Abstract
Object
Management of unruptured arteriovenous malformations is controversial. In the first randomized trial of unruptured AVMs (ARUBA), medically managed had a significantly lower risk of death or stroke and better outcomes. The University of California, San Francisco (UCSF) was one of the participating ARUBA sites. While 473 patients were screened for eligibility, only 4 patients were enrolled in ARUBA. The purpose of this study is to report the treatment and outcomes of all ARUBA eligible patients at UCSF.
Methods
We compared the treatment and outcomes of ARUBA eligible patients using prospectively collected data from the UCSF brain AVM (BAVM) registry. Similar to ARUBA, we compared the rate of stroke or death in observed and treated patients and used the modified Rankin scale to grade outcomes
Results
61 of 74 patients received an intervention and 13 patients were observed. Most treated patients had a surgical resection with or without preoperative embolization (43/61; 70.5%). One observed patient died from AVM hemorrhage (1/13). Nine treated patients had a stroke or died (9/61). There was no significant difference in the rate of stroke or death (HR 1.34 95% C.I. 0.12–14.53 p=0.807) or clinical impairment (Fisher’s exact p=0.68) between observed and treated patients.
Conclusions
The risk of stroke or death and degree of clinical impairment among treated patients was lower than reported in ARUBA. We found no significant difference in outcomes between observed and treated ARUBA eligible patients at the University of California, San Francisco. Results in ARUBA-eligible patients managed outside that trial lead to an entirely different conclusion about AVM intervention, due to the primary role of surgery, judicious surgical selection with established outcome predictors, and technical expertise developed at high-volume AVM centers.
Keywords: arteriovenous malformation, ARUBA trial, observation, microsurgical resection
Introduction
Hemorrhage is the most common presentation of brain arteriovenous malformations (AVMs), yet a large number of AVMs are now discovered incidentally. The management of unruptured AVMs is controversial, as the risk of treatment-associated morbidity and mortality must be weighed against the risk of spontaneous hemorrhage.21,22 While the overall risk of AVM hemorrhage is estimated between 2–4% per year, patients with unruptured AVMs may have a lower risk of spontaneous hemorrhage.1–3,6,7,16,19,23
Mohr et al recently published the first randomized trial of unruptured AVMs (ARUBA: NCT00389181) to better understand their natural history and associated treatment risks.13 They compared 109 patients assigned to medical management alone (pharmacological therapy for existing medical disorders or any coexisting vascular risk factors) to 114 patients assigned to medical management with interventional therapy, consisting of embolization, radiosurgery, microsurgical resection or a combination. After 33 months of follow-up, 30.7% of patients in the intervention arm had a stroke or died, compared to only 10.1% of patients in the medical management arm. In addition, 46.2% in the intervention arm were clinically impaired, defined as a modified Rankin score of 2 or higher, compared to 15.1% in the medical management arm. Thus, unruptured AVM patients in the medical management group had a significantly lower risk of death or stroke and better outcomes than patients in the treatment group.
However, the ARUBA trial faced many difficulties with patient recruitment and was stopped early by the Data Safety and Monitoring Board, which has led some to question the generalizability of the trial results.4,10,18 Only 226 of 1740 screened patients (13%) were randomized. 323 patients refused to participate, while clinicians selected treatment outside of the randomization process for 177 patients. Additionally, although 68% of patients randomized to intervention had low-grade AVMs (76/112), only 18 patients had surgery, despite evidence from observational studies that microsurgical resection of low-grade AVMs is safe and curative.5,14 Instead, most AVMs were treated with embolization or radiosurgery. Both have lower obliteration rates than microsurgical resection.8,12,15,17,20 Thus, the higher rate of stroke or death and clinical impairment in ARUBA’s interventional therapy arm reflects treatment-associated effects, but also complications from partially treated AVMs. Finally, ARUBA’s relatively short follow-up of 33 months favors medical management since curative effects would take longer for the any treatment group, and differences observed between the two arms might dissipate over time.
The purpose of this study is to report the treatment and outcomes of ARUBA eligible patients at the University of California, San Francisco (UCSF), one of the participating ARUBA sites.
Methods
Patients
We used the same inclusion/exclusion criteria as in the ARUBA trial with some additional exclusions as described below.13 During ARUBA’s enrollment period, from April 4, 2007 to April 15, 2013, 473 patients with AVMs were screened for enrollment at UCSF. Patients aged 18 years or older with an unruptured AVM diagnosed by catheter angiography, magnetic resonance imaging (MRI) or MR angiography and computed tomography (CT) angiography were eligible. Patients with evidence of previous hemorrhage, prior treatment or AVMs unsuitable for treatment were excluded. Patients with baseline clinical impairment (defined as a modified Rankin score of 2 or higher) were also excluded. 4 of 87 eligible patients (4.6%) were enrolled in ARUBA.
Of the 87 patients eligible for ARUBA during the enrollment period, three patients were treated elsewhere and were excluded from our analysis. 10 patients with follow-up less than 30 days were also excluded. Five of these patients underwent uncomplicated resections of their AVMs and were discharged home in good condition, but did not follow-up postoperatively at UCSF. Five patients did not return after their initial screening evaluation. The remaining 74 patients were included in our analysis.
All patients were enrolled prospectively in the registry of the UCSF BAVM Study Project. Patient baseline characteristics included age, sex, clinical presentation (seizure, headache or other) and modified Rankin scale score (0 or 1). Nidus size (diameter in cm), venous drainage (superficial only or any deep), and eloquence were determined from preoperative angiograms, CT scans and MRI scans for each AVM. All AVMs were graded using the Spetzler-Martin (SM) scale and the SM-Supplemented scale.9,11
Patients undergoing interventions were treated with microsurgical resection alone, microsurgical resection with preoperative embolization, embolization alone, gamma knife radiosurgery, or a combination. All AVM resections were performed by a single senior neurosurgeon (MTL). AVM obliteration was documented by catheter angiography.
To maintain consistency with ARUBA’s analysis, we analyzed a composite of stroke or death from any cause. We also used the modified Rankin scale to grade outcomes. As in ARUBA, a modified Rankin score of 2 or higher was defined as clinical impairment. A trained study coordinator, under the supervision of a neurologist, performed assessments at presentation, preoperatively, postoperatively at clinic visits, and annually up to 2 years postoperatively in treated patients. Observed patients were assessed at presentation and annually. Follow-up information was obtained during routine clinic visits or telephone interviews.
Statistics
We used Fisher’s exact tests for categorical variables and t tests for continuous variables to evaluate differences in baseline characteristics between observed and treated patients, including sex, age, clinical presentation, modified Rankin score, location, eloquence, venous drainage, size, SM score, and SM-Supplemented score.
We compared the rate of stroke or death in observed and treated patients using Kaplan-Meier survival analysis and the log-rank test. Cox proportional-hazards regression models were performed to estimate hazard ratios, adjusting for SM grade, location, venous drainage pattern, age and time from diagnosis. We compared the proportion of patients with a modified Rankin Scale score of 2 or higher at last follow-up in observed and treated patients with Fisher’s exact test.
All p values reported are 2-sided and regarded as statistically significant if p<0.05. All statistical analysis was performed using STATA 13.1 software.
Results
There were no significant differences in baseline characteristics, including sex, clinical presentation, modified Rankin scale score, location, eloquence, venous drainage, size or Spetzler-Martin score (Table 1). However, observed patients were significantly older than treated patients (59 versus 41 years, p<0.001). Observed patients in our cohort were also significantly older than patients randomized to medical management alone in ARUBA (59 versus 44 years, p<0.001). There were no other significant differences between ARUBA-eligible patients in our cohort and randomized ARUBA patients, including sex, clinical presentation, modified Rankin score, Spetzler-Martin-grade, eloquence and venous drainage pattern.
Table 1.
Baseline characteristics of ARUBA-eligible patients with unruptured AVMs*
| Characteristic | Observation (n=13)† | Treatment (n=61) ‡ | P-value |
|---|---|---|---|
| Sex | |||
| Male | 8 (62%) | 34 (56%) | 0.77 |
| Female | 5 (38%) | 27 (44%) | |
| Mean Age (years) | |||
| Clinical Presentation | 59 | 41 | <0.001 |
| Seizure | 4 (31%) | 28 (46%) | 0.50 |
| Headache | 4 (31%) | 19 (31%) | |
| Other | 5 (38%) | 14 (23%) | |
| mRS score | |||
| 0 | 10 (77%) | 33 (55%) | 0.22 |
| 1 | 3 (23%) | 27 (45%) | |
| Location | |||
| Cortical | 8 (67%) | 47 (80%) | 0.45 |
| Subcortical | 3 (25%) | 19 (18%) | |
| Posterior fossa | 1 (8%) | 1 (2%) | |
| Eloquence | |||
| No | 4 (33%) | 24 (39%) | 0.76 |
| Yes | 8 (67%) | 37 (61%) | |
| Deep venous drainage | |||
| No | 7 (58%) | 42 (69%) | 0.51 |
| Yes | 5 (42%) | 19 (31%) | |
| Size | |||
| <3 cm | 5 (42%) | 25 (41%) | >0.99 |
| 3–6 cm | 7 (58%) | 32 (52%) | |
| >6 cm | 0 | 4 (7%) | |
| Mean Size, cm | |||
| Spetzler-Martin grade | 3.3 | 3.4 | 0.79 |
| I | 1 (8%) | 9 (15%) | 0.71 |
| II | 5 (42%) | 21 (34%) | |
| III | 3 (25%) | 21 (34%) | |
| IV | 3 (25%) | 7 (12%) | |
| V | 0 | 3 (5%) | |
Values represent numbers of patients (%) unless otherwise indicated. Fisher’s exact test was used for categorical variables. Two-sample t test was used for continuous variables. Boldface indicates statistical significance.
The mRS score, Spetzler-Martin grade, and data on location, eloquence, deep venous drainage, and size are missing for 1 observed patient.
The mRS score and location data are missing for 1 treated patient.
61 patients in our cohort had an intervention, while 13 patients were observed. Patients undergoing interventions were treated with surgical resection alone (20/61), surgical resection with pre-operative embolization (23/61), gamma knife radiosurgery (15/61), embolization (1/61) or a combination (2/61) (Table 2).
Table 2.
Treatments of 61 patients with unruptured AVMs
| Treatment | No. of Patients (%) |
|---|---|
| Surgical resection alone | 20 (33) |
| Surgical resection with preoperative embolization | 23 (38) |
| Radiosurgery alone | 15 (25) |
| Embolization alone | 1 (2) |
| Combination | 2 (3) |
Observed patients had a longer mean length of follow-up than treated patients (30 months versus 21 months, p=0.12).
Patients with Spetzler-Martin grade 1 and 2 AVMs or SM-Supplemented grade 4 and 5 AVMs were generally treated with microsurgical resection (27/36 and 16/19 respectively). 24 patients had Spetzler-Martin grade 3 AVMs. Three were observed, 13 had microsurgical resection and eight were treated with radiosurgery. Patients with higher grade AVMs were more likely to be treated with radiosurgery or multi-modality therapy. Only three patients with Spetzler-Martin grade 4 or 5 AVMs had a surgical resection (3/13). Two patients with a SM-Supplemented score higher than 7 had a surgical resection (2/6).
A total of 10 patients had a stroke or died during follow-up period. One observed patient (1/13; 7.7%) had a stroke or died, compared to nine treated patients (9/61; 14.7%). Five of 43 surgical patients had a stroke or died (11.6%) compared to four of 15 patients treated with radiosurgery (26.7%). There was no significant difference in the rate of stroke or death in observed and treated patients (Figure 1; HR 1.34 95% C.I. 0.12–14.53 p=0.807).
Figure 1.
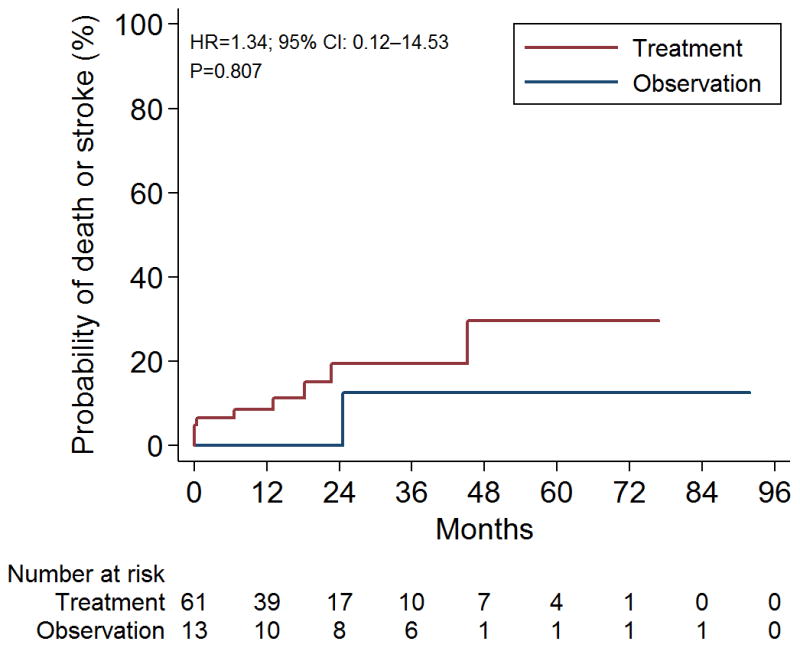
Kaplan-Meier survival estimates for ARUBA eligible patients. Log-rank χ2 0.91 p=0.35. Hazard ratio (HR) 1.34 95% C.I. 0.12–14.53 p=0.807
There were four deaths in the cohort. One observed patient died after AVM hemorrhage (1/13; 7.7%), while three treated patients died (3/61; 4.9%). A patient treated with gamma knife radiosurgery died after AVM hemorrhage. Two patients died from unknown causes, including a patient treated with gamma knife radiosurgery and a patient with an AVM completely obliterated by surgical resection.
There was no significant difference in functional outcome between observed and treated patients. Nine treated patients (9/58; 15.5%) were clinically impaired at last follow up with a modified Rankin score of 2 or higher, compared to one observed patient (1/13; 7.7%) (p=0.68) (Table 3). Six surgical patients were clinically impaired with a modified Rankin score of 2 or higher, but only 2 surgical patients were dead or dependent (modified Rankin score 3 or higher) (2/41; 4.8%) compared to one observed patient (1/13; 7.7%). Three radiosurgery patients were clinically impaired (3/15; 20%), of which two were dead and one had a modified Rankin score of 2.
Table 3.
Proportion of patients who had a stroke or died or had clinical impairment.
| Variable | Observation | Intervention* | p-value† |
|---|---|---|---|
| No. of patients in group | 13 | 61 | |
| No. of patients who had a stroke or died | 1 (7.7%) | 9 (14.8%) | 0.68 |
| No. of patients with mRS score ≥ 2 | 1 (7.7%) | 8 (13.8%) | >0.99 |
The mRS scores at last follow-up were available for 58 of 61 treated patients.
Fisher’s exact test.
Complete AVM obliteration was documented by catheter angiography in 93.0% of cases after surgical resection. Four patients had incompletely resected AVMs. Complete AVM obliteration was documented in four of 14 patients treated with gamma knife radiosurgery (28.6%).
Discussion
While our ARUBA-eligible patients are similar to ARUBA’s patients, their treatment and outcomes are not. Most patients in our cohort had a surgical resection with or without preoperative embolization (43/61; 70.5%, as compared to 18% in the ARUBA trial). Fewer patients were treated with gamma knife radiosurgery (15/61; 24.6%), embolization (1/61; 1.6%) or a combination (2/61; 3.3%). 13 patients were observed.
High-grade AVMs in surgically inaccessible locations were treated with gamma knife radiosurgery or a combination of gamma knife radiosurgery, embolization, and surgery. 86.7% of AVMs treated with gamma knife radiosurgery were high-grade (13/15), while 62.7% of microsurgically resected AVMs were grade I or II (27/43). Given its low cure rate compared to surgical resection and radiosurgery, embolization alone was not offered as a primary therapy. The one patient in this series was embolized adjunctively in preparation for surgery, but then declined surgery after embolization.
Our ARUBA-eligible treated patients were less likely to have a stroke or die than ARUBA patients (14.7% versus 30.7%) and were less likely to be clinically impaired (15.5% versus 46.2%), likely reflecting differences in treatment. Few patients (18%) in ARUBA were treated with surgery, and instead, most were treated with embolization and/or radiosurgery. In contrast, most patients in our cohort had surgery (43/61), while patients with higher grade, surgically inaccessible AVMs received radiosurgery (15/61). Radiosurgery patients had the highest rate of stroke or death (26.7%) and clinical impairment (20%), while fewer surgical patients had a stroke or death (11.6%) or were clinically impaired (14.6%). The one patient who died after surgery had a completely resected AVM and died from unknown causes. There was no significant difference in the rate of stroke or death or clinical impairment between observed and treated patients, which is the critical finding of this analysis because of the 3-fold difference reported in the ARUBA trial. This important finding demonstrates that differences in overall management strategy and surgical expertise between our cohort and the ARUBA trial lead to an entirely different conclusion about AVM intervention.
Stroke or death from any cause and the modified Rankin score used to define clinical impairment may overestimate morbidity. Five of 10 patients who reached the primary outcome in our study had a good neurologic outcome. Of the six patients who had a stroke and survived, one patient had a modified Rankin score of 2, while the other five reported no significant disability (modified Rankin scores of 1) at last follow up. Only 5 treated patients were dead or dependent (modified Rankin score of 3 or higher) at last follow-up (5/60; 8.3%), compared to one observed patient (1/13; 7.7%). Surgical patients had a lower rate of death or dependency than observed patients (4.8% versus 7.7%).
93.0% (39/43) of surgically treated cases in our cohort resulted in AVM obliteration, eliminating the risk of future hemorrhage. While ARUBA did not report obliteration rates, embolization and radiosurgery are known to have lower cure rates and patients with partially treated AVMs remain at risk for hemorrhage. AVM hemorrhage resulting in death occurred in two patients in our study, including one observed patient and one patient treated with gamma knife radiosurgery, illustrating the risk of hemorrhage in both untreated patients and partially treated patients.
The main limitation of our study is the small sample size, particularly in the observed group. Thus, our study is underpowered to detect statistically significant differences between observed and treated patients. A strength of our study is the prospective assessment of ARUBA eligibility and outcomes done independently of treating physicians and the relatively large sample of treated patients from our referral institution that may be more generalizable to other US centers.
Conclusions
Treatment of unruptured AVMs remains controversial, despite evidence from ARUBA suggesting observed patients have better clinical outcomes. Few screened patients were randomized and few patients randomized to intervention had a surgical resection, which is widely considered the gold standard of treatment, particularly for patients with low-grade AVMs. Our treated ARUBA-eligible patients had better outcomes than reported in ARUBA. We found no significant difference in the rate of stroke or death or degree of clinical impairment in observed and treated ARUBA-eligible patients at the University of California, San Francisco, although the wide 95% confidence intervals reflect the estimate uncertainty. The 3-fold increase in stroke and death observed in patients treated in ARUBA, relative to observed patients, was not observed in our cohort, leading to an entirely different conclusion about AVM intervention. This difference was due to utilizing surgery as the primary therapy, selecting surgical patients judiciously with established outcome predictors, and developing surgical expertise through high AVM case volume. While there is a role for observation in patients with unruptured AVMs, longer follow-up and outcomes by treatment type may also reveal low-grade patients who would benefit most from microsurgical resection.
Footnotes
Conflicts of Interest: Authors associated with this submission have no financial conflicts of interest to disclose.
References
- 1.ApSimon HT, Reef H, Phadke RV, Popovic EA. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33:2794–2800. doi: 10.1161/01.str.0000043674.99741.9b. [DOI] [PubMed] [Google Scholar]
- 2.Brown RD, Jr, Wiebers DO, Forbes G, O’Fallon WM, Piepgras DG, Marsh WR, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352–357. doi: 10.3171/jns.1988.68.3.0352. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Mast H, Sciacca RR, Hartmann A, Khaw AV, Mohr JP, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke. 2006;37:1243–1247. doi: 10.1161/01.STR.0000217970.18319.7d. [DOI] [PubMed] [Google Scholar]
- 4.Cockroft KM, Jayaraman MV, Amin-Hanjani S, Derdeyn CP, McDougall CG, Wilson JA. A perfect storm: how a randomized trial of unruptured brain arteriovenous malformations’ (ARUBA’s) trial design challenges notions of external validity. Stroke. 2012;43:1979–1981. doi: 10.1161/STROKEAHA.112.652032. [DOI] [PubMed] [Google Scholar]
- 5.Davidson AS, Morgan MK. How safe is arteriovenous malformation surgery? A prospective, observational study of surgery as first-line treatment for brain arteriovenous malformations. Neurosurgery. 2010;66:498–504. doi: 10.1227/01.NEU.0000365518.47684.98. discussion 504–495. [DOI] [PubMed] [Google Scholar]
- 6.Fults D, Kelly DL., Jr Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658–662. doi: 10.1227/00006123-198411000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Halim AX, Johnston SC, Singh V, McCulloch CE, Bennett JP, Achrol AS, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–1702. doi: 10.1161/01.STR.0000130988.44824.29. [DOI] [PubMed] [Google Scholar]
- 8.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589–597. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Pourmohamad T, Westbroek EM, McCulloch CE, Lawton MT, Young WL. Evaluating performance of the spetzler-martin supplemented model in selecting patients with brain arteriovenous malformation for surgery. Stroke. 2012;43:2497–2499. doi: 10.1161/STROKEAHA.112.661942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knopman J, Stieg PE. Management of unruptured brain arteriovenous malformations. Lancet. 2014;383:581–583. doi: 10.1016/S0140-6736(14)60001-5. [DOI] [PubMed] [Google Scholar]
- 11.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66:702–713. doi: 10.1227/01.NEU.0000367555.16733.E1. discussion 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunsford LD, Kondziolka D, Flickinger JC, Bissonette DJ, Jungreis CA, Maitz AH, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75:512–524. doi: 10.3171/jns.1991.75.4.0512. [DOI] [PubMed] [Google Scholar]
- 13.Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan MK, Rochford AM, Tsahtsarlis A, Little N, Faulder KC. Surgical risks associated with the management of Grade I and II brain arteriovenous malformations. Neurosurgery. 2004;54:832–837. doi: 10.1227/01.neu.0000114264.78966.be. discussion 837–839. [DOI] [PubMed] [Google Scholar]
- 15.Mounayer C, Hammami N, Piotin M, Spelle L, Benndorf G, Kessler I, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518–523. [PMC free article] [PubMed] [Google Scholar]
- 16.Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 17.Pierot L, Cognard C, Herbreteau D, Fransen H, van Rooij WJ, Boccardi E, et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO) Eur Radiol. 2013;23:2838–2845. doi: 10.1007/s00330-013-2870-6. [DOI] [PubMed] [Google Scholar]
- 18.Russin J, Spetzler R. Commentary: The ARUBA Trial. Neurosurgery. 2014 doi: 10.1227/NEU.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 19.Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–1355. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 20.Steiner L, Lindquist C, Adler JR, Torner JC, Alves W, Steiner M. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1–8. doi: 10.3171/jns.1992.77.1.0001. [DOI] [PubMed] [Google Scholar]
- 21.van Beijnum J, Lovelock CE, Cordonnier C, Rothwell PM, Klijn CJ, Al-Shahi Salman R. Outcome after spontaneous and arteriovenous malformation-related intracerebral haemorrhage: population-based studies. Brain. 2009;132:537–543. doi: 10.1093/brain/awn318. [DOI] [PubMed] [Google Scholar]
- 22.van Beijnum J, van der Worp HB, Buis DR, Al-Shahi Salman R, Kappelle LJ, Rinkel GJ, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. Jama. 2011;306:2011–2019. doi: 10.1001/jama.2011.1632. [DOI] [PubMed] [Google Scholar]
- 23.Wedderburn CJ, van Beijnum J, Bhattacharya JJ, Counsell CE, Papanastassiou V, Ritchie V, et al. Outcome after interventional or conservative management of unruptured brain arteriovenous malformations: a prospective, population-based cohort study. Lancet Neurol. 2008;7:223–230. doi: 10.1016/S1474-4422(08)70026-7. [DOI] [PubMed] [Google Scholar]


