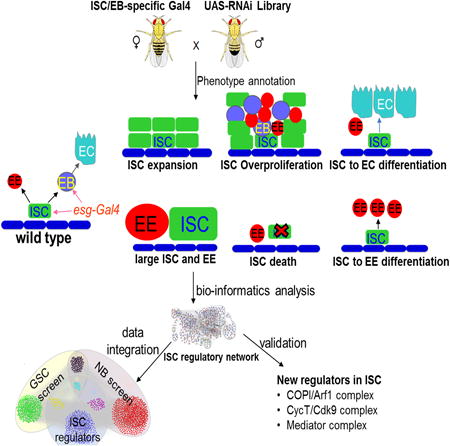
Summary
The intestinal epithelium is the most rapidly self-renewing tissue in adult animals and maintained by intestinal stem cells (ISCs) in both Drosophila and mammals. To comprehensively identify genes and pathways that regulate ISC fates, we performed a genome-wide transgenic RNAi screen in adult Drosophila intestine and identified 405 genes that regulate ISC maintenance and lineage-specific differentiation. Through integrating these genes into publicly available interaction databases, we further developed functional networks that regulate ISC self-renewal; ISC proliferation; ISC maintenance of diploid status; ISC survival; ISC-to-enterocyte (EC) lineage differentiation; and ISC-to-enteroendocrine (EE) lineage differentiation. By comparing regulators among ISCs, female germline stem cells, and neural stem cells, we found that factors related to basic stem cell cellular processes are commonly required in all stem cells, and stem cell–specific, niche-related signals are required only in the unique stem cell type. Our findings provide valuable insights into stem cell maintenance and lineage-specific differentiation.
Graphical abstract
Introduction
Animal tissues and organs are generated and maintained by stem cells. During development, they generate most of the cell types to form an organ, while in adult animals they maintain tissue homeostasis by supplying new cells to replace dying or damaged ones. To accomplish this task, stem cells have to continuously renew themselves and, at the same time, generate daughter cells to produce terminal differentiated cells for their organ-specific functions. Further, the somatic differentiated cells can be reprogrammed into induced pluripotent stem cells (iPSCs) through overexpression of a few transcription factors or the metabolic switch (Ito and Suda, 2014; Takahashi and Yamanaka, 2006; Zhang et al., 2012). The primary functions of activated oncogenes and inactivated tumor suppressors may be to reprogram cellular metabolism and convert somatic cancer cells into pluripotent tumor-initiating cells (also called cancer stem cells) (Ward and Thompson, 2012; Zhang et al., 2012). Therefore, understanding how adult stem cells (particularly somatic adult stem cells) are regulated is important for understanding tissue degeneration and tumorigenesis.
Because the digestive organs are the fastest renewing organs in all animals (Hakim et al., 2010), intestinal stem cells in both adult mouse and Drosophila have been studied extensively. Drosophila intestinal stem cells (ISCs) divide asymmetrically to produce one new ISC (self-renewal) and one immature enteroblast (EB), which further differentiates into an absorptive enterocyte (EC) or one pre-enteroendocrine (pre-EE) cell, which matures into a secretory EE cell (Biteau and Jasper, 2014; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Zeng and Hou, in press). Notch (N) signaling plays a major role in regulating ISC self-renewal and differentiation (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007).
However, a systematic molecular understanding of self-renewal and lineage-specific differentiation of adult somatic stem cells is still lacking. In mammals, lists of stem cell–enriched genes have been identified in both mouse intestinal stem cells and hair follicle stem cells through combined transcriptomics and proteomics (Morris et al., 2004; Muñoz et al., 2012; Tumbar et al., 2004). Yet the functional relevance of these genes is largely unknown. Recent developments in genome-wide RNA interference (RNAi) techniques in Drosophila have enabled the knockdown of near-complete sets of genes involved in cellular processes in living animals (Dietzl et al., 2007; Ni et al., 2011). In addition, genome-wide RNAi screens have been performed to identify regulatory networks function in several somatic tissues, including stem cells (Baumbach et al. 2014; Berns et al. 2014; Neely et al. 2010; Neumüller et al., 2011; Schnorrer et al. 2010; Yan et al., 2014). In this study, we carried out a genome-wide RNAi screen for genes that regulate ISC fates. We identified 405 genes that regulate ISC self-renewal, ISC proliferation, ISC-to-EC differentiation, ISC-to-EE cell differentiation, and ISC survival. Cross-correlation with regulators, neuroblasts (Nbs), and female germline stem cells (GSCs) revealed ISC-specific as well as shared regulators of the stem cells. Our data provide a useful resource for dissecting the regulatory networks of self-renewal and differentiation of adult somatic stem cells.
These different cell types can be identified morphologically as well as by their expression of marker genes. ISCs are diploid, have a small nucleus, and express Delta (Dl), a ligand for the Notch (N) receptor signal transduction pathway. EBs are diploid, have a small nucleus, and express Su(H)GBE-lacZ, a transcriptional reporter of the N pathway. ECs are polyploid, have a large nucleus, and express the transcriptional factor Pdm1. EE cells are diploid, have a small nucleus, and express the transcription factor Prospero (Pros). Like mammalian intestinal epithelium, the Drosophila intestinal epithelium is also constantly undergo turnover and can regenerate after tissue damage (Amcheslavsky et al., 2009; Jiang et al., 2009; reviewed in Jiang and Edgar, 2011).
Results
Test Conditions for High-Throughput Screen of Genes Involved in ISC Regulation
To identify candidate genes involved in ISC regulation, we generated GFP-marked cells that expressed UAS-RNAi in adult Drosophila intestine using esg-Gal4, UAS-GFP/+; tub-Gal80ts/+driver. In the midgut, esg-Gal4 is mainly expressed in the ISCs and EBs (Micchelli and Perrimon, 2006). The temperature-sensitive Gal80 inhibitor, Gal80ts (McGuire et al., 2003), suppresses esg-Gal4 activity at the permissive temperature (18°C). When cultured at 18°C, these flies grew to adulthood with no obvious phenotype and no GFP expression (data not shown). We then shifted the adult flies to the restrictive temperature (29°C). After one week, the flies were dissected and examined under confocal microscope for ISC phenotypes.
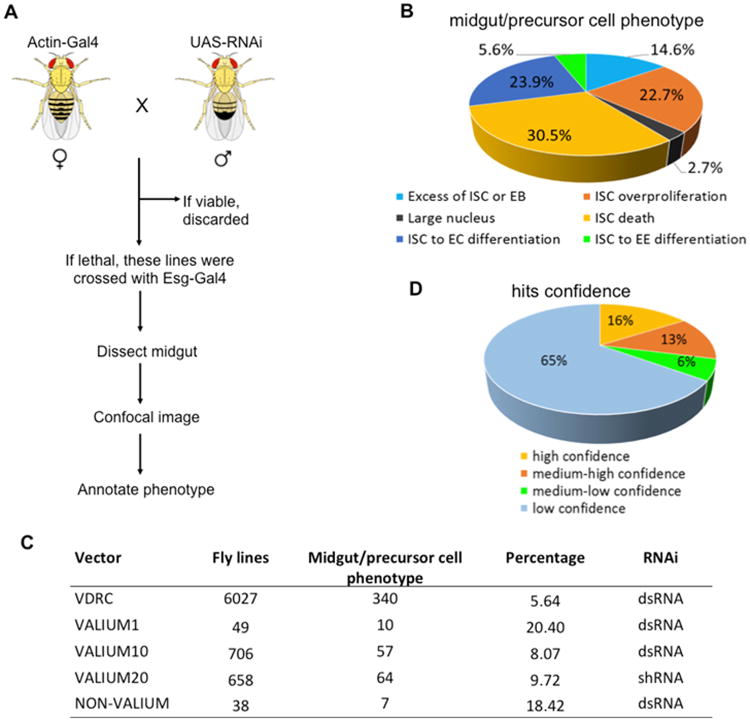
The RNAi methodology has certain restrictions (Dietzl et al., 2007; Ni et al., 2011). First, the P-element-based UAS-hairpin constructs are randomly integrated in the genome, and the level of hairpin expression is affected by its chromosomal location. Second, the RNA level can be reduced only to a variable degree by the RNAi-mediated knockdown, which, in some cases, may insignificantly affect the gene's activity. In addition, there are a large number of nonessential genes whose null mutations have no phenotype (flybase). To reduce the overall false-negative rate and conduct an efficient screen, we first performed a pilot experiment in which we selected 2,000 RNAi lines at random. Each of these lines was crossed in duplicate to Act5C-Gal4 and to esg-Gal4, UAS-GFP/+; tub-Gal80ts/+drivers. The progeny from the cross with Act5C-Gal4 was screened for lethality and any visible adult phenotype. The progeny from the cross with esg-Gal4, UAS-GFP/+; tub-Gal80ts/+ was scored for ISC phenotype. We found that 95.4% of RNAi lines with ISC phenotypes were lethal in the cross with Act5C-Gal4. In the following screen, we first crossed all RNAi lines with Act5C-Gal4 to test lethality and then only crossed the lethal lines with esg-Gal4, UAS-GFP/+; tub-Gal80ts/+ to screen ISC phenotype (Figure 1A).
Figure 1. Transgenic Rnai Screen.
(A) Workflow of the ISC RNAi screen.
(B) The esgts>RNAi female flies were dissected after 7 days at 29°C. Their posterior midguts were stained with antibodies, and analyzed by confocal microscopy. The phenotypes were divided into six categories.
(C) Summary of the screen results.
(D) Confidence of identified 405 genes from the screen. High-confidence genes are identified by two or more independent RNAi lines. Medium-high-confidence genes are identified by one RNAi line, but they co-complex with high-confidence hits. Medium-low-confidence genes are identified by one RNAi line, but they co-complex with other low-confidence hits. Low-confidence hits are identified by one RNAi only.
See also Tables S1, S2, S3, S4 and S5.
Genome-wide RNAi Screen for ISC Phenotype in Adult Drosophila
In total, we screened 16,562 transgenic lines of either long-stranded RNA (dsRNA) or short, small hairpin RNA (shRNA) from both the Vienna Drosophila RNAi Center (VDRC) and Bloomington stock centers (Figure 1C and Table S5), representing 12,705 of the 14,139 protein-coding genes (89.8%) in release 5.7 of the Drosophila genome (Wilson et al., 2008). Among the total 16,562 transgenic lines tested, 7,429 (44.8%) lines corresponding 6,170 genes were lethal, once expressed by the Act5C-Gal4 driver.
We then expressed the 7,429 transgenic lines in ISCs and EBs by crossing each line with esg-Gal4, UAS-GFP/+; tub-Gal80ts/+ (Figure 2A) and analyzed 5–10 flies each for ISC phenotypes by observing the number of green fluorescent protein (GFP) positive cells in midguts. A total of 478 promising lines, which correspondence to 405 genes, were scored (Figure 1C) from the first-round screen were repeatedly screened and stained with molecular markers to confirm the phenotypes.
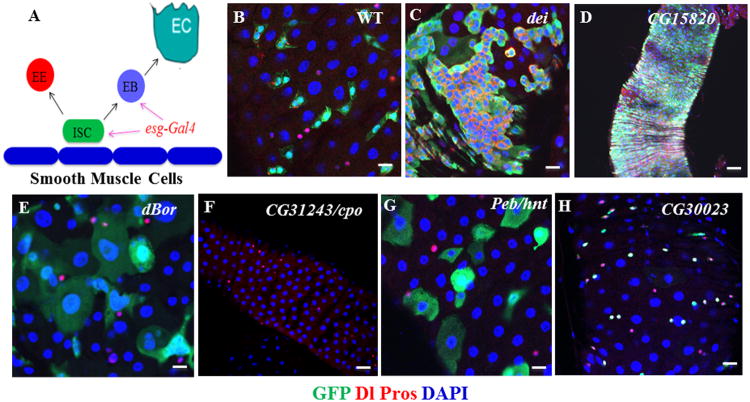
Figure 2. Representive Phenotypes Identified in the RNAi Screen.
(A) Diagram of ISC lineage and expression of esg-Gal4 used in the screen.
(B) Wild-type control.
(C) Knockdown of dei resulted in ISC expansion.
(D) Knockdown of CG15820 resulted in ISC overproliferation.
(E) Knockdown of dBor resulted in large nuclei.
(F) Knockdown of cpo resulted in ISC death.
(G) Knockdown of Peb/hnt resulted in ISC-to-EC differentiation.
(H) Knockdown of CG30023 resulted in ISC-to-EE cell differentiation.
The posterior midguts of corresponding flies were dissected, stained with antibodies of GFP+Dl+Pros+DAPI, and analyzed by confocal microscopy. Scale bars: B, C, E, G, and H–5 μm; D and F–10 μm.
Quality Evaluation
Six lines of evidence suggest that our screen has identified ISC regulators with high confidence. First, the N signal transduction pathway plays a major role in regulating ISC-to-EC differentiation; inactivation of the pathway resulted in an excess of ISCs, and activation of the pathway resulted in premature ISC-to-EC differentiation (Bardin et al., 2010; Beebe et al., 2010; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007; Perdigoto et al., 2011). In this screen, we identified 19 positive regulators in the N pathway whose knockdowns resulted in an excess of ISCs or EBs, and eight negative regulators of the N pathway whose knockdowns resulted in premature ISC-to-EC differentiation (Table S1). Second, we found many previously identified genes regulating ISC functions (Table S1). Third, many of the identified hits (132 genes) whose products are components of protein complexes show a high degree of phenotypic similarity (Figure 1D; Tables S3 and S4). Fourth, Out of 405 genes identified, 310 genes were positive target from VDRC lines, among them, we randomly verified 65 genes by at least two or more independent lines (Figure 1D; Tables S4 and S5), the rest of the lines scored were either from GD or KK libraries with no off-targets as shown in VDRC website (also have similar phenotypes as previously identified genes regulating ISC functions). Among 405 genes, 95 genes were positive target from Bloomington lines screen, which predicted to have no off-targets. Further, we also verified some of the genes identified in RNAi screen by mutant clone analysis. Furthermore, even though many of the GSCs specific genes were lethal in the primary screen, none of them shows any phenotypes in ISCs. These lines of evidence together suggest that our screen has very low percentage of (∼5%) off-targets. Fifth, among the remaining low-confidence genes, nine [elF-5, l(1)10Bb, cas, ial/AurB, dia, pAbp, Ccn, Crn, CSN8] were identified in all three stem cell RNAi screens (Nbs: Neumuller et al., 2011; female GSC: Yan et al., 2014; Tables S4 and S5), and 42 others genes were identified in two of the three stem cell RNAi screens (Table S4). Finally, we showed efficient knockdown of a select set of genes by quantitative PCR (qPCR) analysis or antibody staining (Table S2).
Gene Network That Regulates ISC Fates
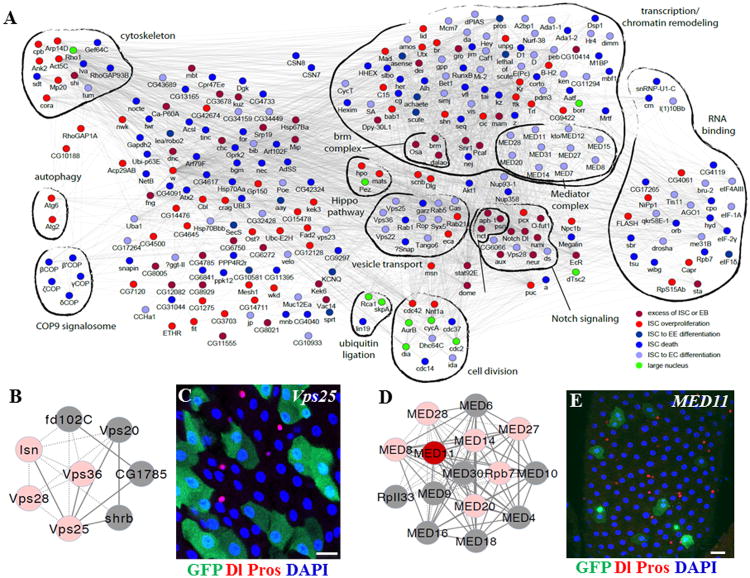
To better analyze our screen results, we generated a gene-interaction network by querying publicly available databases containing yeast–two-hybrid interactions, protein–protein interactions, text-mining data, and genetic interactions between Drosophila genes (Figure 3A). We divided the phenotypes into six categories: (1) genes whose knockdown resulted in an excess of ISCs or EBs; (2) genes important for ISC proliferation; (3) genes important for ISCs to maintain diploid status; (4) genes important for ISC survival; (5) genes important for ISC-to-EC differentiation; and (6) genes important for ISC-to-EE cell differentiation (Figures 1B and 2B-H, Table S1). We performed a complex-enrichment analysis using COMPLEAT (Vinayagam et al., 2013) and identified a number of protein complexes required for ISC fate determination (Figures 3 and S1).
Figure 3. Regulatory Network for Genes Identified from the Screen.
(A) Network of genes identified in the ISC screen. Genes are shown as nodes, and the node colors indicate the observed phenotype in the screen. The edges denote the interactions among the nodes. The distinct molecular complexes are outlined by thick black lines. Red and blue represent genes identified in the screen, and grey represents genes that are not identified in the screen but were identified by querying publicly available databases.
(B) Diagram of the endosome complex.
(C) Knockdown of Vps25 resulted in ISC-to-EC differentiation.
(D) Diagram of the mediator complex.
(E) Knockdown of MED11 resulted in ISC-to-EC differentiation.
Scale bars: C and E–5 μm.
See also Figure S1.
Genes Whose Knockdown Resulted in an Excess of ISCs or EBs
RNAi-mediated knockdowns of genes required for ISC or EB differentiation resulted in the accumulation of undifferentiated esg+ diploid cells. From our phenotype and network analysis, we identified three unique phenotypes that disrupt ISC differentiation; each phenotype involves genes that function in a distinguishing protein complex or pathway.
The Classic N-Signaling Network
RNAi-mediated knockdowns of genes in the N-signaling network resulted in either expansion of both ISCs and EE cells (Dl high and EE cells more) (Table S1A) or expansion of ISCs only (Dl high and EE normal; Figure 2C; Table S1A). Among known components of the N pathway (Bray, 2006; Fortini, 2009; Guruharsha et al., 2012), we found that knockdowns of N, O-fut1, rumi, kuz, psn, neur, shi, nct, aux, Ca-P60A, and mam, resulted in expansion of both ISCs and EEs (Dl high and EE cells more) (Table S1A), while knockdowns of aph-1, chc, and dei resulted in expansion of only ISCs (Dl high and EE cells normal) (Figure 2C and Table S1A). Among the five new components of the N pathway identified in a previous genome-wide RNAi screen (Mummery-Widmer et al., 2009), knockdowns of CG34345 and CG8021 resulted in expansion of both ISCs and EE cells, while knockdowns of CG5608 (Vac14), CG8136, and CG11286 resulted in expansion of only ISCs (Table S1A). These results suggest that components in the N pathway have differential requirement in ISC and EE fate regulation.
In addition to the reported components in the N-signaling network, we identified 26 other genes whose knockdowns resulted in expansion of ISCs (Table S1A). Among them are a histone acetyltransferase ATAC2 and a protein phosphatase type 2A regulator (dPR72, CG4733). Knockdown of ATAC2 resulted in dramatic accumulation of Dl and expansion of ISCs without affecting EE cells (Ma et al., 2013; Table S1A). Protein phosphatase 2A (PP2A) was reported to be a brain tumor suppressor that can inhibit self-renewal of Nbs (Wang et al., 2009). Knockdowns of the remaining 24 genes resulted in either expansion of both ISCs and EE cells or expansion of ISCs only (Table S1A). Further investigations of these genes will significantly advance our knowledge of ISC self-renewal/differentiation and the N-signaling network.
The Osa Complex
RNAi knockdowns of genes in the Osa-containing SWI/SNF chromatin-remodeling complex resulted in ISC expansion and EE cell reduction. In this screen, we identified four components of the SWI/SNF complex, osa, snr1, brm, and dalao (Table S1A). However, these ISCs express low levels of Dl but high levels of another ISC marker, Sanpado (Spno) (Table S1A) (Zeng et al., 2013). In a recent publication (Zeng et al., 2013), we demonstrated that the OSA-containing SWI/SNF chromatin-remodeling complex regulates ISC-to-EC lineage differentiation by controlling Dl transcription and EE cell lineage differentiation by controlling ase transcription.
In addition to the components in the SWI/SNF chromatin-remodeling complex, we identified eight other genes whose knockdowns resulted in ISC expansion and EE cell reduction (Table S1A). Further investigations of these genes will advance our knowledge of Dl and ase regulations.
The JAK-STAT Pathway
RNAi-mediated knockdowns of both dome and stat92E resulted in EB accumulation (Table S1A); the GFP+ cells were Dl- Pros-. This finding is consistent with the previous reports that mutations in the JAK-STAT signal transduction pathway disrupted EB differentiation (Beebe et al., 2010; Jiang et al., 2009).
Genes Important for ISC Proliferation
RNAi-mediated knockdowns of genes negatively regulating ISC proliferation resulted in hyperplasia, a dramatic increase of both ISCs and their differentiated cells (Figures 2D). From our phenotype and network analysis, we identified components in five known pathways, three novel complexes, and many other novel genes whose RNAi-mediated knockdowns resulted in midgut hyperplasia. Among the known pathways, we identified five negative regulators (Cbl, Kek3, ttk, Cic, CG15528) in the EGFR/Map kinase (MAPK) signal transduction pathway (Table S1B). EGFR/Ras/MAPK signaling plays a major role in ISC proliferation (Biteau and Jasper, 2011; Jiang et al., 2011; Xu et al., 2011); two components in the Dpp pathway (Mad, Shn), the Dpp signaling pathway negatively regulate Drosophila midgut homeostasis (Guo et al., 2013). In mouse intestine, EGFR signaling positively and BMP signaling negatively regulate stem cell proliferation (reviewed in Clevers, 2013). Therefore, the functions of the EGFR and Dpp/BMP in intestinal stem cells are conserved in Drosophila and mouse. We also identified a negative regulator (puc) of the JNK pathway, which also regulates ISC proliferation and differentiation (Biteau et al., 2008; Hochmuth et al., 2011); three components of the Hippo pathway (hpo, mats, msn), which negatively regulates ISC proliferation (Li et al., 2014; Karpowicz et al., 2010; Ren et al., 2010; Staley and Irvine, 2010); two components in the Scrib/Dlg tumor-suppressor pathway (dlg, scrib), indicating that the Scrib/Dlg pathway also negatively regulates ISC proliferation.
In addition to the classic pathways, we identified three components in the magnesium transporter complex (CG7830, CG15168, CG11781), two components in the aminopeptidase complex (CG6372, CG4439), and two components in the autophagosome (Atg2, Atg6) whose knockdowns resulted in midgut hyperplasia.
We also identified 73 other genes (Table S1B) whose RNAi-mediated knockdowns resulted in midgut hyperplasia. The information provides a rich resource for investigating ISC proliferation and midgut hyperplasia in future studies.
Genes Necessary for the Maintenance of ISCs' Diploid Status
In this screen, we identified 11 genes whose RNAi-mediated knockdowns resulted in GFP+ cells with much larger nuclei (Figures 2E and 4A-F, and Table S1C), including TSC2. It was previously reported that TSC1/2 and Myc coordinately regulate ISC growth and division in the Drosophila posterior midgut (Amcheslavsky et al., 2011). In TSC2 dsRNA-expressing guts, the size of the ISCs, but not ECs or EE cells, increased by ∼10-fold in 10 days after RNAi initiation. The mutant ISCs expressed the ISC marker Dl but are nonfunctional because they can no longer divide or differentiate (Amcheslavsky et al., 2011). In normal development and adult tissue homeostasis, cells' growth and division are precisely monitored by the checkpoint controls. Cells will divide to maintain the original cell size once they grow in size by approximately twofold. Adult midgut ISCs have a slower intrinsic cell cycle (> 24 h) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006) and differences in checkpoint controls, which may allow the excessive growth to take place in TSC2 dsRNA-expressing cells until the growth passes a critical point that blocks division (Amcheslavsky et al., 2011).
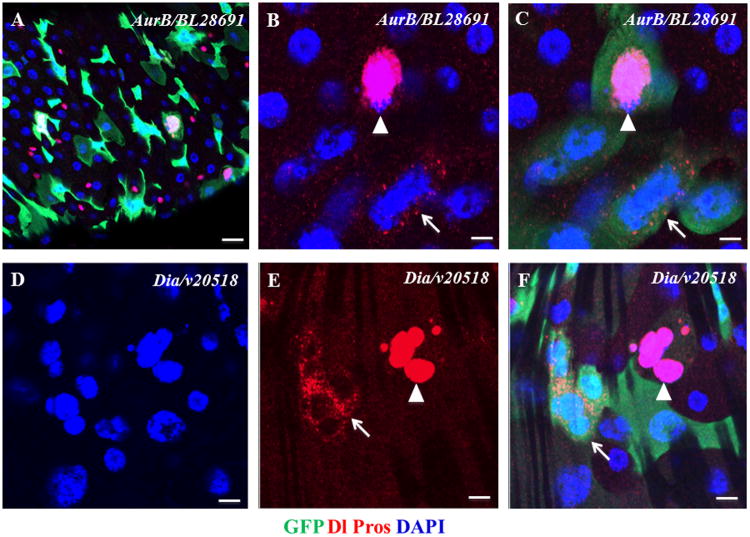
Figure 4. The Larger Nuclei of Knockdowns of AurB and Dia.
(A–C) Knockdown of AurB resulted in larger nuclei of ISCs (arrow in B and C) and EE cells (arrowhead in B and C).
(D–F) Knockdown of Dia resulted in larger nuclei of the ISC cluster (arrow in E and F) and the EE cell cluster (arrowhead in E and F).
The posterior midguts of corresponding flies were dissected, stained with antibodies of GFP+Dl+Pros+DAPI, and analyzed by confocal microscopy. Scale bars: 10 μm in A, 2.5 μm in B and C, 5 μm in D–F.
See also Table S1C.
Consistent with above hypothesis, of the 11 genes identified in our screen, 8 (CG10800/Rca1, CG4454/borr, CG8214/Cep89, CG16983/skpA, CG5363/cdc2, CG5960/cycA, CG6620/ial/AurB, CG1768/dia) regulate mitotic cell cycle or mitotic cytokinesis. Knockdowns of these genes might block mitotic cell division and allow excessive cell growth. However, we found that, unlike the phenotypes of the published TSC2 dsRNA-expressing cells, some of the GFP+ larger-nucleus cells expressed the EE cell marker Pros (Figures 4A-F), and we even identified a cluster of larger-nuclear EE cells in the dia dsRNA-expressing gut (arrowheads in Figures 4E and 4F). These data suggest that excessive DNA amplification and cell growth can happen in both diploid ISCs and EE cells. The data from this screen provide a useful resource for investigating the regulation of the adult stem cell cycle, DNA amplification, and cell growth.
Genes Important for ISC Survival
In this screen, we identified 124 genes whose RNAi-mediated knockdowns resulted in ISC death (Figures 2F and S2C-D, and Table S1D).
The COPI Complex and Lipolysis
Among genes that are required for ISC survival, we identified seven components in the COPI/Arf1 (Arf79F) complex (Figures S2C-D and Table S1D), including Arf79F, Garz (guanine nucleotide exchange factor of Arf79F), and several components of the vesicle-mediated coat protein complex I (COPI) transport complex (ζCOP, βCOP, β'COP, γCOP, and δCOP) (Figures S2C-D and Table S1D). The phenotypes of ζCOP, βCOP, δCOP, and Arf79F were confirmed by using three independent dsRNA or shRNA lines for each gene (Table S1D). The cell survival function of these genes was stem cell–specific, because knockdowns of these genes in ECs using NP1-Gal4 did not result in EC death (compare Figure S2F with S2E, and data not shown).
COPI and COPII complexes are essential components of the trafficking machinery for vesicle transportation between the ER and Golgi (reviewed in Lee et al., 2004). The COPII complex mediates vesicle cargo transport from the ER to the Golgi, while the COPI complex mediates cargo transport from the Golgi back to the ER. In addition to its trafficking function, the COPI complex regulates lipid droplet utilization by transporting enzymes of lipolysis to the lipid droplet surface (Beller et al., 2008; Soni et al., 2009). In this screen, we did not identify any components in the COPII complex, suggesting that lipid droplet utilization (lipolysis), rather than the general trafficking machinery between the ER and the Golgi, is required for stem cell survival. Consistent with this hypothesis, we also identified Acyl-CoA synthetase long-chain (ACSL), an enzyme in the Drosophila lipolysis/β-oxidation pathway (Zhang et al., 2009; Palanker et al., 2009), and bubblegum (bgm), a very-long-chain fatty acid-CoA ligase activity (Min and Benzer, 1999).
In addition, we identified 113 other genes whose RNAi-mediated knockdowns resulted in ISC death (Table S1D). The data provide a rich resource for investigating the molecular mechanisms that specifically regulate stem cell death and survival.
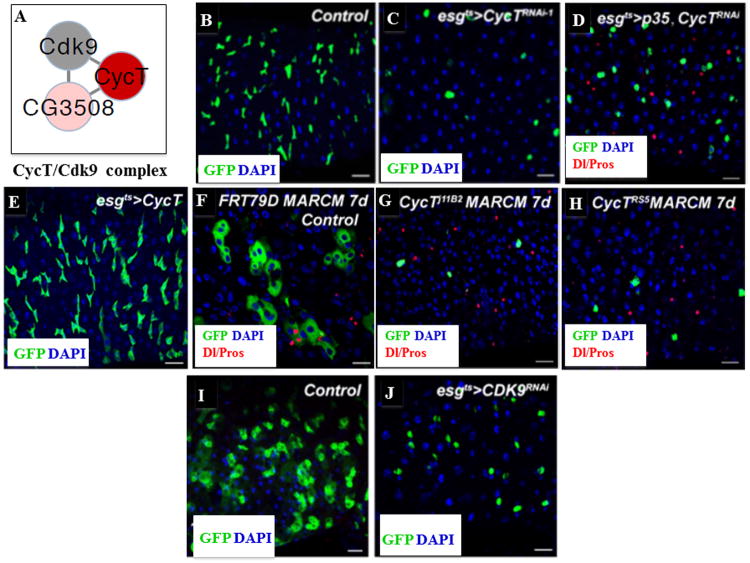
The CycT/Cdk9 Complex
We also identified the Drosophila Cyclin T (CycT) gene (Figure 5A). Knockdowns of CycT by two independent dsRNAs (V37562 and BL31762) and two independent shRNAs (BL32976 and BL35168) all resulted in the ISC quiescence/death phenotype (compare Figure 5C with 5B; Table S1D). Midguts of CycT knockdown contain single and isolated GFP-positive round-shaped cells and they died within two weeks (Figure 5C). Coexpression of the pan-caspase inhibitor p35 could partially slow the death of these GFP-positive cells (Figure 5D). We further generated CycT mutant mosaic clones using the mosaic analysis with a repressible cell marker (MARCM) technique (Lee and Luo, 1999) and found that a few single isolated GFP-postitive round-shaped ISCs were detected in CycT MARCM clones (compare Figures 5G and 5H with the control, 5F), similar to phenotypes observed in CycT RNAi knockdown flies (Figure 5C). Coexpression of p35 in CycT MARCM clones could significantly rescue the ISC quiescence/death phenotypes (Figure S2J), but the rescued cells eventually died 14 days ACI (Figure S2K). Further, overexpression of CycT promoted ISC proliferation (Figure 5E). The CycT protein is ubiquitously expressed in posterior midgut (Figure S2G) and specifically expressed in GFP-positive cells in esgts>CycT flies (Figure S2H). Cdk9/CycT forms a functional complex in vivo and regulates transcriptional elongation and RNA processing through phosphorylating the carboxyl-terminal domain (CTD) of RNA polymerase II (Pol II) (Ni et al., 2004, and Figure 5A). We knocked down Cdk9 activity using its transgenic RNAi line and found a weaker but similar phenotype to that of CycT knockdown (compare Figures 5J with 5I).
Figure 5. The CycT/Cdk9 Complex Regulates ISC Quiescence/Death.
(A) Diagram of the CycT/Cdk9 complex.
(B) esgts wild-type control.
(C) Knockdown of CycT resulted in ISC quiescence/death.
(D) expression of p35 partially rescued the knockdown phenotype of CycT.
(E) Overexpression of CycT promoted ISC proliferation.
(F) Control FRT79D MARCM clones 7 days ACI.
(G) FRT79D-CycT11B2 MARCM clones 7 days ACI.
(H) FRT79D-CycTRS5 MARCM clones 7 days ACI.
(I) esgts wild-type control.
(J) Knockdown of Cdk9 resulted in ISC quiescence/death.
The posterior midguts of corresponding flies were dissected, stained with the indicated antibodies, and analyzed by confocal microscopy. The flies were cultured at 29°C for 7 days in panels A–E, and 14 days in panels I–J. Scale bars, 10 μm.
See also Figure S2, Table S1D.
Genes Whose Knockdowns Resulted in ISC-to-EC Differentiation
Knockdowns of genes required for ISC maintenance or self-renewal would result in the premature differentiation of ISC to EC or EE cells. From our screen, we identified 98 genes whose RNAi-mediated knockdowns resulted in premature ISC-to-EC differentiation (Figures 2G and Table S1E).
Negative Regulators of the N Pathway
Among the 98 genes, we identified eight negative regulators of the N signal transduction network (Vps22/lsn, Vps36, Vps25 (Figure 3C,D), Vps28, Vps23/TSG101, Hey, da, Smr) (Table S1E) (Bray, 2006; Fortini, 2009; Guruharsha et al., 2012). The first five genes function in endosome protein sorting (Figure 3B) and regulate processing of either N or Dl, and the last three are transcriptional factors and control expression of downstream targets of the N signaling. Knockdowns of these genes likely activated the N signaling and promoted ISC-to-EC differentiation. In addition to the known components in the N pathway, we identified five other genes (CG18398/Tango6, CG4722/bib, CG14084/Bet1, CG4214/Syx5, CG15811/Rop) (Table S1E) involved in protein processing or exocytosis. These genes may also negatively regulate the N signaling through regulating the processing of N or Dl.
The Mediator Complex
Among the 98 genes, we also identified 10 components in the mediator complex (CG4184/MED15, CG5121/MED28, CG1245/MED27, CG18780/MED20, CG6884/MED11 (Figure 3C,D), CG31390/MED7, CG1057/MED31, CG12031/MED14, CG13867/MED8, and CG8491/MED12/kto) (Figure 3D, and Table S1E). The mediator complex is a multiple protein complex with 33 identified components in Drosophila (Poss et al., 2013). It is involved in nearly all stages of Pol II transcription, including initiation, promoter escape, elongation, pre-mRNA processing, and termination (Conaway and Conaway, 2013). The mediator complex also generally bridges sequence-specific, DNA-binding transcription factors to the pol II enzyme, thereby converting biological inputs (communicated by TFs) to physiological responses (via changes in gene expression) (Conaway and Conaway, 2013; Poss et al., 2013). In the posterior midgut, the mediator complex may perform a function similar to that of the above negative regulators in the N signal transduction pathway, and restrict activation of the N signaling. It will be interesting to determine how the mediator complex regulates N signaling in future studies.
Nucleosome Remodeling and Histone Modification
In addition to the genes described above, we identified 74 other genes (Table S1E), whose RNAi-mediated knockdowns resulted in premature ISC-to-EC differentiation. Among them are eight genes (CG10272/gpp/dDot1, CG32067/simj, CG31865/Ada1-1, CG8068/dPIAS, CG8103/Mi-2, CG4236/Caf1, CG7776/E(Pc), CG4643/Nurf-38) involved in nucleosome remodeling and histone modification. The data provide a rich resource for investigating ISC-to-EC lineage differentiation in future studies.
Genes Important for ISC-to–EE Cell Differentiation
Extrinsic Slit-Robo2 Signaling from EE Cells to ISCs Regulates the Number of EE Cells through a Negative Feedback Mechanism
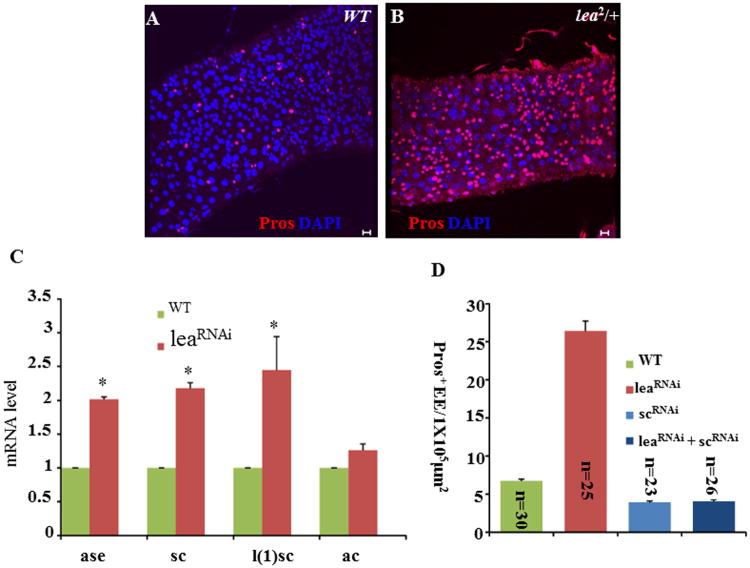
Loss-of-function mutations in the N signal transduction pathway resulted in expansion of both ISCs and EE cells (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Table S1A). Two transcription factors, Scute (Sc) and Asense (Ase), have been shown, by mRNA profiling, to play a major role in EE cell fate determination and to be upregulated in the midgut that expressed a dominant negative form of N (NDN) (Bardin et al., 2010). A recent publication and our manuscript in press demonstrated that EE cells are directly generated from ISCs and the AS-C complex regulates ISCs' commitment to EE cells through Pros (Biteau and Jasper, 2014; Zeng and Hou, in press). The AS-C complex includes four genes (achaete [ac], scute [sc], lethal of scute [l(1)sc], and asense [ase]). Overexpression of each of the four genes resulted in an increase of EE numbers to different degrees (Table S1F). We also identified a Roundabout receptor (Robo2/leak) in the ISC screen (Table S1F), whose knockdown in ISCs (compare Figures S3B with S3A, Figures S3C and S3D), but not in EBs (compare Figure S3F with S3E, Figures S3G and S3H), resulted in a significant increase in the proportion of Pros-positive EE cells. We further generated GFP-marked ISC clones that are homozygous for the loss-of-function allele lea2 (Figure S3J), using the MARCM technique. Seven days ACI, we found that the proportion of Pros+ EE cells was significantly increased in the GFP-marked clones of lea2 (Figures S3J and S3K), as compared with their wild-type counterparts (Figures S3I and S3K), while the ISCs in the GFP-marked clones exhibited normal proliferation and self-renewal (Figure S3L). In addition, we examined posterior midguts of 40-day-old lea2 heterozygous mutant flies and found that the number of Pros+ EE cells was significantly higher in the lea2/+ flies (Figure 6B) than in the wild-type flies (Figure 6A).
Figure 6. The Slit-Robo2 Signaling Regulates EE Cell Fate Specification parallel to AS-C Complex in ISCs.
(A, B) The number of Pros+ EE cells is significantly greater in the lea2/+ flies (B) than in the wild-type flies (A). Scale bars, 10 μm.
(C) mRNA levels of the AS-C complex genes in flies of wild-type and ISCts≫ leaRNAi. (D) Knockdown of both sc and lea in ISCs (ISCts>leaRNAi+scRNAi) suppressed the phenotype of excess EE cells associated with knockdown of lea alone.
Robo2 is one of the three receptors (Robo1, Robo2, and Robo3) of a secreted ligand Slit, and the Slit-Robo signal transduction pathway regulates various biological processes (Ypsilanti et al., 2010). We examined the expression of Robo1, Robo2, Robo3, and Slit using their respective antibodies, and a LacZ reporter line that is under the slit promoter (SlitPZ05248). We found that Robo2 was expressed mainly in Esg-positive ISCs and EBs (Figures S4A and S4A′) in wild-type posterior midgut, but not in the Robo2-depleted posterior midgut (esgts>leaRNAi) (Figures S6D and S6D′), suggesting that the RNAi effectively depleted Robo2 protein expression. We could not detect expression of Robo1 and Robo3 in posterior midgut (Figures S6A–S6C′). Further, in our genome-wide RNAi screen, we screened one Robo1 RNAi line (esgts>robo1 RNAi(v42579)) and two Robo3 RNAi lines (esgts>robo3RNAi(v44702) and esgts>robo3RNAi(JF03331)) and did not find any abnormal phenotype (data not shown). Together, these data suggest that only Robo2 functions in the posterior midgut.
Interestingly, the slit-lacZ reporter was strongly expressed in Pros-positive EE cells (Figures S4B and S4B′) and weakly expressed ISCs (Figures S5A–5E); the Slit protein is strongly expressed in EE cells and also weakly expressed in the periphery of Esg-positive ISCs and EBs (Figures S4C and S4C′). The secreting Slit protein may be diffused from EE cells to ISCs and then trapped there by the Robo2 receptor. To further test this hypothesis, we knocked down lea in ISCs and EBs by expressing the lea RNAi, using esgts. Reducing the expression of Robo2/lea in ISCs and EBs was sufficient to reduce the amount of Slit protein near the periphery of these cells without affecting its expression in EE cells (Figures S4D, S4D′, and S4F). Conversely, we overexpressed Robo2/lea in ISCs and EBs by expressing an UAS-lea (leaEP2582) using esgts. As expected, increasing the expression of Robo2/lea in ISCs and EBs was sufficient to increase the accumulation of Slit protein at the periphery of these cells without affecting its expression in EE cells (Figures S4E, S4E′, and S4F). These data, taken together, indicate that EE cell-produced Slit may prevent new EE cell formation by binding Robo2 and activating the Slit-Robo2 signal transduction pathway in ISCs.
To further test this model, we knocked down Slit expression in EE cells using Gal4 (386Y-Gal4), which is specifically expressed in EE cells during larval stages and in EE cells and other cell types in adult posterior midgut (Reiher et al., 2011), and two independent slit RNAi lines. In both cases, we observed a small but consistent increase in the proportion of EE cells in the posterior midgut epithelium (Figures S7A,B). We also knocked down Slit expression in ISCs (ISCts>slit RNAi) and did not find a significant change of EE cells (Figure S7C). The Slit protein expression could not be detected in the RNAi knocked down posterior midgut (Figures S6E–6H), suggesting that the RNAi effectively depleted Slit protein expression.
In summary, these data suggest that extrinsic Slit-Robo2 signaling from EE cells to ISCs regulates the proportion of EE cells through a negative feedback mechanism to keep the right balance of differentiated cells in the posterior midgut epithelium.
Slit-Robo2 Signaling Regulates EE Cell Fate Specification either upstream or in parallel to AS-C Complex in ISCs
To determine which transcription factor mediates Slit-Robo2 signaling in ISCs to regulate EE cell generation, we first overexpressed pros in ISCs and EBs (esgts>pros) because Pros is an EE cell marker and closely associated with EE cell fate specification (described above). Overexpression of Pros in ISCs and EBs did not affect the proportion of EE cells but rather promoted ISC/EB-to-EC differentiation in the posterior midgut epithelium (Figure S5F), indicating that knocking down the Slit-Robo2 signal does not increase the number of EE cells through inducing pros expression.
Sc and Ase play a major role in EE cell fate determination (Bardin et al., 2010; Zeng et al., 2013; Zeng and Hou, in press). To examine the relationship of Robo2/lea to Sc and Ase, we first compared mRNA levels of the AS-C genes in the midguts of wild-type and ISCts>leaRNAi flies, using qPCR. Among the four AS-C genes, the mRNA levels of ase, sc, and l(1)sc were significantly upregulated in ISCts>leaRNAi midguts, while the mRNA level of ac did not significantly change in comparison to that in wild-type midguts (Figure 6C). We further expressed scRNAi in the ISCts>leaRNAi midgut (ISCts>leaRNAi +scRNAi; Figure 6D) and found that the expression of scRNAi in the leaRNAi midgut suppressed the excess EE cell phenotype of leaRNAi (Figures 6D). Our data so far together suggest that the Slit-Robo2 signaling regulates EE cell fate specification either upstream or in parallel to the AS-C complex in ISCs.
In addition to the genes described above, we identified 16 other genes whose RNAi-mediated knockdowns affect differentiation from ISCs to EE cells (Table S1F). The data provide a rich resource for investigating ISC-to-EE lineage differentiation in future studies.
Comparison of Genes That Regulate Intestinal, Female Germline, and Neural Stem Cells
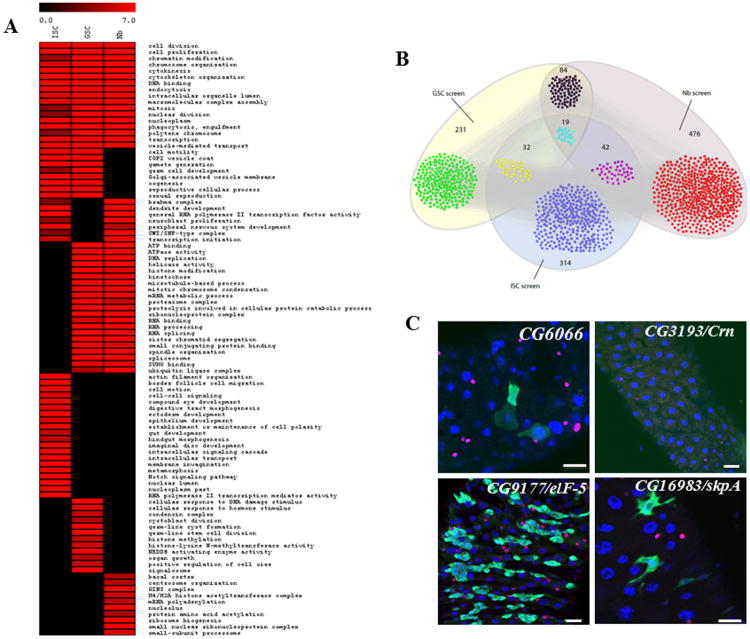
We compared our results with previous screens in Nbs (Neumüller et al., 2011) and female GSCs (Yan et al., 2014) to identify common or unique factors that regulate self-renewal and differentiation of different stem cell systems (Figure 7A). NSC self-renewal and differentiation are controlled through intrinsic asymmetric division, while the fates of female GSCs are regulated through a local niche-dependent mechanism. The adult Drosophila ISCs are regulated by signals from multiple directions, including underlying visceral muscle cells (reviewed in Jiang and Edgar, 2011; O'Brien et al., 2011), differentiated ECs during tissue damage and bacterial infection (Jiang and Edgar, 2011), and trachea-derived Dpp (Li, Z. et al., 2013). Because all three stem cell types are actively dividing, self-renewing, and generating lineage-specific differentiated cells, we expected that some of the basic cellular processes are commonly required and the stem cell–specific regulatory signals are different.
Figure 7. Comparison of ISC, Nb, and female GSC RNAi Screens.
(A) Heatmap display overrepresentation of selected GO terms associated with genes identified in the ISC, Nb, and female GSC screens.
(B) Number of genes identified in the ISC, Nb, and female GSC screens. In all, 19 genes were found in all three screens. Another 32 genes were identified in both ISC and female GSC screens, and the other 42 genes were identified in both ISC and Nb screen.
(C) Phenotypes of common genes identified in ISC, female GSC, and NSC. Scale bars: C and E– 5 μm.
See also Tables S1 and S4.
Among the 405 genes identified in the ISC screen, 19 were identified in all three screens, another 31 genes were identified in both ISC and female GSC screens, and the other 43 genes were identified in both ISC and NSC screens (Figure 7B and Table S4). Most of the shared genes are regulators of basic cellular processes.
Knockdown of several components (osa, brm, Snar1, dalao) of the Brahma (BRM) chromatin-remodeling complex resulted in an expansion of stem cell–like cells in both ISCs and type II Nb lineages (Neumüller et al., 2011; Zeng et al., 2013; Table S1) while knockdown of these genes in female GSC lineage was without detectable phenotypes (Yan et al., 2104). On the other hand, knockdown of several components of the COPI complex resulted in ISC-specific cell death (Figure 7E and Table S1D) and defects in female GSC lineage (Yan et al., 2014), but none of these genes were identified in the Nbs screen (Neumüller et al., 2011). Most of the genes involved in the signal transduction pathways identified in our screen are ISC-specific. As mentioned before, the N signal transduction pathway plays a major role in regulating ISC self-renewal and differentiation. We identified 19 positive and 8 negative regulators of the N pathway in our ISC screen (Tables S1 and S4), but only two positive regulators (Dl, aux) were identified in the female GSC screen and one negative regulator (da) was identified in the Nbs screen (Neumüller et al., 2011; Yan et al., 2014; Table S4). Most of the genes required for ISC proliferation are ISC-specific, including components in the EGFR, JAK-Stat, Dpp, JNK, and Hippo signal transduction pathways (Table S1 and S4).
In summary, factors related to basic stem cell cellular processes are commonly required in all stem cells, and stem cell–specific, niche-related signals are only required in the unique stem cell type.
Discussion
Drosophila midgut stem cells provide an excellent genetic system for studying tissue homeostasis, regeneration, hyperplasia, stress and aging (Amcheslavsky et al., 2009; Buchon, 2009a,b; Biteau et al., 2008; Buszczak et al., 2009; Cronin et al., 2009; Hochmuth et al., 2011; Jiang et al., 2009; Jiang et al., 2011; Jin et al., 2013; Wang et al. 2014; Zeng et al., 2013). In the last few years, using homozygous mutant animals or mosaic analyses, there are several genes have been identified to be required in ISC self-renewal and differentiation. However, a systematic molecular understanding of self-renewal and lineage-specific differentiation of adult ISCs has not been performed. Here, we screened 44.8% of the fly genome and identified 405 genes that regulate various fates of adult Drosophila ISCs. Since we screened the RNAi lines giving an adult lethal phenotype, we might have missed some adult gut-specific genes in this screen. Nevertheless, the genes identified here, we further obtained precise quantification of phenotypic data and identified regulatory networks through a computer network analysis. From our phenotype and network analysis, we reached a number of important conclusions. First, ISC self-renewal, EE cell fate determination, and EB differentiation are regulated by distinct networks or pathways (Table S1A). The JAK-Stat pathway regulates EB differentiation; some components in the N signal transduction network control both ISC self-renewal and EE cell fate determination, and knockdowns of these components resulted in expansion of both ISCs and EE cells. The other components in the N signal transduction network control only ISC self-renewal, and knockdowns of those components resulted only in ISC expansion. The way the Brm/Osa complex regulates ISC self-renewal and EE cell fate determination is different from that of the N signaling; knockdowns of the components in the Brm/Osa complex resulted in ISC expansion and EE cell reduction. Second, ISC proliferation is uniquely regulated. Very few genes whose knockdowns resulted in ISC overproliferation in the ISC screen were also isolated in the screens of Nbs and female GSCs (Neumüller et al., 2011; Yan et al., 2014; Table S4), suggesting that these genes and signal transduction pathways regulate ISC proliferation only. Third, knockdowns of several regulators of mitotic cell cycle and mitotic cytokinesis resulted in large nuclei (polyploidy) of ISCs and EE cells, suggesting that the cell cycle regulators uniquely maintain diploid cells. Fourth, we found that the COPI and CycT/Cdk9 complexes specifically regulate ISC survival. Quiescent CSCs are often resistant to traditional cancer therapies that primarily target dividing and actively metabolizing cells (Trumpp and Wiestler, 2008). Studying stem cell death using the data generated from this screen may lead to the design of new therapies to selectively eliminate stem cells in cancer. Fifth, we identified a large mediator complex that regulates ISC-to-EC differentiation. Sixth, we found that the Slit/Robo2-negative feedback pathway, the N inhibitory pathway, and the Brm/Osa complex together regulate EE cell fate specification in ISCs either upstream or in parallel to AS-C/Pros. Seventh, by comparing genes identified in screens of ISCs, Nbs, and female GSCs, we found that factors related to the cellular processes of basic stem cells are commonly required in all stem cells, and stem cell–specific, niche-related signals are required only in the unique stem cell type. The information obtained from this study will help to further dissect the regulatory networks in stem cell biology.
Experimental Procedures
Fly Stocks
The following fly strains were used: esg-Gal4 line (from Shigeo Hayashi); Su(H)GBE-Gal4 (Zeng et al., 2010), Su(H)GBE-tub-Gal80, and ISCts (esg-Gal4, UAS-GFP; tub-Gal80ts, Su(H)GBE-tub-Gal80) (Zeng and Hou, in Press), and UAS-CycT were generated in our laboratory. The following strains were obtained from the Bloomington Drosophila Stock Center (BDSC): tub-Gal80ts, lea2, leaEP2582, UAS-p35, N264-39, UAS-leaRNAi (BL9286), slit PZ05248, UAS-slitRNAi-1 (BL31468), UAS-robo3 RNAi-1 (BL29398), UAS-CycT RNAi-1 (BL31762), UAS-CycT RNAi-2 (BL32976), UAS-Cdk9RNAi (BL34982), CycT j11B2 (BL12101), and fly lines used for MARCM clones, including FRT2A-piM, FRT40A-piM, SM6,hs-flp, MKRS,hs-flp, FRT2A tub-Gal80, FRT40A tub-Gal80, and FRT19A tub-Gal80. The following transgenic RNAi lines were obtained from VDRC: UAS-slitRNAi-2 (v108853), UAS-robo1RNAi (v42579), and UAS-robo3RNAi-2 (v44702). CycTRS5 (Kyoto125610) was from the Kyoto Drosophila Stock Center. Flies were raised on standard fly food at 25°C and 65% humidity, unless otherwise indicated.
RNAi Stocks Used in the Screen
UAS-RNAi lines were generated by VDRC and the Transgenic RNAi Project (TRiP), and are available at VDRC and the BDSC. The sequences used for VDRC knockdown strains are available for each line at https://stockcenter.vdrc.at) and for Bloomington knock-down strains are available for each line at http://flystocks.bio.indiana.edu.
MARCM Clonal Analysis
To induce MARCM clones of FRT2A-piM (as a wild-type control), FRT2A-CycT j11B2, FRT2A-CycTRS5, FRT40A-piM (as a wild-type control), FRT40A-lea2, FRT19A-sn3 w1118 (as a wild-type control), and FRT19A-N264-39, we generated the following flies: act>y+>Gal4, UAS-GFP/SM6, hs-flp; FRT2A tub-Gal80/FRT2A mutant or FRT40A tub-Gal80/FRT 40A mutant; and MKRS, hs-flp/act>CD2>Gal4, UAS-GFP or hs-flp, tub-Gal80, FRT19A/FRT19A mutant; and act>y+>Gal4, UAS-GFP/+. Three- or four-day-old adult female flies were heat-shocked at 37°C for 45 min twice, at an interval of 8–12 hr. The flies were transferred to fresh food daily after the final heat shock, and their posterior midguts were processed for staining at the indicated times.
RNAi-Mediated Gene Depletion
Male UAS-RNAi transgene flies were crossed with female virgins of esg-Gal4,UAS-GFP; tub-Gal80ts, esg-Gal4,UAS-GFP; Su(H)GBE-Gal80, tub-Gal80ts (for ISC-specific expression), Su(H)GBE-Gal4,UAS-GFP; tub-Gal80ts (for EB-specific expression), or UAS-mCD8.GFP; esg-Gal4,wg-Gal4; tub-Gal80ts (for expression in both posterior midgut and hindgut intestinal stem cells). The flies were cultured at 18°C. Three- to five-day-old adult flies with the appropriate genotype were transferred to new vials at 29°C for 7 days or 14 days before dissection.
Histology and Image Capture
The fly intestines were dissected in PBS and fixed in PBS containing 4% formaldehyde for 20 min. After three 5-min rinses with PBT (PBS + 0.1% Triton X-100), the samples were blocked with PBT containing 5% normal goat serum and kept overnight at 4°C. Then the samples were incubated with primary antibody at room temperature for 2 hr and incubated with the fluorescence-conjugated secondary antibody for 2 hr at room temperature. Samples were mounted in the Vectashield mounting medium with DAPI (Vector Laboratories). We used the following antibodies: mouse anti-β-Gal (1:200; Clontech); mouse anti-Dl (1:50; DSHB); mouse anti-Pros (1:50; DSHB); nc82 (1:20; DSHB); anti-Slit (1:20; DSHB); guinea pig anti-Pros (1:3000, a gift from Tiffany Cook); rabbit anti-Robo2 (1:100, a gift from Barry Dickson); mouse anti-Robo1 (1:50; DSHB); mouse anti-Robo3 cytoplasmic (15H2) (1:50; DSHB); mouse anti-Robo3 extracellular (14C9) (1:50; DSHB); rabbit anti-CycT (1:1000; generated in Xinhua Lin's laboratory), and chicken anti-GFP (1:3,000; Abcam). Secondary antibodies used were goat anti-mouse, anti-chicken, anti-guinea pig, and anti-rabbit IgG conjugated to Alexa 488 or Alexa 568 (1:400; Molecular Probes). Images were captured with the Zeiss LSM 510 confocal system and processed with LSM Image Browser and Adobe Photoshop.
Quantification and Statistical Analysis
To quantify the percentage of Pros+ EE cells, except for Figures S7A-H, in which the Pros+ EE cells and total cells were counted in a 1 × 105 μm2 area of a Z-stack of multiple confocal planes, the Pros+ EE cells and total cells were counted in a 1 × 105 μm2 area of a single confocal plane. To quantify the strength of fluorescence of Slit staining, all the images were taken with the same confocal settings, and the fluorescence intensity was measured using an LSM5 Image Browser (Zeiss). All the data were analyzed using Student's t- test, and sample size (n) is shown in the figure.
Supplementary Material
Acknowledgments
We thank Shigeo Hayashi, Tiffany Cook, VDRC, the Bloomington Stock Centers and TRiP at Harvard Medical School for fly stocks; Barry Dickson, Tiffany Cook, and the Developmental Studies Hybridoma Bank for antibodies; and S. Lockett for help with the confocal microscope. Work in the laboratory of S.H. was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health; work in the laboratory of X. L. was supported by grants from the Nature Sciences Foundation of China [31030049, 81361120382 and 31271582] and the Strategic Priority Research Program of the Chinese Academy of Sciences Grant [XDA01010101].
Footnotes
Author Contributions: X.Z., S.X.H. and X. L. conceived and designed the experiments. X.Z., S.R.S., L.H., H.L., Y.L., W.L., and S.X.H. performed the experiments. R.A.N, D.Y., S.R.S., and Y.H. performed the bioinformatics. X.Z., S.R.S., S.X.H. and X. L. analyzed the data. S.X.H., S.R.S., and X.Z. wrote and revised the manuscript, L.H. and S.R.S made equal contribution, and all authors approved the final version of the manuscript.
Note: Biteau and Jasper (2014) recently published “Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila” while this manuscript was in preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amcheslavsky A, Ito N, Jiang J, Ip YT. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol. 2011;193:695–710. doi: 10.1083/jcb.201103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach J, Hummel P, Bickmeyer I, Kowalczyk KM, Frank M, Knorr K, Hildebrandt A, Riedel D, Jäckle H, Kühnlein RP. A Drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metab. 2014;19:331–343. doi: 10.1016/j.cmet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Beller M, Sztalryd C, Southall N, Bell M, Jäckle H, Auld DS, Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns N, Woichansky I, Friedrichsen S, Kraft N, Riechmann V. A genome-scale in vivo RNAi analysis of epithelial development in Drosophila identifies new proliferation domains outside of the stem cell niche. J Cell Sci. 2014;127:2736–48. doi: 10.1242/jcs.144519. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7:1867–1875. doi: 10.1016/j.celrep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Spradling AC. Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science 323. 2009:248–251. doi: 10.1126/science.1165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009a;25:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009b;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Conaway RC, Conaway JW. The mediator complex and transcription elongation. Biochim Biophys Acta. 2013;1829:69–75. doi: 10.1016/j.bbagrm.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol. 2013;201:945–961. doi: 10.1083/jcb.201302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim RS, Baldwin K, Smagghe G. Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol. 2010;55:593–608. doi: 10.1146/annurev-ento-112408-085450. [DOI] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. Intestinal stem cells in the adult Drosophila midgut. Exp Cell Res. 2011;317:2780–2788. doi: 10.1016/j.yexcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Xu J, Yin MX, Lu Y, Hu L, Li P, Zhang P, Yuan Z, Ho MS, Ji H, Zhao Y, Zhang L. Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. Elife. 2013;2:e00999. doi: 10.7554/eLife.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, Cotton JL, Mao J, McCollum D, Jiang J, Czech MP, Xu L, Ip YT. The conserved misshapen-warts-yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Han L, Shi L, Lin X. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev Cell. 2013;24:133–143. doi: 10.1016/j.devcel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen Z, Jin Y, Liu W. Identification of a histone acetyltransferase as a novel regulator of Drosophila intestinal stem cells. FEBS Lett. 2013;587:1489–1495. doi: 10.1016/j.febslet.2013.03.013. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Min KT, Benzer S. Preventing neurodegeneration in the Drosophila mutant bubblegum. Science. 1999;284:1985–1988. doi: 10.1126/science.284.5422.1985. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ, Knoblich JA. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458:987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumüller RA, Richter C, Fischer A, Novatchkova M, Neumüller KG, Knoblich JA. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell. 2011;8:580–593. doi: 10.1016/j.stem.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely GG, Kuba K, Cammarato A, Isobe K, Amann S, Zhang L, Murata M, Elmén L, Gupta V, Arora S, Sarangi R, Dan D, Fujisawa S, Usami T, Xia CP, Keene AC, Alayari NN, Yamakawa H, Elling U, Berger C, Novatchkova M, Koglgruber R, Nishina H, Isobe M, Pospisilik JA, Imai Y, Pfeufer A, Hicks AA, Pramstaller PP, Subramaniam S, Kimura A, Ocorr K, Bodmer R, Penninger JM. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell. 2010;141:142–153. doi: 10.1016/j.cell.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, Yang-Zhou D, Shim HS, Tao R, Handler D, Karpowicz P, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Schwartz B, Werner J, Suarez JR, Lis JT. Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol Cell. 2004;13:55–65. doi: 10.1016/s1097-2765(03)00526-4. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto CN, Schweisguth F, Bardin AJ. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development. 2011;138:4585–4595. doi: 10.1242/dev.065292. [DOI] [PubMed] [Google Scholar]
- Poss ZC, Ebmeier CC, Taatjes DJ. The mediator complex and transcription regulation. Crit Rev Biochem Mol Biol. 2013;48:575–608. doi: 10.3109/10409238.2013.840259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiher W, Shirras C, Kahnt J, Baumeister S, Isaac RE, Wegener C. Peptidomics and peptide hormone processing in the Drosophila midgut. J Proteome Res. 2011;10:1881–1892. doi: 10.1021/pr101116g. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer F, Schönbauer C, Langer CC, Dietzl G, Novatchkova M, Schernhuber K, Fellner M, Azaryan A, Radolf M, Stark A, Keleman K, Dickson BJ. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature. 2010;464:287–291. doi: 10.1038/nature08799. [DOI] [PubMed] [Google Scholar]
- Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A, Wiestler OD. Mechanisms of disease: cancer stem cells–targeting the evil twin. Nat Clin Pract Oncol. 2008;5:337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- Vinayagam A, Hu Y, Kulkarni M, Roesel C, Sopko R, Mohr SE, Perrimon N. Protein complex-based analysis framework for high-throughput data sets. Sci Signal. 2013;6:rs5. doi: 10.1126/scisignal.2003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chang KC, Somers G, Virshup D, Ang BT, Tang C, Yu F, Wang H. Protein phosphatase 2A regulates self-renewal of Drosophila neural stem cells. Development. 2009;136:2287–2296. doi: 10.1242/dev.035758. [DOI] [PubMed] [Google Scholar]
- Wang L, Karpac J, Jasper H. Promoting longevity by maintaining metabolic and proliferative homeostasis. J Exp Biol. 2014;217:109–118. doi: 10.1242/jeb.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Goodman JL, Strelets VB FlyBase Consortium. FlyBase: integration and improvements to query tools. Nucleic Acids Res. 2008;36(Database issue):D588–593. doi: 10.1093/nar/gkm930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Yan D, Neumüller RA, Buckner M, Ayers K, Li H, Hu Y, Yang-Zhou D, Pan L, Wang X, Kelley C, Vinayagam A, Binari R, Randklev S, Perkins LA, Xie T, Cooley L, Perrimon N. A regulatory network of Drosophila germline stem cell self-renewal. Dev Cell. 2014;28:459–473. doi: 10.1016/j.devcel.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y, Chédotal A. Moving away from the midline: new developments for Slit and Robo. Development. 2010;137:1939–1952. doi: 10.1242/dev.044511. [DOI] [PubMed] [Google Scholar]
- Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis. 2010;48:607–611. doi: 10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Lin X, Hou SX. The Osa-containing SWI/SNF chromatin-remodeling complex regulates stem cell commitment in the adult Drosophila intestine. Development. 2013;140:3532–3540. doi: 10.1242/dev.096891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen D, Wang Z. Analyses of mental dysfunction-related ACSl4 in Drosophila reveal its requirement for Dpp/BMP production and visual wiring in the brain. Hum Mol Genet. 2009;18:3894–3905. doi: 10.1093/hmg/ddp332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.