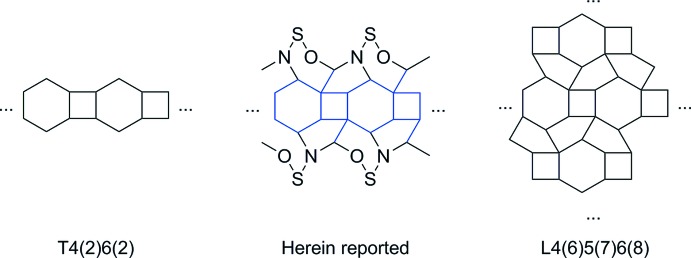
The zwitterionic title compound shows a major disorder of the triisopropylphenyl group over two equally occupied locations. An interesting feature is the uncommon hydrate structure, exhibiting a tape-like motif which can be classified as a transition of the one-dimensional T4(2)6(2) motif into the two-dimensional L4(6)5(7)6(8) motif.
Keywords: crystal structure, zwitterion, hydrate, tape-like motif, hydrogen bonding
Abstract
The zwitterionic title compound, C23H42N2O2S·4H2O, crystallized as a tetrahydrate from a solution of N-[(2,4,6-triisopropylphenyl)sulfonyl]aziridine in triethylamine, diethyl ether and pentane in the presence of moist air. It is formed by a nucleophillic ring-opening that is assumed to be reversible. The molecular structure shows a major disorder of the triisopropylphenyl group over two equally occupied locations. An interesting feature is the uncommon hydrate structure, exhibiting a tape-like motif which can be classified as a transition of the one-dimensional T4(2)6(2) motif into the two-dimensional L4(6)5(7)6(8) motif.
Chemical context
The title compound was isolated as by-product while purifying the corresponding sulfonylaziridine via column chromatography using a solvent mixture containing triethylamine. Interestingly, the zwitterionic title compound was formed by the nucleophilic ring-opening of the aziridine. This is so far undocumented for tertiary amines but well known for primary or secondary amines (Hu, 2003 ▸). We assume that this ring-opening reaction is reversible, since the aziridine was isolated in the absence of water. Possibly, the zwitterionic structure is stabilized by the water molecules and/or by crystallization, preventing the reverse reaction. Furthermore, the four incorporated solvent water molecules in the crystal structure form a tape-like hydrate structure comparable to some known hydrogen-bonding motifs (Infantes et al., 2003 ▸). This is discussed further in the Supramolecular features section.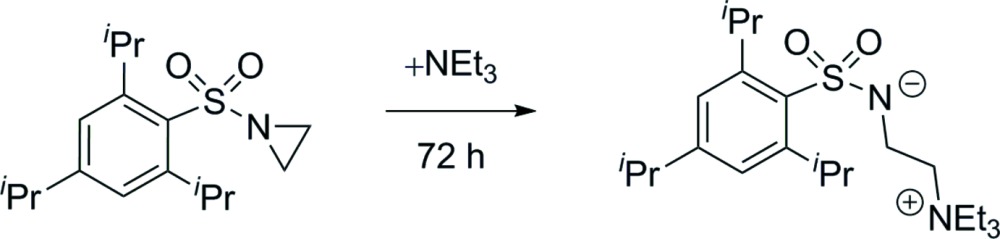
Structural commentary
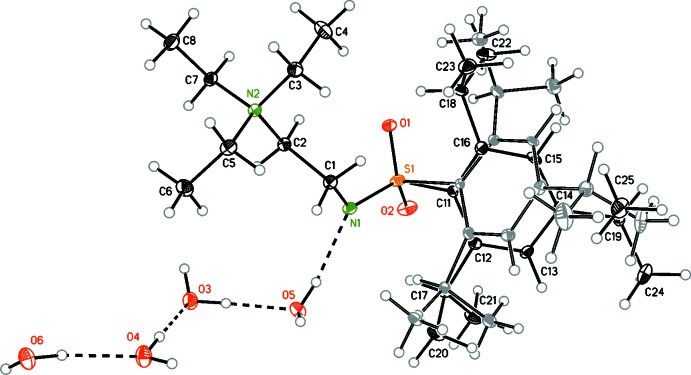
The asymmetric unit consists of a [2-(triethylammonio)ethyl][(2,4,6-triisopropylphenyl)sulfonyl]amide and four water molecules (Fig. 1 ▸). The triisopropylphenyl substituent is disordered over two slightly tilted locations with almost equal occupancies. No superlattice could be found and statistical disorder was assumed. Furthermore, the benzene ring appears to be bent towards the sulfur, which was also observed in the corresponding aziridine compound; for the structure of rac-2-phenyl-1-[(2,4,6-triisopropylbenzene)sulfonyl]aziridine, see Golz et al. (2014 ▸) and for isopropyl 2,4,6-triisopropylphenyl sulfone see Sandrock et al. (2004 ▸). This seems to be typical of the triisoproylphenylsulfonyl group, though that will not be discussed further due to the disorder. The C2—N2 bond involving the cationic N atom is long [1.521 (2) Å], significantly exceeding the sum of the van der Waals radii (1.47 Å), while the C1—N1 bond [1.475 (2) Å], involving the anionic N atom, is close to the sum of the van der Waals radii. In contrast, the S—N1 bond [1.571 (1) Å] is shortened significantly, with the sum of the van der Waals radii being 1.73 Å. Both nitrogen groups are in an almost perfect antiperiplanar conformation [N1—C1—C2—N2 = 179.7 (1)°].
Figure 1.
The molecular structure and atom numbering for the title compound with displacement ellipsoids drawn at the 30% probability level. Atoms of the minor disorder component are drawn with grey-coloured C atoms.
Supramolecular features
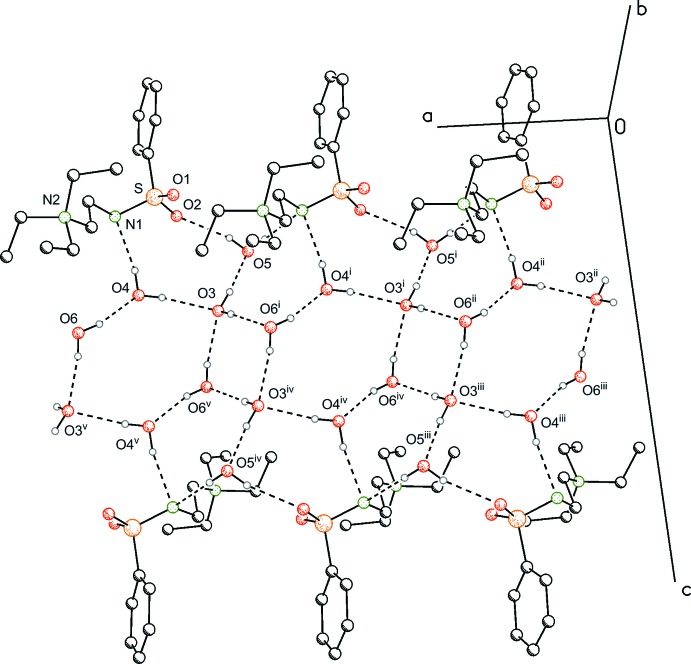
Intermolecular interactions occur mostly through hydrogen bonding of the water molecules among themselves and with the zwitterionic compound (Table 1 ▸). Three of the four water molecules form an infinite tape of interconnected four- and six-membered rings known as the T4(2)6(2) motif. Each ring contains a centre of symmetry and the tape expands in the [100] direction. Interestingly, the border of the tape is lined with the zwitterionic compound and one additional water molecule, thus expanding the tape with five- and six-membered rings involving the O4–O6–O3–O5–N1 and O4–O3–O5–O2–S1–N1 atoms, respectively (Fig. 2 ▸ and Fig. 3 ▸). The structure is comparable to the L4(6)5(7)6(8) motif, building up two-dimensional sheets, which are limited here by the zwitterionic amide. In summary, the hydrate structure discussed herein represents a transition between a one-dimensional tape and a two-dimensional sheet.
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| O4H4DO3 | 0.83(2) | 2.04(2) | 2.867(2) | 171(2) |
| O3H3CO5 | 0.90(2) | 1.83(2) | 2.725(2) | 174(2) |
| O3H3DO6i | 0.85(3) | 2.08(3) | 2.912(2) | 169(2) |
| O5H5CO2ii | 0.83(3) | 2.09(3) | 2.901(2) | 165(2) |
| O6H6DO4 | 0.86(2) | 1.95(2) | 2.787(2) | 167(2) |
| O6H6EO3iii | 0.82(3) | 2.03(3) | 2.845(2) | 170(2) |
| O5H5DN1 | 0.84(3) | 2.05(3) | 2.881(2) | 170(2) |
| O4H4EN1ii | 0.92(3) | 2.06(3) | 2.959(2) | 165(3) |
Symmetry codes: (i) 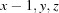 ; (ii)
; (ii) 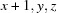 ; (iii)
; (iii) 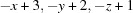 .
.
Figure 2.
A view of the hydrate structure expanding along (100). H atoms not involved in hydrogen bonds and the isopropyl groups have been omitted for clarity. [Symmetry codes: (i) x − 1, y, z; (ii) x − 2, y, z; (iii) x − 1, y − 2, z − 1; (iv) x − 2, y − 2, z − 1; (v) x − 3, y − 2, z − 1.]
Figure 3.
Hydrate-structure motifs already known (left and right) (Infantes et al., 2003 ▸) and the structure reported here (centre).
Some recent structures involving water forming the T4(2)6(2) hydrogen-bonding motif have been published (Li, Li, Su et al., 2006 ▸; Li, Chen et al., 2006 ▸; Song et al., 2007 ▸; Kostakis et al., 2009 ▸). There are only a few examples of two-dimensional hydrogen-bond networks known, but among these the L4(6)5(7)6(8) motif is the most common. For recent examples, see Born et al. (1995 ▸) and Gómez-Saiz et al. (2002 ▸).
Database survey
Comparable zwitterionic structures with neighbouring amide and ammonium groups are quite uncommon. Only one related structure was found in the Cambridge Structural database (Version 5.35, November 2013; Groom & Allen 2014 ▸). In the molecule reported here, the N1—C1 bond length [1.475 (2) Å] involving the anionic N atom is normal [sum of van der Waals radii = 1.479 (2) Å], while the C2—N2 bond to the cationic N atom [1.521 (2) Å] is unusually long. This contrasts sharply with the structure of zwitterionic 1-amino-2-nitraminoethane (Vasiliev et al., 2001 ▸), where these observations are reversed, with the C—N bond to the anionic N atom reduced to 1.455 (2) Å.
Synthesis and crystallization
N-[(2,4,6-Triisopropylphenyl)sulfonyl]aziridine was synthesized from ethanolamine as described in the recent literature (Buckley et al., 2013 ▸). Crystals of the title compound were obtained after a test tube containing small amounts of the sulfonylaziridine dissolved in a mixture of diethyl ether, pentane and triethylamine was left to evaporate over a period of 3 d.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms not involved in hydrogen bonds were positioned geometrically and refined using a riding model, with U iso(H) = 1.5U eq(C) for terminal and 1.2U eq(C) for non-terminal H atoms, with C—H = 0.98 Å. H atoms involved in hydrogen bonds were located in a difference Fourier synthesis map and were freely refined.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C23H42N2O2S4H2O |
| M r | 482.71 |
| Crystal system, space group | Triclinic, P
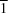
|
| Temperature (K) | 173 |
| a, b, c () | 6.6797(4), 8.7345(5), 23.3973(14) |
| , , () | 96.579(5), 93.734(5), 95.570(5) |
| V (3) | 1345.69(14) |
| Z | 2 |
| Radiation type | Mo K |
| (mm1) | 0.16 |
| Crystal size (mm) | 0.34 0.25 0.08 |
| Data collection | |
| Diffractometer | Agilent Xcalibur Sapphire3 |
| Absorption correction | Multi-scan (CrysAlis PRO; Oxford Diffraction, 2013 ▸) |
| T min, T max | 0.981, 1.000 |
| No. of measured, independent and observed [I > 2(I)] reflections | 34730, 5881, 4239 |
| R int | 0.075 |
| (sin /)max (1) | 0.639 |
| Refinement | |
| R[F 2 > 2(F 2)], wR(F 2), S | 0.049, 0.102, 1.01 |
| No. of reflections | 5881 |
| No. of parameters | 472 |
| No. of restraints | 36 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| max, min (e 3) | 0.24, 0.33 |
The disorder of the triisopropylphenyl group was refined by a free variable to an occupancy ratio of 0.502 (2):0.498 (2). To ensure the stability of the phenyl ring in the refinement, the standard FLAT restraint was applied to atoms C11–C19 and a DELU restraint to atoms C11, C12 and C16, in both of the disorder domains. In addition, atoms C11, C11′ and C16′ required an additional ISOR restraint with a reduced deviation (s = 0.001 and st = 0.002).
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015008105/sj5448sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015008105/sj5448Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989015008105/sj5448Isup3.cml
CCDC reference: 1061352
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We are grateful to the Forschungsgemeinschaft (DFG) and the Fonds der Chemischen Industrie (VCI) for financial support.
supplementary crystallographic information
Crystal data
| C23H42N2O2S·4H2O | Z = 2 |
| Mr = 482.71 | F(000) = 532 |
| Triclinic, P1 | Dx = 1.191 Mg m−3 |
| a = 6.6797 (4) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 8.7345 (5) Å | Cell parameters from 5123 reflections |
| c = 23.3973 (14) Å | θ = 2.6–28.2° |
| α = 96.579 (5)° | µ = 0.16 mm−1 |
| β = 93.734 (5)° | T = 173 K |
| γ = 95.570 (5)° | Plate, clear colourless |
| V = 1345.69 (14) Å3 | 0.34 × 0.25 × 0.08 mm |
Data collection
| Agilent Xcalibur Sapphire3 diffractometer | 5881 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 4239 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.075 |
| Detector resolution: 16.0560 pixels mm-1 | θmax = 27.0°, θmin = 2.4° |
| ω scans | h = −8→8 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2013) | k = −11→11 |
| Tmin = 0.981, Tmax = 1.000 | l = −29→29 |
| 34730 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.049 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.102 | w = 1/[σ2(Fo2) + (0.0428P)2 + 0.1372P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max = 0.001 |
| 5881 reflections | Δρmax = 0.24 e Å−3 |
| 472 parameters | Δρmin = −0.33 e Å−3 |
| 36 restraints |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S1 | 0.46930 (6) | 0.84811 (5) | 0.73798 (2) | 0.01893 (12) | |
| O1 | 0.32141 (17) | 0.72141 (14) | 0.71224 (5) | 0.0225 (3) | |
| O2 | 0.39889 (18) | 1.00090 (14) | 0.73798 (6) | 0.0295 (3) | |
| O3 | 1.1266 (2) | 1.00237 (18) | 0.57740 (7) | 0.0300 (3) | |
| O4 | 1.5372 (2) | 0.93656 (17) | 0.59417 (7) | 0.0322 (4) | |
| O5 | 1.0118 (2) | 1.06162 (16) | 0.68669 (6) | 0.0262 (3) | |
| O6 | 1.7836 (2) | 0.81480 (17) | 0.51262 (7) | 0.0312 (4) | |
| N1 | 0.6732 (2) | 0.85102 (16) | 0.70803 (6) | 0.0186 (3) | |
| C1 | 0.7592 (3) | 0.70129 (19) | 0.70154 (7) | 0.0186 (4) | |
| H1A | 0.7122 | 0.6373 | 0.7313 | 0.022* | |
| H1B | 0.9083 | 0.7186 | 0.7062 | 0.022* | |
| N2 | 0.7612 (2) | 0.46079 (15) | 0.62443 (6) | 0.0155 (3) | |
| C2 | 0.6901 (2) | 0.61873 (18) | 0.64150 (7) | 0.0164 (4) | |
| H2A | 0.5408 | 0.6063 | 0.6379 | 0.020* | |
| H2B | 0.7347 | 0.6877 | 0.6130 | 0.020* | |
| C3 | 0.6882 (3) | 0.3436 (2) | 0.66417 (8) | 0.0216 (4) | |
| H3A | 0.7416 | 0.2440 | 0.6522 | 0.026* | |
| H3B | 0.7462 | 0.3807 | 0.7039 | 0.026* | |
| C4 | 0.4624 (3) | 0.3141 (2) | 0.66513 (9) | 0.0320 (5) | |
| H4A | 0.4073 | 0.4116 | 0.6774 | 0.048* | |
| H4B | 0.4298 | 0.2395 | 0.6922 | 0.048* | |
| H4C | 0.4032 | 0.2722 | 0.6264 | 0.048* | |
| C5 | 0.9904 (2) | 0.4708 (2) | 0.62907 (8) | 0.0213 (4) | |
| H5A | 1.0404 | 0.5092 | 0.6694 | 0.026* | |
| H5B | 1.0311 | 0.3652 | 0.6203 | 0.026* | |
| C6 | 1.0912 (3) | 0.5746 (2) | 0.58966 (8) | 0.0291 (5) | |
| H6A | 1.0489 | 0.5345 | 0.5494 | 0.044* | |
| H6B | 1.2380 | 0.5770 | 0.5960 | 0.044* | |
| H6C | 1.0522 | 0.6798 | 0.5980 | 0.044* | |
| C7 | 0.6761 (3) | 0.4100 (2) | 0.56254 (7) | 0.0198 (4) | |
| H7A | 0.5270 | 0.4016 | 0.5615 | 0.024* | |
| H7B | 0.7194 | 0.4918 | 0.5386 | 0.024* | |
| C8 | 0.7380 (3) | 0.2575 (2) | 0.53547 (8) | 0.0281 (5) | |
| H8A | 0.8850 | 0.2656 | 0.5344 | 0.042* | |
| H8B | 0.6745 | 0.2333 | 0.4961 | 0.042* | |
| H8C | 0.6946 | 0.1750 | 0.5584 | 0.042* | |
| C11 | 0.5230 (8) | 0.8325 (6) | 0.8086 (2) | 0.0128 (14) | 0.5020 (15) |
| C12 | 0.6299 (5) | 0.9594 (4) | 0.84523 (15) | 0.0160 (7) | 0.5020 (15) |
| C13 | 0.6466 (5) | 0.9557 (4) | 0.90455 (14) | 0.0179 (8) | 0.5020 (15) |
| H13 | 0.7179 | 1.0416 | 0.9285 | 0.021* | 0.5020 (15) |
| C14 | 0.5630 (5) | 0.8313 (4) | 0.93024 (14) | 0.0171 (8) | 0.5020 (15) |
| C15 | 0.4656 (9) | 0.7066 (5) | 0.8933 (3) | 0.0181 (11) | 0.5020 (15) |
| H15 | 0.4075 | 0.6203 | 0.9100 | 0.022* | 0.5020 (15) |
| C16 | 0.4474 (9) | 0.6995 (8) | 0.8334 (3) | 0.0168 (12) | 0.5020 (15) |
| C17 | 0.7393 (5) | 1.1005 (4) | 0.82250 (15) | 0.0172 (8) | 0.5020 (15) |
| H17 | 0.7186 | 1.0852 | 0.7794 | 0.021* | 0.5020 (15) |
| C18 | 0.3527 (5) | 0.5456 (4) | 0.80066 (16) | 0.0203 (8) | 0.5020 (15) |
| H18 | 0.3777 | 0.5485 | 0.7591 | 0.024* | 0.5020 (15) |
| C19 | 0.5849 (5) | 0.8221 (4) | 0.99530 (15) | 0.0218 (8) | 0.5020 (15) |
| H19 | 0.4485 | 0.7879 | 1.0074 | 0.026* | 0.5020 (15) |
| C20 | 0.9656 (5) | 1.1076 (5) | 0.83905 (17) | 0.0293 (10) | 0.5020 (15) |
| H20A | 0.9905 | 1.1284 | 0.8811 | 0.044* | 0.5020 (15) |
| H20B | 1.0375 | 1.1906 | 0.8211 | 0.044* | 0.5020 (15) |
| H20C | 1.0139 | 1.0083 | 0.8255 | 0.044* | 0.5020 (15) |
| C21 | 0.6519 (6) | 1.2506 (4) | 0.84431 (19) | 0.0331 (10) | 0.5020 (15) |
| H21A | 0.5071 | 1.2408 | 0.8328 | 0.050* | 0.5020 (15) |
| H21B | 0.7197 | 1.3375 | 0.8276 | 0.050* | 0.5020 (15) |
| H21C | 0.6731 | 1.2693 | 0.8865 | 0.050* | 0.5020 (15) |
| C22 | 0.1266 (10) | 0.5259 (6) | 0.8042 (3) | 0.0256 (14) | 0.5020 (15) |
| H22A | 0.0962 | 0.5164 | 0.8441 | 0.038* | 0.5020 (15) |
| H22B | 0.0684 | 0.4322 | 0.7792 | 0.038* | 0.5020 (15) |
| H22C | 0.0686 | 0.6163 | 0.7914 | 0.038* | 0.5020 (15) |
| C23 | 0.4510 (6) | 0.4075 (4) | 0.82033 (17) | 0.0281 (9) | 0.5020 (15) |
| H23A | 0.5970 | 0.4234 | 0.8172 | 0.042* | 0.5020 (15) |
| H23B | 0.3947 | 0.3125 | 0.7958 | 0.042* | 0.5020 (15) |
| H23C | 0.4243 | 0.3980 | 0.8605 | 0.042* | 0.5020 (15) |
| C24 | 0.6614 (6) | 0.9742 (4) | 1.03185 (16) | 0.0297 (9) | 0.5020 (15) |
| H24A | 0.6546 | 0.9616 | 1.0728 | 0.045* | 0.5020 (15) |
| H24B | 0.5775 | 1.0549 | 1.0221 | 0.045* | 0.5020 (15) |
| H24C | 0.8015 | 1.0042 | 1.0243 | 0.045* | 0.5020 (15) |
| C25 | 0.7232 (11) | 0.6981 (7) | 1.0074 (3) | 0.0307 (14) | 0.5020 (15) |
| H25A | 0.8594 | 0.7299 | 0.9969 | 0.046* | 0.5020 (15) |
| H25B | 0.6711 | 0.5996 | 0.9845 | 0.046* | 0.5020 (15) |
| H25C | 0.7278 | 0.6854 | 1.0485 | 0.046* | 0.5020 (15) |
| C11' | 0.5178 (9) | 0.8031 (6) | 0.8164 (2) | 0.0110 (13) | 0.4980 (15) |
| C16' | 0.3975 (9) | 0.6931 (8) | 0.8416 (3) | 0.0122 (15) | 0.4980 (15) |
| C12' | 0.7007 (5) | 0.8740 (4) | 0.84657 (14) | 0.0149 (7) | 0.4980 (15) |
| C13' | 0.7689 (5) | 0.8181 (4) | 0.89710 (15) | 0.0175 (8) | 0.4980 (15) |
| H13' | 0.8919 | 0.8650 | 0.9171 | 0.021* | 0.4980 (15) |
| C14' | 0.6617 (5) | 0.6952 (4) | 0.91930 (14) | 0.0165 (8) | 0.4980 (15) |
| C15' | 0.4741 (8) | 0.6404 (5) | 0.8922 (2) | 0.0129 (10) | 0.4980 (15) |
| H15' | 0.3936 | 0.5636 | 0.9086 | 0.015* | 0.4980 (15) |
| C17' | 0.8201 (5) | 1.0221 (4) | 0.83205 (14) | 0.0149 (7) | 0.4980 (15) |
| H17' | 0.7618 | 1.0467 | 0.7941 | 0.018* | 0.4980 (15) |
| C18' | 0.1755 (5) | 0.6347 (5) | 0.82125 (16) | 0.0201 (8) | 0.4980 (15) |
| H18' | 0.1335 | 0.6925 | 0.7887 | 0.024* | 0.4980 (15) |
| C19' | 0.7393 (6) | 0.6336 (5) | 0.97372 (18) | 0.0202 (8) | 0.4980 (15) |
| H19' | 0.6572 | 0.5330 | 0.9764 | 0.024* | 0.4980 (15) |
| C20' | 1.0444 (5) | 1.0021 (4) | 0.82672 (18) | 0.0213 (8) | 0.4980 (15) |
| H20D | 1.1134 | 1.0970 | 0.8155 | 0.032* | 0.4980 (15) |
| H20E | 1.0571 | 0.9148 | 0.7974 | 0.032* | 0.4980 (15) |
| H20F | 1.1056 | 0.9817 | 0.8639 | 0.032* | 0.4980 (15) |
| C21' | 0.7914 (6) | 1.1560 (4) | 0.87833 (16) | 0.0231 (8) | 0.4980 (15) |
| H21D | 0.8426 | 1.1322 | 0.9162 | 0.035* | 0.4980 (15) |
| H21E | 0.6476 | 1.1696 | 0.8790 | 0.035* | 0.4980 (15) |
| H21F | 0.8657 | 1.2516 | 0.8694 | 0.035* | 0.4980 (15) |
| C22' | 0.0387 (5) | 0.6696 (5) | 0.87050 (16) | 0.0266 (9) | 0.4980 (15) |
| H22D | −0.1029 | 0.6520 | 0.8552 | 0.040* | 0.4980 (15) |
| H22E | 0.0701 | 0.7778 | 0.8875 | 0.040* | 0.4980 (15) |
| H22F | 0.0618 | 0.6011 | 0.9001 | 0.040* | 0.4980 (15) |
| C23' | 0.1484 (11) | 0.4617 (6) | 0.7993 (3) | 0.0250 (13) | 0.4980 (15) |
| H23D | 0.1894 | 0.4026 | 0.8305 | 0.037* | 0.4980 (15) |
| H23E | 0.2321 | 0.4417 | 0.7669 | 0.037* | 0.4980 (15) |
| H23F | 0.0064 | 0.4298 | 0.7864 | 0.037* | 0.4980 (15) |
| C24' | 0.7064 (13) | 0.7460 (9) | 1.0273 (3) | 0.0349 (15) | 0.4980 (15) |
| H24D | 0.7516 | 0.7031 | 1.0622 | 0.052* | 0.4980 (15) |
| H24E | 0.5627 | 0.7599 | 1.0280 | 0.052* | 0.4980 (15) |
| H24F | 0.7840 | 0.8463 | 1.0257 | 0.052* | 0.4980 (15) |
| C25' | 0.9588 (6) | 0.6021 (5) | 0.97315 (18) | 0.0364 (11) | 0.4980 (15) |
| H25D | 0.9743 | 0.5244 | 0.9405 | 0.055* | 0.4980 (15) |
| H25E | 1.0009 | 0.5633 | 1.0092 | 0.055* | 0.4980 (15) |
| H25F | 1.0428 | 0.6982 | 0.9694 | 0.055* | 0.4980 (15) |
| H4D | 1.414 (3) | 0.946 (2) | 0.5910 (9) | 0.036 (7)* | |
| H3C | 1.081 (3) | 1.025 (3) | 0.6124 (11) | 0.048 (7)* | |
| H3D | 1.036 (4) | 0.937 (3) | 0.5591 (12) | 0.065 (9)* | |
| H5C | 1.110 (4) | 1.033 (3) | 0.7046 (10) | 0.049 (8)* | |
| H6D | 1.695 (4) | 0.853 (3) | 0.5332 (10) | 0.048 (7)* | |
| H6E | 1.794 (4) | 0.866 (3) | 0.4853 (11) | 0.056 (9)* | |
| H5D | 0.909 (4) | 1.010 (3) | 0.6956 (11) | 0.065 (9)* | |
| H4E | 1.565 (4) | 0.923 (3) | 0.6322 (14) | 0.094 (11)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0136 (2) | 0.0180 (2) | 0.0234 (2) | 0.00158 (17) | −0.00203 (18) | −0.00337 (18) |
| O1 | 0.0173 (6) | 0.0259 (7) | 0.0210 (7) | −0.0049 (5) | −0.0018 (5) | −0.0027 (5) |
| O2 | 0.0202 (7) | 0.0226 (7) | 0.0441 (9) | 0.0081 (6) | −0.0053 (6) | −0.0041 (6) |
| O3 | 0.0257 (8) | 0.0411 (9) | 0.0229 (8) | 0.0026 (7) | 0.0028 (7) | 0.0030 (7) |
| O4 | 0.0290 (9) | 0.0445 (9) | 0.0259 (8) | 0.0138 (7) | 0.0042 (7) | 0.0067 (7) |
| O5 | 0.0211 (7) | 0.0311 (8) | 0.0278 (8) | 0.0013 (6) | 0.0031 (6) | 0.0106 (6) |
| O6 | 0.0274 (8) | 0.0390 (9) | 0.0310 (9) | 0.0115 (7) | 0.0086 (7) | 0.0103 (7) |
| N1 | 0.0162 (7) | 0.0162 (7) | 0.0225 (8) | 0.0018 (6) | 0.0007 (6) | −0.0012 (6) |
| C1 | 0.0165 (9) | 0.0195 (9) | 0.0196 (9) | 0.0031 (7) | 0.0001 (7) | 0.0010 (7) |
| N2 | 0.0138 (7) | 0.0157 (7) | 0.0168 (8) | 0.0030 (6) | 0.0000 (6) | 0.0008 (6) |
| C2 | 0.0167 (9) | 0.0151 (9) | 0.0180 (9) | 0.0040 (7) | 0.0014 (7) | 0.0024 (7) |
| C3 | 0.0273 (10) | 0.0165 (9) | 0.0223 (10) | 0.0042 (8) | 0.0034 (8) | 0.0050 (7) |
| C4 | 0.0281 (11) | 0.0324 (11) | 0.0382 (12) | 0.0005 (9) | 0.0113 (9) | 0.0125 (9) |
| C5 | 0.0133 (9) | 0.0240 (10) | 0.0257 (10) | 0.0055 (7) | −0.0027 (8) | −0.0011 (8) |
| C6 | 0.0157 (9) | 0.0426 (12) | 0.0270 (11) | −0.0029 (9) | −0.0011 (8) | 0.0025 (9) |
| C7 | 0.0153 (9) | 0.0247 (10) | 0.0173 (9) | −0.0005 (7) | −0.0009 (7) | −0.0028 (7) |
| C8 | 0.0256 (10) | 0.0279 (10) | 0.0281 (11) | 0.0022 (8) | 0.0035 (9) | −0.0080 (9) |
| C11 | 0.0123 (16) | 0.0136 (16) | 0.0127 (16) | 0.0009 (9) | 0.0033 (9) | 0.0017 (9) |
| C12 | 0.0134 (17) | 0.0168 (19) | 0.0178 (19) | 0.0013 (15) | 0.0003 (14) | 0.0027 (15) |
| C13 | 0.0161 (17) | 0.0200 (18) | 0.0156 (18) | 0.0007 (14) | −0.0019 (14) | −0.0033 (14) |
| C14 | 0.0153 (17) | 0.0248 (19) | 0.0129 (17) | 0.0077 (14) | 0.0028 (14) | 0.0037 (15) |
| C15 | 0.020 (2) | 0.011 (2) | 0.025 (2) | 0.000 (2) | 0.0075 (17) | 0.008 (3) |
| C16 | 0.013 (3) | 0.020 (3) | 0.018 (3) | 0.003 (2) | 0.002 (2) | −0.0006 (19) |
| C17 | 0.0174 (19) | 0.0157 (19) | 0.0170 (19) | −0.0038 (15) | 0.0005 (15) | 0.0003 (15) |
| C18 | 0.0231 (19) | 0.0165 (18) | 0.0203 (19) | −0.0058 (15) | −0.0003 (15) | 0.0057 (15) |
| C19 | 0.0206 (19) | 0.030 (2) | 0.0155 (18) | 0.0016 (16) | 0.0041 (15) | 0.0043 (16) |
| C20 | 0.019 (2) | 0.038 (2) | 0.031 (2) | −0.0049 (19) | −0.0003 (17) | 0.0127 (19) |
| C21 | 0.036 (2) | 0.019 (2) | 0.046 (3) | 0.0010 (17) | 0.013 (2) | 0.0055 (18) |
| C22 | 0.023 (3) | 0.018 (4) | 0.035 (3) | −0.003 (3) | −0.009 (2) | 0.009 (3) |
| C23 | 0.038 (2) | 0.0172 (19) | 0.028 (2) | 0.0010 (17) | 0.0011 (18) | 0.0013 (16) |
| C24 | 0.039 (2) | 0.035 (2) | 0.0153 (19) | 0.0106 (19) | −0.0028 (17) | −0.0014 (17) |
| C25 | 0.045 (3) | 0.026 (4) | 0.022 (4) | 0.008 (3) | −0.006 (3) | 0.008 (3) |
| C11' | 0.0113 (15) | 0.0120 (16) | 0.0097 (15) | 0.0002 (9) | 0.0018 (9) | 0.0016 (9) |
| C16' | 0.0128 (18) | 0.0110 (16) | 0.0132 (17) | 0.0016 (10) | 0.0013 (10) | 0.0022 (9) |
| C12' | 0.0190 (18) | 0.0113 (18) | 0.0152 (18) | 0.0029 (15) | 0.0066 (14) | 0.0008 (14) |
| C13' | 0.0144 (17) | 0.0195 (18) | 0.0176 (18) | −0.0010 (14) | −0.0005 (14) | 0.0013 (14) |
| C14' | 0.0174 (18) | 0.0186 (18) | 0.0136 (17) | 0.0035 (14) | 0.0013 (14) | 0.0007 (14) |
| C15' | 0.013 (2) | 0.011 (2) | 0.014 (2) | −0.003 (2) | 0.0009 (15) | 0.005 (2) |
| C17' | 0.0159 (18) | 0.0177 (19) | 0.0104 (17) | −0.0023 (16) | −0.0014 (14) | 0.0040 (15) |
| C18' | 0.0189 (19) | 0.023 (2) | 0.0183 (19) | −0.0041 (16) | 0.0020 (15) | 0.0064 (17) |
| C19' | 0.023 (2) | 0.021 (2) | 0.017 (2) | 0.0015 (16) | −0.0015 (17) | 0.0060 (17) |
| C20' | 0.0162 (18) | 0.023 (2) | 0.024 (2) | −0.0024 (16) | 0.0063 (16) | 0.0035 (16) |
| C21' | 0.026 (2) | 0.0180 (18) | 0.025 (2) | −0.0012 (15) | 0.0074 (16) | 0.0033 (16) |
| C22' | 0.0156 (18) | 0.037 (2) | 0.028 (2) | 0.0002 (16) | 0.0021 (16) | 0.0072 (18) |
| C23' | 0.026 (3) | 0.022 (3) | 0.026 (3) | −0.004 (3) | 0.0007 (19) | 0.002 (3) |
| C24' | 0.045 (3) | 0.049 (5) | 0.014 (3) | 0.016 (3) | 0.006 (3) | 0.005 (3) |
| C25' | 0.034 (2) | 0.054 (3) | 0.027 (2) | 0.022 (2) | 0.0028 (19) | 0.018 (2) |
Geometric parameters (Å, º)
| S1—O1 | 1.4563 (12) | C19—C24 | 1.520 (5) |
| S1—O2 | 1.4574 (13) | C19—C25 | 1.529 (7) |
| S1—N1 | 1.5708 (14) | C20—H20A | 0.9800 |
| S1—C11 | 1.692 (6) | C20—H20B | 0.9800 |
| S1—C11' | 1.934 (6) | C20—H20C | 0.9800 |
| O3—H3C | 0.90 (2) | C21—H21A | 0.9800 |
| O3—H3D | 0.85 (3) | C21—H21B | 0.9800 |
| O4—H4D | 0.83 (2) | C21—H21C | 0.9800 |
| O4—H4E | 0.92 (3) | C22—H22A | 0.9800 |
| O5—H5C | 0.83 (3) | C22—H22B | 0.9800 |
| O5—H5D | 0.84 (3) | C22—H22C | 0.9800 |
| O6—H6D | 0.86 (2) | C23—H23A | 0.9800 |
| O6—H6E | 0.82 (3) | C23—H23B | 0.9800 |
| N1—C1 | 1.475 (2) | C23—H23C | 0.9800 |
| C1—H1A | 0.9900 | C24—H24A | 0.9800 |
| C1—H1B | 0.9900 | C24—H24B | 0.9800 |
| C1—C2 | 1.525 (2) | C24—H24C | 0.9800 |
| N2—C2 | 1.521 (2) | C25—H25A | 0.9800 |
| N2—C3 | 1.527 (2) | C25—H25B | 0.9800 |
| N2—C5 | 1.521 (2) | C25—H25C | 0.9800 |
| N2—C7 | 1.521 (2) | C11'—C16' | 1.399 (8) |
| C2—H2A | 0.9900 | C11'—C12' | 1.419 (7) |
| C2—H2B | 0.9900 | C16'—C15' | 1.403 (9) |
| C3—H3A | 0.9900 | C16'—C18' | 1.546 (7) |
| C3—H3B | 0.9900 | C12'—C13' | 1.396 (5) |
| C3—C4 | 1.508 (2) | C12'—C17' | 1.536 (5) |
| C4—H4A | 0.9800 | C13'—H13' | 0.9500 |
| C4—H4B | 0.9800 | C13'—C14' | 1.401 (5) |
| C4—H4C | 0.9800 | C14'—C15' | 1.383 (7) |
| C5—H5A | 0.9900 | C14'—C19' | 1.518 (5) |
| C5—H5B | 0.9900 | C15'—H15' | 0.9500 |
| C5—C6 | 1.510 (3) | C17'—H17' | 1.0000 |
| C6—H6A | 0.9800 | C17'—C20' | 1.536 (5) |
| C6—H6B | 0.9800 | C17'—C21' | 1.535 (5) |
| C6—H6C | 0.9800 | C18'—H18' | 1.0000 |
| C7—H7A | 0.9900 | C18'—C22' | 1.538 (5) |
| C7—H7B | 0.9900 | C18'—C23' | 1.529 (5) |
| C7—C8 | 1.514 (2) | C19'—H19' | 1.0000 |
| C8—H8A | 0.9800 | C19'—C24' | 1.542 (6) |
| C8—H8B | 0.9800 | C19'—C25' | 1.518 (5) |
| C8—H8C | 0.9800 | C20'—H20D | 0.9800 |
| C11—C12 | 1.422 (6) | C20'—H20E | 0.9800 |
| C11—C16 | 1.420 (8) | C20'—H20F | 0.9800 |
| C12—C13 | 1.390 (5) | C21'—H21D | 0.9800 |
| C12—C17 | 1.537 (5) | C21'—H21E | 0.9800 |
| C13—H13 | 0.9500 | C21'—H21F | 0.9800 |
| C13—C14 | 1.389 (5) | C22'—H22D | 0.9800 |
| C14—C15 | 1.390 (6) | C22'—H22E | 0.9800 |
| C14—C19 | 1.532 (5) | C22'—H22F | 0.9800 |
| C15—H15 | 0.9500 | C23'—H23D | 0.9800 |
| C15—C16 | 1.392 (9) | C23'—H23E | 0.9800 |
| C16—C18 | 1.526 (7) | C23'—H23F | 0.9800 |
| C17—H17 | 1.0000 | C24'—H24D | 0.9800 |
| C17—C20 | 1.529 (5) | C24'—H24E | 0.9800 |
| C17—C21 | 1.534 (5) | C24'—H24F | 0.9800 |
| C18—H18 | 1.0000 | C25'—H25D | 0.9800 |
| C18—C22 | 1.512 (7) | C25'—H25E | 0.9800 |
| C18—C23 | 1.530 (5) | C25'—H25F | 0.9800 |
| C19—H19 | 1.0000 | ||
| O1—S1—O2 | 113.94 (7) | C17—C20—H20C | 109.5 |
| O1—S1—N1 | 112.66 (7) | H20A—C20—H20B | 109.5 |
| O1—S1—C11 | 110.2 (2) | H20A—C20—H20C | 109.5 |
| O1—S1—C11' | 103.48 (18) | H20B—C20—H20C | 109.5 |
| O2—S1—N1 | 107.70 (8) | C17—C21—H21A | 109.5 |
| O2—S1—C11 | 104.22 (18) | C17—C21—H21B | 109.5 |
| O2—S1—C11' | 109.94 (16) | C17—C21—H21C | 109.5 |
| N1—S1—C11 | 107.6 (2) | H21A—C21—H21B | 109.5 |
| N1—S1—C11' | 108.99 (18) | H21A—C21—H21C | 109.5 |
| H3C—O3—H3D | 105 (2) | H21B—C21—H21C | 109.5 |
| H4D—O4—H4E | 105 (2) | C18—C22—H22A | 109.5 |
| H5C—O5—H5D | 107 (2) | C18—C22—H22B | 109.5 |
| H6D—O6—H6E | 107 (2) | C18—C22—H22C | 109.5 |
| C1—N1—S1 | 114.54 (11) | H22A—C22—H22B | 109.5 |
| N1—C1—H1A | 110.1 | H22A—C22—H22C | 109.5 |
| N1—C1—H1B | 110.1 | H22B—C22—H22C | 109.5 |
| N1—C1—C2 | 108.09 (14) | C18—C23—H23A | 109.5 |
| H1A—C1—H1B | 108.4 | C18—C23—H23B | 109.5 |
| C2—C1—H1A | 110.1 | C18—C23—H23C | 109.5 |
| C2—C1—H1B | 110.1 | H23A—C23—H23B | 109.5 |
| C2—N2—C3 | 111.46 (12) | H23A—C23—H23C | 109.5 |
| C2—N2—C7 | 106.21 (12) | H23B—C23—H23C | 109.5 |
| C5—N2—C2 | 110.78 (13) | C19—C24—H24A | 109.5 |
| C5—N2—C3 | 106.66 (13) | C19—C24—H24B | 109.5 |
| C5—N2—C7 | 110.95 (12) | C19—C24—H24C | 109.5 |
| C7—N2—C3 | 110.85 (13) | H24A—C24—H24B | 109.5 |
| C1—C2—H2A | 107.8 | H24A—C24—H24C | 109.5 |
| C1—C2—H2B | 107.8 | H24B—C24—H24C | 109.5 |
| N2—C2—C1 | 117.89 (14) | C19—C25—H25A | 109.5 |
| N2—C2—H2A | 107.8 | C19—C25—H25B | 109.5 |
| N2—C2—H2B | 107.8 | C19—C25—H25C | 109.5 |
| H2A—C2—H2B | 107.2 | H25A—C25—H25B | 109.5 |
| N2—C3—H3A | 108.5 | H25A—C25—H25C | 109.5 |
| N2—C3—H3B | 108.5 | H25B—C25—H25C | 109.5 |
| H3A—C3—H3B | 107.5 | C16'—C11'—S1 | 123.9 (5) |
| C4—C3—N2 | 115.21 (15) | C16'—C11'—C12' | 119.4 (5) |
| C4—C3—H3A | 108.5 | C12'—C11'—S1 | 116.3 (4) |
| C4—C3—H3B | 108.5 | C11'—C16'—C15' | 118.7 (6) |
| C3—C4—H4A | 109.5 | C11'—C16'—C18' | 124.9 (6) |
| C3—C4—H4B | 109.5 | C15'—C16'—C18' | 116.1 (5) |
| C3—C4—H4C | 109.5 | C11'—C12'—C17' | 124.8 (4) |
| H4A—C4—H4B | 109.5 | C13'—C12'—C11' | 118.9 (4) |
| H4A—C4—H4C | 109.5 | C13'—C12'—C17' | 115.8 (3) |
| H4B—C4—H4C | 109.5 | C12'—C13'—H13' | 118.9 |
| N2—C5—H5A | 108.6 | C12'—C13'—C14' | 122.1 (3) |
| N2—C5—H5B | 108.6 | C14'—C13'—H13' | 118.9 |
| H5A—C5—H5B | 107.6 | C13'—C14'—C19' | 121.6 (3) |
| C6—C5—N2 | 114.60 (14) | C15'—C14'—C13' | 117.1 (3) |
| C6—C5—H5A | 108.6 | C15'—C14'—C19' | 121.1 (3) |
| C6—C5—H5B | 108.6 | C16'—C15'—H15' | 118.5 |
| C5—C6—H6A | 109.5 | C14'—C15'—C16' | 122.9 (5) |
| C5—C6—H6B | 109.5 | C14'—C15'—H15' | 118.5 |
| C5—C6—H6C | 109.5 | C12'—C17'—H17' | 108.2 |
| H6A—C6—H6B | 109.5 | C12'—C17'—C20' | 112.1 (3) |
| H6A—C6—H6C | 109.5 | C20'—C17'—H17' | 108.2 |
| H6B—C6—H6C | 109.5 | C21'—C17'—C12' | 108.5 (3) |
| N2—C7—H7A | 108.5 | C21'—C17'—H17' | 108.2 |
| N2—C7—H7B | 108.5 | C21'—C17'—C20' | 111.6 (3) |
| H7A—C7—H7B | 107.5 | C16'—C18'—H18' | 108.1 |
| C8—C7—N2 | 115.17 (15) | C22'—C18'—C16' | 110.1 (4) |
| C8—C7—H7A | 108.5 | C22'—C18'—H18' | 108.1 |
| C8—C7—H7B | 108.5 | C23'—C18'—C16' | 112.1 (5) |
| C7—C8—H8A | 109.5 | C23'—C18'—H18' | 108.1 |
| C7—C8—H8B | 109.5 | C23'—C18'—C22' | 110.3 (4) |
| C7—C8—H8C | 109.5 | C14'—C19'—H19' | 107.6 |
| H8A—C8—H8B | 109.5 | C14'—C19'—C24' | 110.1 (4) |
| H8A—C8—H8C | 109.5 | C14'—C19'—C25' | 113.0 (3) |
| H8B—C8—H8C | 109.5 | C24'—C19'—H19' | 107.6 |
| C12—C11—S1 | 119.8 (4) | C25'—C19'—H19' | 107.6 |
| C16—C11—S1 | 121.0 (4) | C25'—C19'—C24' | 110.8 (5) |
| C16—C11—C12 | 118.9 (5) | C17'—C20'—H20D | 109.5 |
| C11—C12—C17 | 123.3 (4) | C17'—C20'—H20E | 109.5 |
| C13—C12—C11 | 119.7 (4) | C17'—C20'—H20F | 109.5 |
| C13—C12—C17 | 116.9 (3) | H20D—C20'—H20E | 109.5 |
| C12—C13—H13 | 118.7 | H20D—C20'—H20F | 109.5 |
| C14—C13—C12 | 122.5 (3) | H20E—C20'—H20F | 109.5 |
| C14—C13—H13 | 118.7 | C17'—C21'—H21D | 109.5 |
| C13—C14—C15 | 116.5 (4) | C17'—C21'—H21E | 109.5 |
| C13—C14—C19 | 123.6 (3) | C17'—C21'—H21F | 109.5 |
| C15—C14—C19 | 119.8 (4) | H21D—C21'—H21E | 109.5 |
| C14—C15—H15 | 117.8 | H21D—C21'—H21F | 109.5 |
| C14—C15—C16 | 124.5 (5) | H21E—C21'—H21F | 109.5 |
| C16—C15—H15 | 117.8 | C18'—C22'—H22D | 109.5 |
| C11—C16—C18 | 126.4 (5) | C18'—C22'—H22E | 109.5 |
| C15—C16—C11 | 117.6 (5) | C18'—C22'—H22F | 109.5 |
| C15—C16—C18 | 115.9 (5) | H22D—C22'—H22E | 109.5 |
| C12—C17—H17 | 108.0 | H22D—C22'—H22F | 109.5 |
| C20—C17—C12 | 109.2 (3) | H22E—C22'—H22F | 109.5 |
| C20—C17—H17 | 108.0 | C18'—C23'—H23D | 109.5 |
| C20—C17—C21 | 112.3 (3) | C18'—C23'—H23E | 109.5 |
| C21—C17—C12 | 111.3 (3) | C18'—C23'—H23F | 109.5 |
| C21—C17—H17 | 108.0 | H23D—C23'—H23E | 109.5 |
| C16—C18—H18 | 107.1 | H23D—C23'—H23F | 109.5 |
| C16—C18—C23 | 112.2 (4) | H23E—C23'—H23F | 109.5 |
| C22—C18—C16 | 111.4 (4) | C19'—C24'—H24D | 109.5 |
| C22—C18—H18 | 107.1 | C19'—C24'—H24E | 109.5 |
| C22—C18—C23 | 111.6 (3) | C19'—C24'—H24F | 109.5 |
| C23—C18—H18 | 107.1 | H24D—C24'—H24E | 109.5 |
| C14—C19—H19 | 107.6 | H24D—C24'—H24F | 109.5 |
| C24—C19—C14 | 114.7 (3) | H24E—C24'—H24F | 109.5 |
| C24—C19—H19 | 107.6 | C19'—C25'—H25D | 109.5 |
| C24—C19—C25 | 109.8 (4) | C19'—C25'—H25E | 109.5 |
| C25—C19—C14 | 109.3 (3) | C19'—C25'—H25F | 109.5 |
| C25—C19—H19 | 107.6 | H25D—C25'—H25E | 109.5 |
| C17—C20—H20A | 109.5 | H25D—C25'—H25F | 109.5 |
| C17—C20—H20B | 109.5 | H25E—C25'—H25F | 109.5 |
| S1—N1—C1—C2 | 94.86 (14) | C13—C12—C17—C20 | 60.6 (4) |
| S1—C11—C12—C13 | 170.0 (3) | C13—C12—C17—C21 | −63.9 (4) |
| S1—C11—C12—C17 | −13.0 (6) | C13—C14—C15—C16 | 0.0 (7) |
| S1—C11—C16—C15 | −168.3 (4) | C13—C14—C19—C24 | 14.0 (5) |
| S1—C11—C16—C18 | 13.7 (8) | C13—C14—C19—C25 | −109.8 (4) |
| S1—C11'—C16'—C15' | 163.2 (4) | C14—C15—C16—C11 | −3.9 (9) |
| S1—C11'—C16'—C18' | −22.9 (8) | C14—C15—C16—C18 | 174.2 (4) |
| S1—C11'—C12'—C13' | −164.4 (3) | C15—C14—C19—C24 | −170.1 (4) |
| S1—C11'—C12'—C17' | 23.3 (5) | C15—C14—C19—C25 | 66.1 (5) |
| O1—S1—N1—C1 | −50.19 (14) | C15—C16—C18—C22 | 75.9 (6) |
| O1—S1—C11—C12 | −166.8 (3) | C15—C16—C18—C23 | −50.0 (6) |
| O1—S1—C11—C16 | 7.5 (5) | C16—C11—C12—C13 | −4.3 (6) |
| O2—S1—N1—C1 | −176.69 (12) | C16—C11—C12—C17 | 172.6 (4) |
| O2—S1—C11—C12 | −44.1 (4) | C17—C12—C13—C14 | −176.8 (3) |
| O2—S1—C11—C16 | 130.1 (4) | C19—C14—C15—C16 | −176.3 (5) |
| N1—S1—C11—C12 | 70.0 (4) | C11'—S1—N1—C1 | 64.1 (2) |
| N1—S1—C11—C16 | −115.7 (5) | C11'—C16'—C15'—C14' | 1.7 (8) |
| N1—C1—C2—N2 | 179.67 (13) | C11'—C16'—C18'—C22' | −120.3 (6) |
| C2—N2—C3—C4 | 60.00 (19) | C11'—C16'—C18'—C23' | 116.5 (6) |
| C2—N2—C5—C6 | −62.17 (18) | C11'—C12'—C13'—C14' | −0.6 (5) |
| C2—N2—C7—C8 | 176.93 (14) | C11'—C12'—C17'—C20' | −129.7 (4) |
| C3—N2—C2—C1 | 61.08 (19) | C11'—C12'—C17'—C21' | 106.5 (4) |
| C3—N2—C5—C6 | 176.37 (15) | C16'—C11'—C12'—C13' | 8.1 (6) |
| C3—N2—C7—C8 | −61.84 (18) | C16'—C11'—C12'—C17' | −164.2 (4) |
| C5—N2—C2—C1 | −57.52 (18) | C12'—C11'—C16'—C15' | −8.7 (8) |
| C5—N2—C3—C4 | −178.96 (15) | C12'—C11'—C16'—C18' | 165.2 (5) |
| C5—N2—C7—C8 | 56.47 (19) | C12'—C13'—C14'—C15' | −6.2 (5) |
| C7—N2—C2—C1 | −178.09 (14) | C12'—C13'—C14'—C19' | 178.9 (3) |
| C7—N2—C3—C4 | −58.08 (19) | C13'—C12'—C17'—C20' | 57.7 (4) |
| C7—N2—C5—C6 | 55.54 (19) | C13'—C12'—C17'—C21' | −66.0 (4) |
| C11—S1—N1—C1 | 71.5 (2) | C13'—C14'—C15'—C16' | 5.6 (7) |
| C11—C12—C13—C14 | 0.3 (5) | C13'—C14'—C19'—C24' | 75.7 (5) |
| C11—C12—C17—C20 | −116.4 (4) | C13'—C14'—C19'—C25' | −48.8 (5) |
| C11—C12—C17—C21 | 119.1 (4) | C15'—C16'—C18'—C22' | 53.7 (6) |
| C11—C16—C18—C22 | −106.2 (6) | C15'—C16'—C18'—C23' | −69.5 (6) |
| C11—C16—C18—C23 | 127.9 (6) | C15'—C14'—C19'—C24' | −99.0 (5) |
| C12—C11—C16—C15 | 6.0 (8) | C15'—C14'—C19'—C25' | 136.5 (4) |
| C12—C11—C16—C18 | −172.0 (4) | C17'—C12'—C13'—C14' | 172.4 (3) |
| C12—C13—C14—C15 | 1.9 (5) | C18'—C16'—C15'—C14' | −172.7 (4) |
| C12—C13—C14—C19 | 178.0 (3) | C19'—C14'—C15'—C16' | −179.4 (5) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H4D···O3 | 0.83 (2) | 2.04 (2) | 2.867 (2) | 171 (2) |
| O3—H3C···O5 | 0.90 (2) | 1.83 (2) | 2.725 (2) | 174 (2) |
| O3—H3D···O6i | 0.85 (3) | 2.08 (3) | 2.912 (2) | 169 (2) |
| O5—H5C···O2ii | 0.83 (3) | 2.09 (3) | 2.901 (2) | 165 (2) |
| O6—H6D···O4 | 0.86 (2) | 1.95 (2) | 2.787 (2) | 167 (2) |
| O6—H6E···O3iii | 0.82 (3) | 2.03 (3) | 2.845 (2) | 170 (2) |
| O5—H5D···N1 | 0.84 (3) | 2.05 (3) | 2.881 (2) | 170 (2) |
| O4—H4E···N1ii | 0.92 (3) | 2.06 (3) | 2.959 (2) | 165 (3) |
Symmetry codes: (i) x−1, y, z; (ii) x+1, y, z; (iii) −x+3, −y+2, −z+1.
References
- Born, M., Mootz, D. & Schaefgen, S. (1995). Z. Naturforsch. Teil B, 50, 101–105.
- Buckley, B. R., Patel, A. P. & Wijayantha, K. G. U. (2013). J. Org. Chem. 78, 1289–1292. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Golz, C., Preut, H. & Strohmann, C. (2014). Acta Cryst. E70, o153. [DOI] [PMC free article] [PubMed]
- Gómez-Saiz, P., García-Tojal, J., Maestro, M. A., Arnaiz, F. J. & Rojo, T. (2002). Inorg. Chem. 41, 1345–1347. [DOI] [PubMed]
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Hu, X. E. (2003). Tetrahedron, 60, 2701–2743.
- Infantes, L., Chisholm, J. & Motherwell, S. (2003). CrystEngComm, 5, 480–486.
- Kostakis, G. E., Abbas, G., Anson, C. E. & Powell, A. K. (2009). CrystEngComm, 11, 82–86.
- Li, M., Chen, S., Xiang, J., He, H., Yuan, L. & Sun, J. (2006). Cryst. Growth Des. 6, 1250–1252.
- Li, F., Li, T.-H., Su, W., Gao, S.-Y. & Cao, R. (2006). Eur. J. Inorg. Chem. pp. 1582–1587.
- Oxford Diffraction (2013). CrysAlis PRO. Oxford Diffraction Ltd, Yarnton, England.
- Sandrock, P. B., Meyers, C. Y., Rath, N. P. & Robinson, P. D. (2004). Acta Cryst. E60, o544–o546.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Song, X.-Y., Li, L.-C., Liao, D.-Z., Jiang, Z.-H. & Yan, S.-P. (2007). Cryst. Growth Des. 7, 1220–1222.
- Vasiliev, A. D., Astachov, A. M., Kekin, Y. V., Kruglyakova, L. A. & Stepanov, R. S. (2001). Acta Cryst. C57, 1192–1193. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015008105/sj5448sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015008105/sj5448Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989015008105/sj5448Isup3.cml
CCDC reference: 1061352
Additional supporting information: crystallographic information; 3D view; checkCIF report