The CrIII atoms in the title compound show a distorted octahedral coordination with four N atoms of the cyclam ligand in the equatorial plane and two N-coordinated NCS− groups in axial positions. The macrocyclic ligands adopt trans-III configurations. The crystal packing is stabilized by N—H⋯S and N—H⋯Cl hydrogen bonds.
Keywords: crystal structure, synchrotron radiation, cyclam, thiocyanate ligand, trans-III configuration, chromium(III) complex, hydrogen bonding
Abstract
The structure of the title compound, [Cr(NCS)2(cyclam)]2[ZnCl4] (cyclam = 1,4,8,11-tetraazacyclotetradecane, C10H24N4), has been determined from synchrotron data. The asymmetric unit contains two independent halves of the CrIII complex cations and half of a tetrachloridozincate anion. In each complex cation, the CrIII atom is coordinated by the four N atoms of the cyclam ligand in the equatorial plane and by two N-bound NCS− anions in a trans axial arrangement, displaying a distorted octahedral geometry with crystallographic inversion symmetry. The mean Cr—N(cyclam) and Cr—N(NCS) bond lengths are 2.065 (4) and 1.995 (6) Å, respectively. The macrocyclic cyclam moieties adopt centrosymmetric trans-III configurations with six- and five-membered chelate rings in chair and gauche configurations, respectively. The [ZnCl4]2− anion, which lies about a twofold rotation axis, has a slightly distorted tetrahedral geometry. The crystal packing is stabilized by hydrogen-bonding interactions between the N—H groups of the cyclam ligands, the S atoms of the NCS− groups and the Cl− ligands of the anion.
Chemical context
In recent years, it has been found that cyclam (1,4,8,11-tetraazacyclotetradecane, C10H24N4) derivatives and their metal complexes exhibit anti-HIV activity (Ronconi & Sadler, 2007 ▸; De Clercq, 2010 ▸; Ross et al., 2012 ▸). The cyclam derivatives inhibit the entry of the virus into white cells by binding to CXCR4, a chemokine receptor in the outer membrane. The strength of binding to the CXCR4 receptor correlates with the anti-HIV activity. The cyclam ligand has a moderately flexible structure, and can adopt both planar (trans) and folded (cis) configurations (Poon & Pun, 1980 ▸). There are five configurational trans isomers for this type of macrocycle, Fig. 1 ▸, that differ in the chirality of the sec-NH groups (Choi, 2009 ▸). The trans-V configuration can also fold to form the cis-V isomer (Subhan et al., 2011 ▸). In addition, the thiocyanate anion can be present in complexes as either a ligand or a non-coordinating anion (Moon et al., 2013 ▸). Furthermore it can coordinate to metals as a terminal ligand through either the nitrogen or the sulfur atoms, or can use both donor atoms and function as a bridging ligand.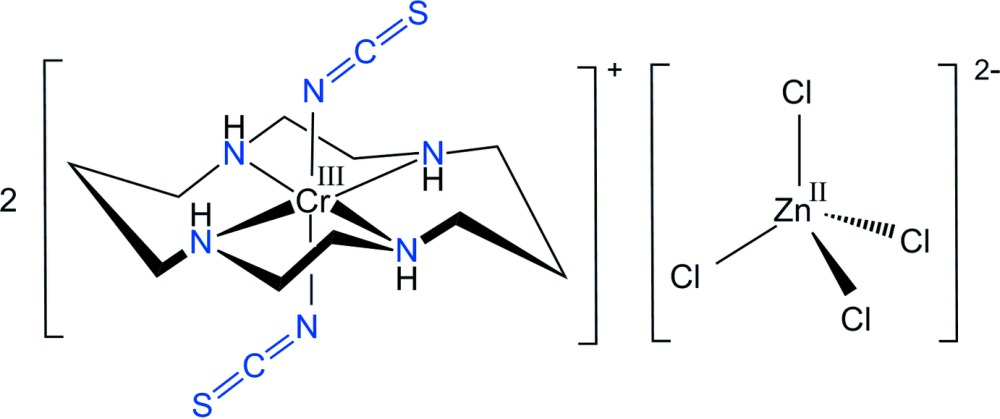
Figure 1.
Possible configurations for trans-cyclam complexes with the trans-III configuration adopted by the title compound highlighted in blue.
Counter-anionic species play a very important role in the coordination chemistry, pharmacy and biology (Fabbrizzi & Poggi, 2013 ▸) of metal complexes. Thus, we describe here the synthesis and structural characterization of trans-[Cr(NCS)2(cyclam)]2[ZnCl4], (I).
Structural commentary
Each of the two trans-[Cr(NCS)2(cyclam)]+ cations in the structure of the title compound are generated by inversion symmetry, hence the configurations of the cyclam ligands can be described as trans-III, Fig. 1 ▸. The CrIII cations, which are located on discrete inversion centres, are coordinated by the nitrogen atoms of the cyclam ligands that occupy equatorial sites. Two thiocyanate anions complete the distorted octahedral coordination sphere binding through their N atoms in a trans configuration. The single [ZnCl4]2− anion, which lies about a twofold rotation axis, has slightly distorted tetrahedral geometry and completes the complex salt. Fig. 2 ▸ shows an ellipsoid plot of (I), with the atom-numbering scheme. This is a second example of the structure of a trans-[Cr(NCS)]2(cyclam)]+ salt, but the previous example had a perchlorate counter-anion (Friesen et al., 1997 ▸).
Figure 2.
A perspective view (30% probability ellipsoids) of the two independent chromium(III) complex cations and the tetrachloridozincate anion in (I). [Symmetry codes: (A′) x − 1, y, z; (B′) x, −y, z +  ; (C′) −x + 1, −y + 1, −z + 2.]
; (C′) −x + 1, −y + 1, −z + 2.]
The Cr—N bond lengths from the donor atoms of the cyclam ligand range from 2.0614 (10) to 2.0700 (10) Å, and these lengths are comparable to those found in a range of related [CrL 2(cyclam)]+ complexes (Flores-Velez et al., 1991 ▸; Friesen et al., 1997 ▸; Choi, 2009 ▸; Choi, Oh, Suzuki et al., 2004 ▸; Subhan et al., 2011 ▸; Choi, Oh, Lim et al., 2004 ▸). However, they are shorter than the bonds to a primary amine as found in the related complex trans-[CrCl2(Me2tn)2]2[ZnCl4] (Me2tn = [2,2-dimethylpropane-1,3-diamine]; Choi et al., 2011 ▸). Furthermore, the mean Cr—N(NCS) distance of 1.9951 (11) Å is close the values found in other trans/cis-[Cr(NCS)2N4]+ cations (Moon & Choi, 2015 ▸; Choi & Lee, 2009 ▸; Moon et al., 2013 ▸). As is normally found with cyclam complexes, the five-membered chelate rings adopt gauche configurations while the six-membered rings are in chair configurations. The average bite angles of the five- and six-membered chelate rings around the chromium(III) atoms are 85.51 (4) and 94.49 (4)°, respectively. The N-coordinated NCS ligands are almost linear, with N—C—S angles of 177.42 (12)° in cation A and 178.66 (12)° in cation B. The C6A—S1A bond length [1.6126 (12) Å] in the Cr1A complex cation is slightly longer than the C6B–-S1B bond length [1.6056 (12) Å] in the Cr2B complex cation. This elongation may be attributed to the weak hydrogen bond formed by S1A with the N2A—H2A group of the cyclam ligand.
Supramolecular features
Each complex molecule forms three classical N—H⋯Cl hydrogen bonds between the amine groups of the cyclam ligand in each complex cation and the Cl atoms of the tetrachloridozincate anion, Table 1 ▸ (Steed & Atwood, 2009 ▸). These hydrogen bonds link the cations and anions into a three-dimensional network as shown in Fig. 3 ▸ and help to stabilize the crystal structure.
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| N1AH1ACl2C i | 0.98 | 2.66 | 3.4510(12) | 138 |
| N2AH2AS1A ii | 0.98 | 2.60 | 3.4884(13) | 151 |
| N1BH1BCl1C i | 0.98 | 2.58 | 3.4120(12) | 143 |
| N2BH2BCl1C iii | 0.98 | 2.57 | 3.3944(13) | 142 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Figure 3.
The molecular packing in (I), viewed along the a axis. Dashed lines represent hydrogen-bonding interactions N—H⋯Cl (cyan) and N—H⋯S (purple), respectively. H atoms bound to C have been omitted.
Database survey
A search of the Cambridge Structural Database (Version 5.36, last update February 2015; Groom & Allen, 2014 ▸) gave only three hits for the [Cr(NCS)2(cyclam)]+ cation. Of these structures, trans-[Cr(NCS)2(cyclam)](ClO4) (Friesen et al., 1997 ▸) adopts the trans-III configuration, similar to that adopted by the title compound, while cis-[Cr(NCS)2(cyclam)](ClO4) (Friesen et al., 1997 ▸) and cis-[Cr(NCS)2(cyclam)](NCS) (Moon et al., 2013 ▸), both adopt the folded cis-V configuration. No structure of a salt of [Cr(NCS)2(cyclam)]+ with the [ZnCl4]2− anion was found.
Synthesis and crystallization
The free ligand cyclam was purchased from Strem Chemicals and used as provided. All chemicals were reagent-grade materials and were used without further purification. The starting material, trans-[Cr(NCS)2(cyclam)]ClO4, was prepared according to the literature (Friesen et al., 1997 ▸). The perchlorate salt (0.33 g) was dissolved in 10 mL of 0.1 M HCl at 333 K and added to 7.5 mL of 6 M HCl containing 0.75 g of solid ZnCl2. The resulting solution was filtered, and allowed to stand at room temperature for two days to give pale-yellow crystals of (I) suitable for X-ray structural analysis.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.97 Å and N—H = 0.98 Å, and with U iso(H) values of 1.2U eq of the parent atoms.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Cr(NCS)2(C10H24N4)]2[ZnCl4] |
| M r | 944.15 |
| Crystal system, space group | Monoclinic, P2/c |
| Temperature (K) | 260 |
| a, b, c () | 7.9990(16), 16.532(3), 15.430(3) |
| () | 101.36(3) |
| V (3) | 2000.5(7) |
| Z | 2 |
| Radiation type | Synchrotron, = 0.610 |
| (mm1) | 1.07 |
| Crystal size (mm) | 0.22 0.19 0.12 |
| Data collection | |
| Diffractometer | ADSC Q210 CCD area detector diffractometer |
| Absorption correction | Empirical (using intensity measurements) (HKL3000sm SCALEPACK; Otwinowski Minor, 1997 ▸) |
| T min, T max | 0.801, 0.883 |
| No. of measured, independent and observed [I > 2(I)] reflections | 20982, 5738, 5500 |
| R int | 0.018 |
| (sin /)max (1) | 0.706 |
| Refinement | |
| R[F 2 > 2(F 2)], wR(F 2), S | 0.025, 0.072, 1.06 |
| No. of reflections | 5738 |
| No. of parameters | 217 |
| H-atom treatment | H-atom parameters constrained |
| max, min (e 3) | 0.60, 0.58 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698901500746X/sj5452sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901500746X/sj5452Isup2.hkl
CCDC reference: 1059896
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The X-ray crystallography experiment at the PLS-II BL2D-SMC beamline was supported in part by MSIP and POSTECH.
supplementary crystallographic information
Crystal data
| [Cr(NCS)2(C10H24N4)]2[ZnCl4] | F(000) = 972 |
| Mr = 944.15 | Dx = 1.567 Mg m−3 |
| Monoclinic, P2/c | Synchrotron radiation, λ = 0.610 Å |
| a = 7.9990 (16) Å | Cell parameters from 92486 reflections |
| b = 16.532 (3) Å | θ = 0.4–33.7° |
| c = 15.430 (3) Å | µ = 1.07 mm−1 |
| β = 101.36 (3)° | T = 260 K |
| V = 2000.5 (7) Å3 | Block, pale yellow |
| Z = 2 | 0.22 × 0.19 × 0.12 mm |
Data collection
| ADSC Q210 CCD area-detector diffractometer | 5500 reflections with I > 2σ(I) |
| Radiation source: PLSII 2D bending magnet | Rint = 0.018 |
| ω scan | θmax = 25.5°, θmin = 2.4° |
| Absorption correction: empirical (using intensity measurements) (HKL3000smSCALEPACK; Otwinowski & Minor, 1997) | h = −11→11 |
| Tmin = 0.801, Tmax = 0.883 | k = −23→23 |
| 20982 measured reflections | l = −21→21 |
| 5738 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.025 | w = 1/[σ2(Fo2) + (0.0404P)2 + 0.6258P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.072 | (Δ/σ)max = 0.001 |
| S = 1.06 | Δρmax = 0.60 e Å−3 |
| 5738 reflections | Δρmin = −0.58 e Å−3 |
| 217 parameters | Extinction correction: SHELXL2014/7 (Sheldrick 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.013 (2) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cr1A | 0.0000 | 0.0000 | 0.5000 | 0.01897 (7) | |
| S1A | 0.26852 (5) | 0.12583 (3) | 0.28897 (3) | 0.04579 (10) | |
| N1A | 0.02449 (13) | 0.11498 (5) | 0.55355 (7) | 0.02520 (18) | |
| H1A | −0.0189 | 0.1129 | 0.6086 | 0.030* | |
| N2A | 0.22186 (12) | −0.04305 (6) | 0.57760 (7) | 0.02639 (18) | |
| H2A | 0.1956 | −0.0559 | 0.6355 | 0.032* | |
| N3A | 0.12441 (13) | 0.03450 (6) | 0.40583 (7) | 0.0289 (2) | |
| C1A | −0.09496 (17) | 0.16709 (7) | 0.49075 (9) | 0.0327 (2) | |
| H1A1 | −0.0461 | 0.1798 | 0.4396 | 0.039* | |
| H1A2 | −0.1155 | 0.2174 | 0.5193 | 0.039* | |
| C2A | 0.20038 (17) | 0.14817 (7) | 0.57588 (9) | 0.0340 (3) | |
| H2A1 | 0.1976 | 0.2001 | 0.6049 | 0.041* | |
| H2A2 | 0.2421 | 0.1571 | 0.5217 | 0.041* | |
| C3A | 0.32286 (17) | 0.09229 (9) | 0.63584 (10) | 0.0386 (3) | |
| H3A1 | 0.4276 | 0.1217 | 0.6577 | 0.046* | |
| H3A2 | 0.2733 | 0.0786 | 0.6865 | 0.046* | |
| C4A | 0.36743 (16) | 0.01431 (9) | 0.59357 (10) | 0.0359 (3) | |
| H4A1 | 0.4006 | 0.0267 | 0.5378 | 0.043* | |
| H4A2 | 0.4639 | −0.0110 | 0.6319 | 0.043* | |
| C5A | 0.26019 (17) | −0.12134 (7) | 0.53750 (9) | 0.0334 (2) | |
| H5A1 | 0.3391 | −0.1528 | 0.5803 | 0.040* | |
| H5A2 | 0.3122 | −0.1113 | 0.4868 | 0.040* | |
| C6A | 0.18232 (14) | 0.07189 (7) | 0.35542 (8) | 0.0263 (2) | |
| Cr2B | 0.5000 | 0.5000 | 1.0000 | 0.01881 (7) | |
| S1B | 0.26292 (6) | 0.31238 (2) | 1.17097 (3) | 0.04295 (10) | |
| N1B | 0.33254 (12) | 0.45724 (6) | 0.89068 (6) | 0.02683 (18) | |
| H1B | 0.2412 | 0.4284 | 0.9118 | 0.032* | |
| N2B | 0.35373 (12) | 0.60051 (6) | 1.01461 (7) | 0.02692 (19) | |
| H2B | 0.2655 | 0.5822 | 1.0461 | 0.032* | |
| N3B | 0.37356 (14) | 0.43650 (7) | 1.07593 (7) | 0.0310 (2) | |
| C1B | 0.43163 (18) | 0.39638 (8) | 0.85091 (8) | 0.0351 (3) | |
| H1B1 | 0.5087 | 0.4235 | 0.8191 | 0.042* | |
| H1B2 | 0.3548 | 0.3627 | 0.8095 | 0.042* | |
| C2B | 0.25141 (18) | 0.51949 (9) | 0.82674 (9) | 0.0377 (3) | |
| H2B1 | 0.1736 | 0.4934 | 0.7787 | 0.045* | |
| H2B2 | 0.3385 | 0.5468 | 0.8020 | 0.045* | |
| C3B | 0.15434 (18) | 0.58129 (10) | 0.87041 (10) | 0.0429 (3) | |
| H3B1 | 0.0815 | 0.5525 | 0.9034 | 0.051* | |
| H3B2 | 0.0809 | 0.6119 | 0.8244 | 0.051* | |
| C4B | 0.26361 (18) | 0.64063 (8) | 0.93259 (10) | 0.0379 (3) | |
| H4B1 | 0.3467 | 0.6650 | 0.9026 | 0.045* | |
| H4B2 | 0.1917 | 0.6834 | 0.9479 | 0.045* | |
| C5B | 0.46800 (18) | 0.65534 (8) | 1.07613 (9) | 0.0353 (3) | |
| H5B1 | 0.4012 | 0.6952 | 1.1006 | 0.042* | |
| H5B2 | 0.5447 | 0.6835 | 1.0450 | 0.042* | |
| C6B | 0.32530 (14) | 0.38445 (7) | 1.11521 (8) | 0.0262 (2) | |
| Zn1C | 1.0000 | 0.28259 (2) | 0.7500 | 0.02829 (7) | |
| Cl1C | 0.94451 (4) | 0.36436 (2) | 0.86092 (2) | 0.03394 (8) | |
| Cl2C | 0.77981 (5) | 0.20141 (2) | 0.68975 (3) | 0.04336 (9) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cr1A | 0.02262 (11) | 0.01669 (11) | 0.01964 (12) | −0.00101 (7) | 0.00917 (8) | 0.00082 (7) |
| S1A | 0.0498 (2) | 0.0544 (2) | 0.03621 (19) | −0.01306 (16) | 0.01597 (15) | 0.01604 (15) |
| N1A | 0.0311 (4) | 0.0197 (4) | 0.0267 (4) | −0.0016 (3) | 0.0104 (4) | −0.0017 (3) |
| N2A | 0.0272 (4) | 0.0272 (4) | 0.0255 (5) | 0.0023 (3) | 0.0069 (3) | −0.0002 (3) |
| N3A | 0.0344 (5) | 0.0289 (4) | 0.0269 (5) | −0.0025 (4) | 0.0142 (4) | 0.0021 (4) |
| C1A | 0.0423 (6) | 0.0199 (5) | 0.0370 (6) | 0.0043 (4) | 0.0105 (5) | 0.0026 (4) |
| C2A | 0.0372 (6) | 0.0261 (5) | 0.0404 (7) | −0.0106 (4) | 0.0122 (5) | −0.0077 (5) |
| C3A | 0.0330 (6) | 0.0408 (7) | 0.0402 (7) | −0.0073 (5) | 0.0030 (5) | −0.0122 (5) |
| C4A | 0.0248 (5) | 0.0400 (6) | 0.0426 (7) | −0.0012 (5) | 0.0059 (5) | −0.0065 (5) |
| C5A | 0.0346 (6) | 0.0278 (5) | 0.0381 (6) | 0.0093 (4) | 0.0079 (5) | −0.0004 (4) |
| C6A | 0.0269 (5) | 0.0287 (5) | 0.0246 (5) | 0.0001 (4) | 0.0080 (4) | 0.0015 (4) |
| Cr2B | 0.02030 (11) | 0.02070 (11) | 0.01588 (12) | 0.00103 (7) | 0.00471 (8) | 0.00120 (7) |
| S1B | 0.0606 (2) | 0.02721 (15) | 0.0445 (2) | −0.01031 (14) | 0.01873 (16) | 0.00437 (13) |
| N1B | 0.0273 (4) | 0.0307 (5) | 0.0210 (4) | −0.0015 (3) | 0.0011 (3) | −0.0001 (3) |
| N2B | 0.0268 (4) | 0.0263 (4) | 0.0282 (5) | 0.0053 (3) | 0.0067 (3) | −0.0006 (3) |
| N3B | 0.0345 (5) | 0.0326 (5) | 0.0276 (5) | −0.0021 (4) | 0.0106 (4) | 0.0039 (4) |
| C1B | 0.0422 (6) | 0.0376 (6) | 0.0252 (6) | −0.0019 (5) | 0.0058 (5) | −0.0106 (5) |
| C2B | 0.0404 (7) | 0.0427 (7) | 0.0243 (6) | 0.0018 (5) | −0.0070 (5) | 0.0040 (5) |
| C3B | 0.0340 (6) | 0.0483 (8) | 0.0407 (7) | 0.0109 (6) | −0.0067 (5) | 0.0067 (6) |
| C4B | 0.0388 (6) | 0.0322 (6) | 0.0404 (7) | 0.0132 (5) | 0.0024 (5) | 0.0062 (5) |
| C5B | 0.0401 (6) | 0.0274 (5) | 0.0390 (7) | 0.0029 (5) | 0.0087 (5) | −0.0086 (5) |
| C6B | 0.0292 (5) | 0.0258 (5) | 0.0244 (5) | −0.0010 (4) | 0.0073 (4) | −0.0035 (4) |
| Zn1C | 0.03449 (11) | 0.02582 (10) | 0.02644 (11) | 0.000 | 0.01061 (7) | 0.000 |
| Cl1C | 0.03853 (15) | 0.03419 (15) | 0.03262 (15) | −0.00012 (11) | 0.01562 (12) | −0.00580 (10) |
| Cl2C | 0.05010 (19) | 0.04102 (17) | 0.04059 (19) | −0.01715 (14) | 0.01290 (14) | −0.00567 (13) |
Geometric parameters (Å, º)
| Cr1A—N3Ai | 1.9991 (11) | Cr2B—N1Bii | 2.0614 (11) |
| Cr1A—N3A | 1.9991 (11) | Cr2B—N1B | 2.0614 (11) |
| Cr1A—N2A | 2.0622 (11) | Cr2B—N2Bii | 2.0700 (10) |
| Cr1A—N2Ai | 2.0623 (11) | Cr2B—N2B | 2.0700 (10) |
| Cr1A—N1Ai | 2.0664 (10) | S1B—C6B | 1.6056 (12) |
| Cr1A—N1A | 2.0665 (10) | N1B—C2B | 1.4836 (16) |
| S1A—C6A | 1.6126 (12) | N1B—C1B | 1.4861 (16) |
| N1A—C2A | 1.4861 (16) | N1B—H1B | 0.9800 |
| N1A—C1A | 1.4931 (16) | N2B—C4B | 1.4838 (17) |
| N1A—H1A | 0.9800 | N2B—C5B | 1.4887 (17) |
| N2A—C4A | 1.4842 (16) | N2B—H2B | 0.9800 |
| N2A—C5A | 1.4921 (15) | N3B—C6B | 1.1614 (16) |
| N2A—H2A | 0.9800 | C1B—C5Bii | 1.512 (2) |
| N3A—C6A | 1.1591 (15) | C1B—H1B1 | 0.9700 |
| C1A—C5Ai | 1.5106 (19) | C1B—H1B2 | 0.9700 |
| C1A—H1A1 | 0.9700 | C2B—C3B | 1.519 (2) |
| C1A—H1A2 | 0.9700 | C2B—H2B1 | 0.9700 |
| C2A—C3A | 1.521 (2) | C2B—H2B2 | 0.9700 |
| C2A—H2A1 | 0.9700 | C3B—C4B | 1.522 (2) |
| C2A—H2A2 | 0.9700 | C3B—H3B1 | 0.9700 |
| C3A—C4A | 1.5186 (19) | C3B—H3B2 | 0.9700 |
| C3A—H3A1 | 0.9700 | C4B—H4B1 | 0.9700 |
| C3A—H3A2 | 0.9700 | C4B—H4B2 | 0.9700 |
| C4A—H4A1 | 0.9700 | C5B—C1Bii | 1.512 (2) |
| C4A—H4A2 | 0.9700 | C5B—H5B1 | 0.9700 |
| C5A—C1Ai | 1.5107 (19) | C5B—H5B2 | 0.9700 |
| C5A—H5A1 | 0.9700 | Zn1C—Cl2Ciii | 2.2632 (6) |
| C5A—H5A2 | 0.9700 | Zn1C—Cl2C | 2.2632 (6) |
| Cr2B—N3Bii | 1.9911 (11) | Zn1C—Cl1Ciii | 2.2919 (5) |
| Cr2B—N3B | 1.9911 (11) | Zn1C—Cl1C | 2.2919 (5) |
| N3Ai—Cr1A—N3A | 180.0 | N3Bii—Cr2B—N1B | 91.31 (5) |
| N3Ai—Cr1A—N2A | 88.52 (4) | N3B—Cr2B—N1B | 88.69 (5) |
| N3A—Cr1A—N2A | 91.48 (4) | N1Bii—Cr2B—N1B | 180.0 |
| N3Ai—Cr1A—N2Ai | 91.48 (5) | N3Bii—Cr2B—N2Bii | 89.75 (5) |
| N3A—Cr1A—N2Ai | 88.52 (4) | N3B—Cr2B—N2Bii | 90.25 (5) |
| N2A—Cr1A—N2Ai | 180.0 | N1Bii—Cr2B—N2Bii | 94.26 (4) |
| N3Ai—Cr1A—N1Ai | 90.38 (4) | N1B—Cr2B—N2Bii | 85.74 (4) |
| N3A—Cr1A—N1Ai | 89.62 (4) | N3Bii—Cr2B—N2B | 90.25 (5) |
| N2A—Cr1A—N1Ai | 85.28 (4) | N3B—Cr2B—N2B | 89.75 (5) |
| N2Ai—Cr1A—N1Ai | 94.72 (4) | N1Bii—Cr2B—N2B | 85.74 (4) |
| N3Ai—Cr1A—N1A | 89.62 (4) | N1B—Cr2B—N2B | 94.26 (4) |
| N3A—Cr1A—N1A | 90.38 (4) | N2Bii—Cr2B—N2B | 180.0 |
| N2A—Cr1A—N1A | 94.72 (4) | C2B—N1B—C1B | 113.21 (10) |
| N2Ai—Cr1A—N1A | 85.28 (4) | C2B—N1B—Cr2B | 115.78 (8) |
| N1Ai—Cr1A—N1A | 180.0 | C1B—N1B—Cr2B | 104.87 (7) |
| C2A—N1A—C1A | 113.14 (10) | C2B—N1B—H1B | 107.5 |
| C2A—N1A—Cr1A | 116.31 (7) | C1B—N1B—H1B | 107.5 |
| C1A—N1A—Cr1A | 105.85 (7) | Cr2B—N1B—H1B | 107.5 |
| C2A—N1A—H1A | 107.0 | C4B—N2B—C5B | 113.94 (10) |
| C1A—N1A—H1A | 107.0 | C4B—N2B—Cr2B | 117.13 (8) |
| Cr1A—N1A—H1A | 107.0 | C5B—N2B—Cr2B | 105.60 (7) |
| C4A—N2A—C5A | 113.86 (10) | C4B—N2B—H2B | 106.5 |
| C4A—N2A—Cr1A | 115.67 (8) | C5B—N2B—H2B | 106.5 |
| C5A—N2A—Cr1A | 106.39 (8) | Cr2B—N2B—H2B | 106.5 |
| C4A—N2A—H2A | 106.8 | C6B—N3B—Cr2B | 163.19 (10) |
| C5A—N2A—H2A | 106.8 | N1B—C1B—C5Bii | 108.88 (10) |
| Cr1A—N2A—H2A | 106.8 | N1B—C1B—H1B1 | 109.9 |
| C6A—N3A—Cr1A | 164.12 (10) | C5Bii—C1B—H1B1 | 109.9 |
| N1A—C1A—C5Ai | 108.06 (10) | N1B—C1B—H1B2 | 109.9 |
| N1A—C1A—H1A1 | 110.1 | C5Bii—C1B—H1B2 | 109.9 |
| C5Ai—C1A—H1A1 | 110.1 | H1B1—C1B—H1B2 | 108.3 |
| N1A—C1A—H1A2 | 110.1 | N1B—C2B—C3B | 111.50 (11) |
| C5Ai—C1A—H1A2 | 110.1 | N1B—C2B—H2B1 | 109.3 |
| H1A1—C1A—H1A2 | 108.4 | C3B—C2B—H2B1 | 109.3 |
| N1A—C2A—C3A | 112.57 (10) | N1B—C2B—H2B2 | 109.3 |
| N1A—C2A—H2A1 | 109.1 | C3B—C2B—H2B2 | 109.3 |
| C3A—C2A—H2A1 | 109.1 | H2B1—C2B—H2B2 | 108.0 |
| N1A—C2A—H2A2 | 109.1 | C2B—C3B—C4B | 115.66 (12) |
| C3A—C2A—H2A2 | 109.1 | C2B—C3B—H3B1 | 108.4 |
| H2A1—C2A—H2A2 | 107.8 | C4B—C3B—H3B1 | 108.4 |
| C4A—C3A—C2A | 115.59 (12) | C2B—C3B—H3B2 | 108.4 |
| C4A—C3A—H3A1 | 108.4 | C4B—C3B—H3B2 | 108.4 |
| C2A—C3A—H3A1 | 108.4 | H3B1—C3B—H3B2 | 107.4 |
| C4A—C3A—H3A2 | 108.4 | N2B—C4B—C3B | 111.82 (11) |
| C2A—C3A—H3A2 | 108.4 | N2B—C4B—H4B1 | 109.3 |
| H3A1—C3A—H3A2 | 107.4 | C3B—C4B—H4B1 | 109.3 |
| N2A—C4A—C3A | 111.76 (10) | N2B—C4B—H4B2 | 109.3 |
| N2A—C4A—H4A1 | 109.3 | C3B—C4B—H4B2 | 109.3 |
| C3A—C4A—H4A1 | 109.3 | H4B1—C4B—H4B2 | 107.9 |
| N2A—C4A—H4A2 | 109.3 | N2B—C5B—C1Bii | 107.44 (10) |
| C3A—C4A—H4A2 | 109.3 | N2B—C5B—H5B1 | 110.2 |
| H4A1—C4A—H4A2 | 107.9 | C1Bii—C5B—H5B1 | 110.2 |
| N2A—C5A—C1Ai | 108.33 (10) | N2B—C5B—H5B2 | 110.2 |
| N2A—C5A—H5A1 | 110.0 | C1Bii—C5B—H5B2 | 110.2 |
| C1Ai—C5A—H5A1 | 110.0 | H5B1—C5B—H5B2 | 108.5 |
| N2A—C5A—H5A2 | 110.0 | N3B—C6B—S1B | 178.66 (12) |
| C1Ai—C5A—H5A2 | 110.0 | Cl2Ciii—Zn1C—Cl2C | 107.27 (3) |
| H5A1—C5A—H5A2 | 108.4 | Cl2Ciii—Zn1C—Cl1Ciii | 114.02 (2) |
| N3A—C6A—S1A | 177.42 (12) | Cl2C—Zn1C—Cl1Ciii | 107.01 (2) |
| N3Bii—Cr2B—N3B | 180.00 (5) | Cl2Ciii—Zn1C—Cl1C | 107.01 (2) |
| N3Bii—Cr2B—N1Bii | 88.69 (5) | Cl2C—Zn1C—Cl1C | 114.02 (2) |
| N3B—Cr2B—N1Bii | 91.31 (4) | Cl1Ciii—Zn1C—Cl1C | 107.71 (2) |
| C2A—N1A—C1A—C5Ai | 171.46 (10) | C2B—N1B—C1B—C5Bii | 170.70 (11) |
| Cr1A—N1A—C1A—C5Ai | 42.96 (11) | Cr2B—N1B—C1B—C5Bii | 43.59 (12) |
| C1A—N1A—C2A—C3A | −176.86 (10) | C1B—N1B—C2B—C3B | −178.93 (11) |
| Cr1A—N1A—C2A—C3A | −53.99 (13) | Cr2B—N1B—C2B—C3B | −57.79 (14) |
| N1A—C2A—C3A—C4A | 69.83 (15) | N1B—C2B—C3B—C4B | 72.48 (16) |
| C5A—N2A—C4A—C3A | −179.00 (11) | C5B—N2B—C4B—C3B | 177.65 (11) |
| Cr1A—N2A—C4A—C3A | 57.31 (14) | Cr2B—N2B—C4B—C3B | 53.73 (14) |
| C2A—C3A—C4A—N2A | −71.67 (16) | C2B—C3B—C4B—N2B | −69.85 (17) |
| C4A—N2A—C5A—C1Ai | −169.58 (11) | C4B—N2B—C5B—C1Bii | −172.02 (11) |
| Cr1A—N2A—C5A—C1Ai | −40.99 (11) | Cr2B—N2B—C5B—C1Bii | −42.08 (11) |
Symmetry codes: (i) −x, −y, −z+1; (ii) −x+1, −y+1, −z+2; (iii) −x+2, y, −z+3/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1A—H1A···Cl2Civ | 0.98 | 2.66 | 3.4510 (12) | 138 |
| N2A—H2A···S1Av | 0.98 | 2.60 | 3.4884 (13) | 151 |
| N1B—H1B···Cl1Civ | 0.98 | 2.58 | 3.4120 (12) | 143 |
| N2B—H2B···Cl1Cii | 0.98 | 2.57 | 3.3944 (13) | 142 |
Symmetry codes: (ii) −x+1, −y+1, −z+2; (iv) x−1, y, z; (v) x, −y, z+1/2.
References
- Arvai, A. J. & Nielsen, C. (1983). ADSC Quantum-210 ADX. Area Detector System Corporation, Poway, CA, USA.
- Choi, J.-H. (2009). Inorg. Chim. Acta, 362, 4231–4236.
- Choi, J.-H., Joshi, T. & Spiccia, L. (2011). Z. Anorg. Allg. Chem. 637, 1194–1198.
- Choi, J.-H. & Lee, S. H. (2009). J. Mol. Struct. 932, 84–89.
- Choi, J.-H., Oh, I.-G., Lim, W.-T. & Park, K.-M. (2004). Acta Cryst. C60, m238–m240. [DOI] [PubMed]
- Choi, J.-H., Oh, I.-G., Suzuki, T. & Kaizaki, S. (2004). J. Mol. Struct. 694, 39–44.
- De Clercq, E. (2010). J. Med. Chem. 53, 1438–1450. [DOI] [PubMed]
- Fabbrizzi, L. & Poggi, A. (2013). Chem. Soc. Rev. 42, 1681–1699. [DOI] [PubMed]
- Flores-Velez, L. M., Sosa-Rivadeneyra, J., Sosa-Torres, M. E., Rosales-Hoz, M. J. & Toscanoh, R. A. (1991). J. Chem. Soc. Dalton Trans. pp. 3243–3247.
- Friesen, D. A., Quail, J. W., Waltz, W. L. & Nashiem, R. E. (1997). Acta Cryst. C53, 687–691.
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Moon, D. & Choi, J.-H. (2015). Acta Cryst. E71, 100–103. [DOI] [PMC free article] [PubMed]
- Moon, D., Choi, J.-H., Ryoo, K. S. & Hong, Y. P. (2013). Acta Cryst. E69, m376–m377. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Poon, C. K. & Pun, K. C. (1980). Inorg. Chem. 19, 568–569.
- Putz, H. & Brandenburg, K. (2014). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Ronconi, L. & Sadler, P. J. (2007). Coord. Chem. Rev. 251, 1633–1648.
- Ross, A., Choi, J.-H., Hunter, T. M., Pannecouque, C., Moggach, S. A., Parsons, S., De Clercq, E. & Sadler, P. J. (2012). Dalton Trans. 41, 6408–6418. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Steed, J. W. & Atwood, J. L. (2009). Supramolecular Chemistry, 2nd ed., New York: John Wiley & Sons.
- Subhan, M. A., Choi, J.-H. & Ng, S. W. (2011). Z. Anorg. Allg. Chem. 637, 2193–2197.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698901500746X/sj5452sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901500746X/sj5452Isup2.hkl
CCDC reference: 1059896
Additional supporting information: crystallographic information; 3D view; checkCIF report





