypoxic-ischemic damage. J Neuropathol Exp Neurol. 1998 Jun;57(6):554–562. doi: 10.1097/00005072-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ishiyama G, Lopez IA, Beltran-Parrazal L, Ishiyama A. Immunohistochemical localization and mRNA expression of aquaporins in the macula utricle of patients with Meniere’s disease and acoustic neuroma. Cell Tissue Research. 2010;340:407–419. doi: 10.1007/s00441-010-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin ZH, Kikuchi T, Tanaka K, Kobayashi T. Expression of glutamate transporter GLAST in the developing mouse cochlea. Tohoku J Exp Med. 2003;200:137–144. doi: 10.1620/tjem.200.137. [DOI] [PubMed] [Google Scholar]
- 15.Johnsson LG, Hawkins JE. Sensory and neural degeneration with aging, as seen in microdissections of the human inner ear. Ann Otol Rhinol Laryngol. 1972;81:179–193. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]
- 16.Kariya S, Cureoglu S, Fukushima H, Kusunoki T, Schachern PA, Nishizaki K, Paparella MM. Histopathologic Changes of Contralateral Human Temporal Bone in Unilateral Meniere’s Disease. Otology & Neurotology. 2007;28:1063–1068. doi: 10.1097/MAO.0b013e31815a8433. [DOI] [PubMed] [Google Scholar]
- 17.Lehre KP, Davanger S, Danbolt NC. Localization of the glutamate transporter protein GLAST in rat retina. Brain Res. 1997 Jan 2;744(1):129–137. doi: 10.1016/s0006-8993(96)01022-0. [DOI] [PubMed] [Google Scholar]
- 18.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995 Mar;15(3 Pt 1):1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li HS, Niedzielski AS, Beisel KW, Hiel H, Wenthold RJ, Morley BJ. Identification of a glutamate/aspartate transporter in the rat cochlea. Hear Res. 1994;78:235–242. doi: 10.1016/0378-5955(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 20.Lopez I, Ishiyama G, Lee M, Baloh RW, Ishiyama A. Immunohistochemical localization of aquaporins in the human inner ear. Cell & Tissue Research. 2007;328:453–460. doi: 10.1007/s00441-007-0380-z. [DOI] [PubMed] [Google Scholar]
- 21.Lopez I, Ishiyama G, Tang Y, Frank M, Baloh RW, Ishiyama A. Estimation of the number of nerve fiber in the human vestibular endorgans using unbiased stereology and immunohistochemistry. Journal of Neuroscience Methods. 2005a;145:37–46. doi: 10.1016/j.jneumeth.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Minowa O, Ikeda K, Sugitani Y, Oshima T, Nakai S, Katori Y, Suzuki M, Furukawa M, Kawase T, Zheng Y, Ogura M, Asada Y, Watanabe K, Yamanaka H, Gotoh S, Nishi-Takeshima M, Sugimoto T, Kikuchi T, Takasaka T, Noda T. Altered cochlear fibrocytes in a mouse model of DFN3 nonsyndromic deafness. Science. 1999;285:1408–1411. doi: 10.1126/science.285.5432.1408. [DOI] [PubMed] [Google Scholar]
- 23.Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006 Sep;116(9 Pt 3) Suppl 112:1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- 24.Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H. Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J Neurosci. 2003 Jun 15;23(12):4831–4841. doi: 10.1523/JNEUROSCI.23-12-04831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangan GK, Tesch GH. Quantification of renal pathology by image analysis. Nephrology. 2007;12:553–558. doi: 10.1111/j.1440-1797.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 26.Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996;100:80–100. doi: 10.1016/0378-5955(96)00106-2. [DOI] [PubMed] [Google Scholar]
- 27.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright JL, Schuknecht HF. Atrophy of the spiral ligament. Arch Otolaryngol. 1972;96:16–21. doi: 10.1001/archotol.1972.00770090054005. [DOI] [PubMed] [Google Scholar]
- 29.Wu T, Marcus DC. Age-related changes in cochlear endolymphatic potassium and potential in CD-1 and CBA/CaJ mice. J Assoc Res Otolaryngol. 2003;4:353–362. doi: 10.1007/s10162-002-3026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]