Abstract
The cross-linking Mass Spectrometry (XL-MS) technique extracts structural information from protein complexes without requiring highly purified samples, crystallinity, or large amounts of material. However, there are challenges to applying the technique to protein complexes in vitro, and those challenges become more daunting with in vivo experiments. Issues include effective detection and identification of cross-linked peptides from complex mixtures. While MS-cleavable cross-linkers facilitate the sequencing and identification of cross-linked peptides, enrichable cross-linkers increase their detectability by allowing their separation from non-cross-linked peptides prior to MS analysis. Although a number of cross-linkers with single functionality have been developed in recent years, an ideal reagent would incorporate both capabilities for XL-MS studies. Therefore, two new cross-linkers have been designed and prepared that incorporate an azide (azide-A-DSBSO) or alkyne (alkyne-A-DSBSO) to enable affinity purification strategies based on click chemistry. The integration of an acid cleavage site next to the enrichment handle allows easy recovery of cross-linked products during affinity purification. In addition, these sulfoxide containing cross-linking reagents possess robust MS-cleavable bonds to facilitate fast and easy identification of cross-linked peptides using MS analysis. Optimized, gram-scale syntheses of these cross-linkers have been developed and the azide-A-DSBSO cross-linker has been evaluated with peptides and proteins to demonstrate its utility in XL-MS analysis.
Keywords: proteomics, sulfoxide, lysine-reactive, cross-linker, NHS ester, CID-cleavable
Most proteins act in association with other proteins to form protein complexes stably or transiently in cells, and mapping these interactions is essential to understand their cellular functions. Protein complexes represent functional entities that are often difficult to analyze using conventional structural tools due to their heterogeneous and dynamic nature. Recently, cross-linking Mass Spectrometry (XL-MS) has been recognized as a valuable tool for the structural analysis of protein assemblies,1 which can be used alone and in combination with other techniques.1,2 In addition to in vitro studies, XL-MS approaches have been extended to capture protein interactions in living cells.3 Identification of cross-linked peptides by MS analysis can provide distance constraints to assist computational modeling and yield structural information at amino acid resolution.4 The advantages of cross-linking studies include small sample size, robust tolerance for size and environment of the protein complex, instrument accessibility, and the speed of handling and data collection. Although successful, inherent limitations in current XL-MS strategies require further developments to enable MS detection and identification of cross-linked peptides with better efficiency, accuracy, sensitivity and speed. Among various approaches to improve existing XL-MS workflow,5 developing new cross-linking reagents holds the greatest promise towards the ultimate goal of mapping protein-protein interactions in living cells at the systems level. We report the chemical synthesis of two new cross-linking agents whose effectiveness has recently been demonstrated for in vivo protein-protein analysis.6
Unambiguous identification of cross-linked peptides can be greatly facilitated by the introduction of a MS cleavable bond in a cross-linking reagent, which can fragment during collision induced dissociation (CID) prior to peptide backbone breakage.7 Previously, we have successfully developed a new class of robust MS-cleavable reagents that contain labile C-S sulfoxide bonds (e.g. DSSO (DiSuccinimidyl-SulfOxide), Figure 1), and thus enables fast and accurate identification of cross-linked peptides using liquid chromatography-multistage tandem mass spectrometry analysis (LC/MSn).8,9 With DSSO as an example, this new XL-MS workflow involves protein DSSO cross-linking, trypsin digestion of cross-linked proteins, and LC/MSn analysis of resulting peptide mixtures. During MSn analysis, the cross-linked peptides are first detected in MS1 and selected for subsequent MS2 analysis. The CID-fragmentation site, i.e. one of the C–S sulfoxide bonds, is selectively fragmented in MS2, allowing the physical separation of the two DSSO cross-linked peptide constituents for subsequent sequencing. The resulting peptide fragments in MS2 are then analyzed in MS3 for unambiguous identification. The integration of these three types of MS data (MS1, MS2, MS3) enables simplified analysis of DSSO cross-linked peptides with improved speed and accuracy. This strategy has been demonstrated to be effective in the structural analysis of purified protein complexes in vitro.4
Figure 1.
CID cleavable cross-linker DSSO is based on the sulfoxide functional group. The red arrow points to the bond that is broken during the CID process. The workflow for cross-linking proteins is shown. After protein cross-linking, trypsin digest generates the cross-linked peptide for LC/MSn analysis. CID leads to selective cleavage of the bonds adjacent to the sulfoxide functional group.
The analytical problem with effectively detecting and identifying cross-linked peptides becomes much more daunting with large, complex protein assemblies and especially when studying protein-protein interactions in living cells. A strategy to improve the sensitivity and efficiency of XL-MS analysis is to incorporate an affinity purification handle into the cross-linker itself. To this end, we have developed an azide-tagged cross-linking reagent that allows the incorporation of an affinity purification handle based on click chemistry for enriching cross-linked peptides prior to MS analysis, thus improving their detection and identification.4a In comparison to other enrichment handles incorporated in cross-linking reagents,10 the azide group is advantageous as it is small and bioorthogonal, and click chemistry has been proven effective in enriching biological samples for various proteomic analyses including cross-linking studies.11 In order to combine these unique features in a multifunctional cross-linking reagent that can advance current XL-MS workflow for studying protein-protein interactions in vivo as well as in vitro, we have developed a new class of low molecular weight, membrane permeable, enrichable and MS-cleavable cross-linkers.
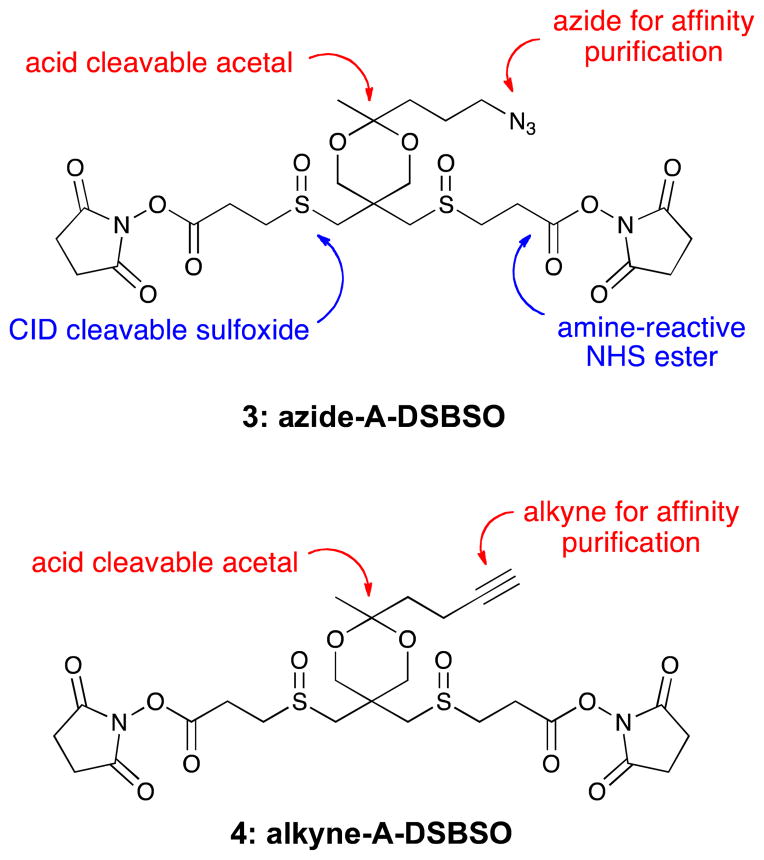
In order to explore the flexibility of using azide-alkyne click chemistry in the XL-MS workflow, we have designed and synthesized two new cross-linkers, i.e. azide-tagged and an alkyne-tagged reagents as presented in Figure 2. The structure of Azide-A-DSBSO (Azide-tagged, Acid-cleavable DiSuccinimidyl-BisSulfOxide) 3 incorporates a number of important design elements. The N-hydroxysuccinimidyl (NHS) esters are designed to react with lysine side chains thus cross-linking the proteins in the complex. The sulfoxide groups provide MS-cleavable bonds, and because only one side of each sulfoxide has β-hydrogen atoms, the elimination must take place regioselectively at the outer C–S bond. The design incorporates an azide functional group to be used in click reactions with strained alkynes or in a copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC).12 The click and CuAAC reactions enable several strategies for affinity purification, including direct coupling with alkyne or azide-functionalized beads or by linking with common affinity ligands such as biotin.11 Finally, the azide portion of the molecule is joined to the cross-linker with an acid labile acetal bond, which can be cleaved under aqueous acidic conditions to facilitate selective elution from an affinity column. The same elements are incorporated into the alkyne-A-DSBSO (Alkyne-tagged, Acid-cleavable DiSuccinimidyl-BisSulfOxide) 4, except that the azide functional group has been exchanged for the complementary alkyne. One other design feature is that both of these cross-linkers, prior to the introduction of the sulfoxides, are achiral and exist as single stereoisomers. This feature offers considerable simplification in the preparation and analysis of the synthetic intermediates, and decreases the chance of any stereoselective behavior in the crosslinking environment. These reagents have been under investigation for several years in our program and their applications in mapping protein-protein interactions at the systems level in living cells were recently described.6 In this report, the syntheses of these reagents are described in full along with foundational studies on the cross-linking effectiveness and LC/MSn sequencing.
Figure 2.
Protein cross-linkers designed with CID cleavable sulfoxide groups, azide (3) or alkyne (4) groups for clickable enrichment strategies, and an acid labile acetal to facilitate affinity purification.
Results and Discussion
Synthesis of the cross-linkers 3 and 4
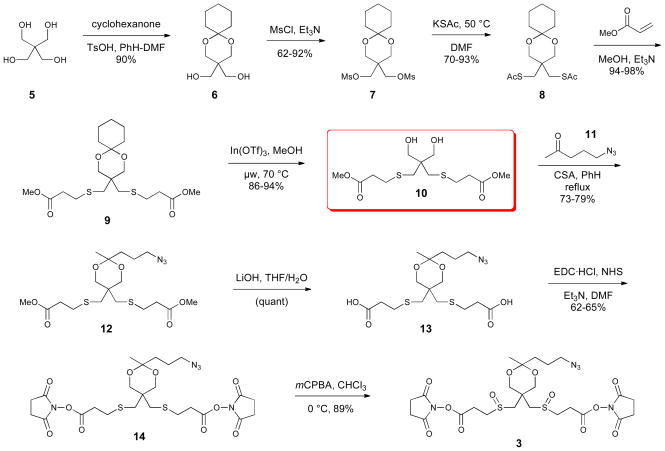
The original synthesis of Azide-A-DSBSO 3 began with pentaerythritol (5) and is presented in Scheme 1. Selective protection of 5 as cyclohexanone acetal 6 represented an improvement of the literature procedure.13 Mesylation followed by displacement with potassium thioacetate produced the bisthioacetate 8 in good overall yield. All three intermediates, 6–8, were crystalline solids that were isolated and purified on multi-gram scale without chromatography. Thioacetate methanolysis and Michael addition of 8 into methyl acrylate was accomplished in a single step in the presence of triethylamine and methanol. The key intermediate 9 was isolated in nearly quantitative yield and used without further purification. Careful hydrolysis of the cyclohexanone acetal using In(OTf)3 catalysis produced 86% of the diol 10 and 8% recovered starting material, which were separated by chromatography. Partial hydrolysis of the acetal was a recurring problem in this step when using Brønsted or Lewis acids in refluxing solvent, which required isolation and recycling of a small amount of the recovered acetal 9. By carefully optimizing the catalyst quantity, time, and temperature in microwave reaction conditions the conversion to diol 10 could be pushed to completion with isolated yields of ~94%. Diol 10 is a key intermediate in the synthesis, and it is the branch point for the preparation of the azide cross-linker 3 and the alkyne cross-linker 4. It was prepared in five steps following this route in 64% overall yield with only one chromatographic purification.14
Scheme 1.
Synthesis of azide-A-DSBSO (3) from pentaerythritol (5)
The remainder of the synthesis of azide cross-linker 3 is outlined in Scheme 1. The diol 10 was reacted with 5-azidopentan-2-one (11)15 with acid catalysis using a Dean-Stark trap to remove water. The acetal 12 was isolated in 77% yield by chromatography. The NHS esters were introduced in two steps: hydrolysis of the methyl ester and coupling with N-hydroxysuccinimide using EDC•HCl in dimethylformamide (DMF). The overall yield was typically around 60% and the bis-NHS ester 14 was isolated by chromatography. The final oxidation was carried out with meta-chloroperoxybenzoic acid (m-CPBA); the oxidant was added in aliquots and conversion was monitored by ESI-MS until the starting material and monosulfoxide were no longer present. Azide cross-linker 3 was isolated by extraction. This route produced several grams of the cross-linker 3 in ca. 18% overall yield for the sequence.14
In the course of our in vivo studies,6,11 we found that the azide 3 crossed the membrane and produced cross-links in targeted protein complexes.6 The studies required a large excess of cross-linker, and led to an ongoing demand for more material. Although the original optimized synthesis in Scheme 1 was effective, it did require nine steps. A shorter route was developed that incorporated several improvements in the individual transformations and avoided the use of protecting groups. The new route is presented in Scheme 2.
Scheme 2.

Improved synthesis of azide-A-DSBSO (3) beginning with 2,2-bis(bromomethyl)propane-1,3-diol (17).
The new route begins with the commercially available and inexpensive dibromide 15 and thiol 16. Direct alkylation with K2CO3 in DMF generated the key intermediate 10 in a single step. Diol 10 could be purified by chromatography on silica gel to produce 75% of pure 10, but the crude product was carried on in the sequence. By comparison to the original route, this method eliminates four steps in the sequence. The acetal synthesis was carried out using the Noyori protocol,16 which was found to be more reliable than the original acid-catalyzed method. Diol 10 was silylated and then combined with ketone 11 in the presence of TMSOTf to give acetal 12 in 65% overall yield. Hydrolysis of the dimethyl ester 12 used LiOH as before. The bis-NHS ester 14 was prepared using in situ generated TFA-NHS,17 which lead to similar overall yields but shorter reaction times, fewer side products, and a more reliable purification. Finally, oxidation to the bissulfoxide as previously described gave azide 3. The new route requires only six steps, three chromatographic purifications, and led to an overall yield of 38%.14 It is more convenient and reliable than the prior route and has been used to produce multiple grams of azide-A-DSBSO 3.
The 5-azidoentan-2-one was initially prepared by the alkylation of commercially available bromide 18 with NaN3.15 The very high cost of bromide 18 led us to develop a more economical approach starting with lactone 17 (Scheme 3). The lactone 17 was treated with HBr to generate the required 5-bromopentan-2-one (18). The standard displacement with sodium azide gave the desired ketone 11 in good overall yield. Scaling up the synthesis of azide-A-DSBSO 3 required a significant quantity of the volatile azide 11, and the starting with lactone 17 was both effective and economical.
Scheme 3.
Improved synthesis of 5-azidopentan-2-one (11)
The alkyne 4 was prepared from diol 10 using a very similar route (Scheme 4). The acetal 19 was formed from 5-hexyn-2-one and the diol under Dean-Stark conditions. The dimethyl ester was hydrolyzed to a diacid using LiOH, and the di-NHS ester was prepared using TFA-NHS reagent.17 Di-NHS ester 20 was isolated in 60% yield using this method. The same compound was also prepared using an EDCI coupling, but the yield was lower and the purification was more difficult. The m-CPBA oxidation was conducted as described for the azide substrate to give the Alkyne-A-DSBSO 4. The route required only four steps from diol 10 and made cross-linker 4 available on gram scale.
Scheme 4.
Synthesis of alkyne-A-DSBSO (4) from diol 10
MSn analysis of Azide-A-DSBSO cross-linked Ac-myelin peptide
Given the similarity of cleavable C–S bonds in azide-A-DSBSO and DSSO, we anticipated that azide-A-DSBSO cross-linked peptides would display comparable fragmentation characteristics to DSSO cross-linked peptides (Figure 3A).3a In such experiments, MS2 produces peptide fragments that are modified with remnant portions of the cross-linking reagents. These remnants are not identical thus producing two products with a separation of 254 Da, the αA and the αT fragments. The αA fragment has an alkene group, while the other half of the cleaved linker results in a terminal thiol group (after hydrolysis of the sulfenic acid intermediate). Although azide-A-DSBSO contains four C–S bonds due to the presence of two sulfoxide groups, the two central C–S bonds cannot undergo fragmentation due to the lack of β-hydrogens. Therefore, only the two C–S bonds closer to cross-linked residues are expected to fragment during MS2. To examine MS2 fragmentation patterns of azide-A-DSBSO cross-linked peptides during MSn analysis, we have first cross-linked and analyzed model peptide Ac-myelin. MS1 analysis detected azide-A-DSBSO cross-linked Ac-myelin (α-α) homodimer at four different charge states (m/z 501.416+, 601.455+, 751.624+, 1001.823+) (Figure 3B). MS2 analyses of inter-linked Ac-myelin homodimer at different charge states yielded the expected fragmentation of two identical inter-linked peptides, i.e. a characteristic fragment pair (αA/αT). As an example, the fragment pair αA/αT detected in MS/MS spectra of the quadruply (m/z 751.624+) and sextuply (m/z 501.416+) charged inter-linked Ac-myelin (α-α) was displayed in Figure 3C and 3D, respectively. The results demonstrate that the MS-cleavable CS bonds in azide-A-DSBSO cross-linked peptides are preferentially fragmented during MS2 analysis prior to the breakage of peptide backbones. Similar results were observed with Alkyne-A-DSBSO cross-linked Ac-myelin peptide (data not shown) as Azide-A-DSBSO and Alkene-A-DSBSO are almost identical in structures.
Figure 3. MSn analysis of Azide-A-DSBSO cross-linked Ac-Myelin synthetic peptide.

(A). Schematic illustration of MS2 analysis of a Azide-A-DSBSO interlinked homodimeric peptide (α-α). During collision-induced dissociation in MS2, cleavage of either of the two symmetric MS-cleavable C-S bonds leads to physical separation of the two inter-linked peptides, thus generating pairs of peptide fragments: i.e. αA and αT; (B) The interlinked Ac-Myelin was detected as multiple charged ions (m/z 1001.823+, m/z 751.624+, m/z 601.455+, m/z 501.416+) in MS1. (C-D) MS2 spectra of interlinked Ac-Myelin at two different charge states: (C) [α-α]4+ and (D) [α-α]6+. As shown, two predicted peptide fragment pairs were observed as αT2+/βA2+ (m/z 674.352+/819.892+) and αT3+/βA3+ (449.903+/546.933+) in (C ) and (D) respectively.
LC/MSn analysis of DSBSO cross-linked peptides of cytochrome C after enrichment
To demonstrate the applicability of azide-A-DSBSO for XL-MS studies, we have cross-linked model protein cytochrome C with azide-A-DSBSO. Cytochrome C has been used extensively by us and other groups for evaluating cross-linking reagents because it is a small protein with a high number of lysine residues. Given its success in the past for cross-linking studies,2d,18 we decided to use it as the model protein for characterizing our new cross-linking reagent. The resulting cross-linked cytochrome C products were conjugated with BARAC-biotin,19 affinity purified by binding to Streptavidin beads, and digested with trypsin. The cross-linked peptides were eluted from the beads with acid, and thus became acid-cleaved products of azide-A-DSBSO cross-linked peptides, i.e. DSBSO cross-linked peptides, which were then subjected to LC/MSn analysis. The general workflow and the structure of the cross-linked peptides leading up to LC/MSn analysis are illustrated in Figure 4. As illustrated, the acid-cleaved products are the final analytes for LC/MSn analysis. It is noted that the acid-cleaved products of azide-A-DSBSO and alkyne-A-DSBSO cross-linked peptides are the same, because the differentiated group is lost during the acid elution of cross-linked peptides from affinity matrix during enrichment. Since the cleavable C–S bonds in DSBSO are similar to those in DSSO, the general data analysis workflow for the identification of DSBSO cross-linked peptides by LC/MSn is similar to the analysis of DSSO cross-linked peptides.4 There are three types of cross-linked peptides, i.e. dead-end, intra-linked, and interlinked peptides. Among them, inter-linked peptides provide most informative structural details for defining protein-protein interaction interfaces. Therefore, we are most interested in identifying inter-linked peptides between the same and/or different proteins. As an example, Figure 5 describes a representative MSn analysis of a DSBSO inter-linked cytochrome C peptide (α-β) that was detected as a quadruply charged ion (m/z, 510.01664+). As shown, MS2 analysis resulted in two pairs of peptide fragments (i.e. αA/βT or αT/βA), characteristic fragmentation of inter-linked heterodimeric peptide. Subsequent MS3 analysis of MS2 fragment αA (m/z 430.752+) and βA (m/z 489.282+) determined their sequences as KAYIPGTK and M(ox)IFAGIKAK respectively, in which KA is modified with the alkene moiety. Integration of MS1, MS2, and MS3 results has unambiguously determined this DSBSO cross-linked cytochrome C peptide as [74KYIPGTK80 inter-linked to 81M(ox)IFAGIKK88], in which a cross-link was formed between K74 and K87 in cytochrome C.
Figure 4.
Work flow for affinity purification of cross-linked cytochrome C proteins. The MS2 fragments resulting from CID cleavage sites are shown.
Figure 5.
MSn Analysis of a representative DSBSO 3 Inter-Linked Peptide (α-β) of Cytochrome C (m/z 510.024+). A) MS1 and B) MS2 spectra of the selected peptide. In MS2 spectrum, two pairs of peptide fragments: i.e. αA/βT (m/z 430.752+/580.282+) and αT/βA(521.752+/489.282+) were detected. Note: αA, βA, and KA are alkene modified species (+54 Da); αT and βT are unsaturated thiol modified species (+236 Da). C-D) Respective MS3 spectra of αA (m/z 430.752+) and βA (489.282+) fragments detected in MS2. The detection of a series of y and b ions has unambiguously identified their sequences as KAYIPGTK and MoxIFAGIKAK respectively. Mox: oxidized methionine.
In total, LC/MSn analysis of enriched cross-linked cytochrome C identified 7 unique inter-linked peptides (Supplemental Table 1). In addition, 11 unique dead-end and 5 unique intra-linked cytochrome C peptides were identified since all types of cross-linked peptides can be selectively enriched (data not shown). The results are comparable to those obtained using DSSO cross-linking,4 demonstrating the effectiveness of Azide-A-DSBSO based XL-MS strategy. Although it is not necessary to enrich cross-linked peptides for simple proteins like cytochrome C, it is evident that such a process is essential for mapping protein interaction interfaces at the systems level.6
Conclusion
Full experimental details are presented for the preparation of two CID-cleavable lysine cross-linkers, an azide (azide-A-DSBSO) and an alkyne (alkyne-A-DSBSO), along with initial characterization using a model peptide and a model protein. The syntheses are not trivial, but the optimized procedures reported herein make these useful compounds available on multigram scale. The azide and alkyne functional groups are suitable for click enrichment strategies. The cross-linkers described here have been utilized in mammalian HEK-293 cells6 and to facilitate the study of the interaction of subunits in the proteasome complex, which is responsible for degradation of ubiquitin tagged proteins.6 The importance of developing XL-MS reagents that are applicable for in vivo studies is significant because protein-protein interactions are involved in most cell function and are not well understood. We hope that this report detailing the synthesis of these cross-linking agents will enable others to prepare and utilize them to study protein-protein interactions.
Methods
See Supporting Information for experimental details for the synthesis of each cross-linking reagent.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants RO1GM074830-06A1 and R21CA161807 to L.H., and Biomedical Informatics Training predoctoral fellowship to A.K.(5T15LM007443-10 to Pierre Baldi). Eric J. Novitsky was supported by an Institutional Chemical and Structural Biology Training Grant predoctoral fellowship (T32-GM10856).
ABBREVIATIONS
- Ac
acetate
- Alkyne-A-DSBSO
Alkyne-tagged, Acid-cleavable DiSuccinimidyl-BisSulfOxide
- Azide-A-DSBSO
Azide-tagged, Acid-cleavable DiSuccinimidyl-BisSulfOxide
- BARAC
biarylazacyclooctynone
- CID
collision-induced dissociation
- CSA
camphorsulfonic acid
- CuAAC
copper (I) catalyzed azide-alkyne cycloaddition
- DIPEA
N,N-diisopropylethylamine
- DMF
dimethylformamide
- DSSO
DiSuccin-imidylSulfOxide
- EDC·HCl
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- Et3N
tri-ethylamine
- HB
His-Bio
- ESI-MS
electrospray ionization-mass spectrometry
- In(OTf)3
indium(III) tri-fluoromethanesulfonate
- KAYIPGTK
lysine(alkene modified)-tyrosine-isoleucine-proline-glycine-threonine-lysine peptide
- LC/MS
liquid chromatography-mass spectrometry
- MeOH
methanol
- M(ox)IFAGIKAK
methionine(oxidized)-isoleucine-phenylalanine-alanine-glycine-isoleucine-lysine(alkene modified)-lysine
- m-CPBA
meta-chloroperoxybenzoic acid
- MsCl
methanesulfonyl chloride
- NHS
N-hydroxysuccinimidyl
- Ph-H
benzene
- pyr
pyridine
- QTAX
quantitative analysis of tandem affinity purified in vivo cross-linked (X) protein complexes
- TFAA
trifluoroacetic anhydride
- TFA-NHS
N-trifluoroacetoxy succinimide
- THF
tetrahydrofuran
- TMSCl
trimethylsilyl chloride
- TMSOTf
tri-methylsilyl trifluoromethanesulfonate
- TsOH
para-toluenesulfonic acid
- XL-MS
cross-linking mass spectrometry
- μw
microwave
Footnotes
General synthetic methods, experimental data, and spectral data are presented in pdf format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T, Leitner A, Beck M, Hartl FU, Ban N, Malmstrom L, Aebersold R. Science. 2012;337:1348–1352. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]; (b) Politis A, Stengel F, Hall Z, Hernández H, Leitner A, Walzthoeni T, Robinson CV, Aebersold R. Nat Meth. 2014;11:403–406. doi: 10.1038/nmeth.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Robinson CV, Sali A, Baumeister W. Nature. 2007;450:973–982. doi: 10.1038/nature06523. [DOI] [PubMed] [Google Scholar]; (b) Erzberger JP, Stengel F, Pellarin R, Zhang S, Schaefer T, Aylett CHS, Cimermančič P, Boehringer D, Sali A, Aebersold R, Ban N. Cell. 2014;158:1123–1135. doi: 10.1016/j.cell.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kao A, Randall A, Yang Y, Patel VR, Kandur W, Guan S, Rychnovsky SD, Baldi P, Huang L. Mol Cell Proteomics. 2012;11:1566–1577. doi: 10.1074/mcp.M112.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Guerrero C, Tagwerker C, Kaiser P, Huang L. Mol Cell Proteomics. 2006;5:366–378. doi: 10.1074/mcp.M500303-MCP200. [DOI] [PubMed] [Google Scholar]; (b) Chavez JD, Weisbrod CR, Zheng C, Eng JK, Bruce JE. Mol Cell Proteomics. 2013;12:1451–1467. doi: 10.1074/mcp.M112.024497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao A, Chiu C-L, Vellucci D, Yang Y, Patel VR, Guan S, Randall A, Baldi P, Rychnovsky SD, Huang L. Mol Cell Proteomics. 2011;10:M110.002212. doi: 10.1074/mcp.M110.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitner A, Walzthoeni T, Kahraman A, Herzog F, Rinner O, Beck M, Aebersold R. Mol Cell Proteomics. 2010;9:1634–1649. doi: 10.1074/mcp.R000001-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaake RM, Wang X, Burke A, Yu C, Kandur W, Yang Y, Novitsky EJ, Second T, Duan J, Kao A, Guan S, Vellucci D, Rychnovsky SD, Huang L. Mol Cell Proteomics. 2014;13:3533–3543. doi: 10.1074/mcp.M114.042630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paramelle D, Miralles G, Subra G, Martinez J. J Proteomics. 2013;13:438–456. doi: 10.1002/pmic.201200305. [DOI] [PubMed] [Google Scholar]
- 8.(a) Petrotchenko EV, Serpa JJ, Borchers CH. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Muller MQ, Dreiocker F, Ihling CH, Schafer M, Sinz A. J Mass Spectrom. 2010;45:880–891. doi: 10.1002/jms.1775. [DOI] [PubMed] [Google Scholar]; (c) Dreiocker F, Muller MQ, Sinz A, Schafer M. J Mass Spectrom. 2010;45:178–189. doi: 10.1002/jms.1702. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Tanasova M, Borhan B, Reid GE. Anal Chem. 2008;80:9279–9287. doi: 10.1021/ac801625e. [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Fishburn J, Hahn S, Ranish J. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Szychowski J, Mahdavi A, Hodas JJL, Bagert JD, Ngo JT, Landgraf P, Dieterich DC, Schuman EM, Tirrell DA. J Am Chem Soc. 2010;132:18351–18360. doi: 10.1021/ja1083909. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nessen MA, Kramer G, Back J, Baskin JM, Smeenk LEJ, de Koning LJ, van Maarseveen JH, de Jong L, Bertozzi CR, Hiemstra H, de Koster CG. J Proteome Res. 2009;8:3702–3711. doi: 10.1021/pr900257z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chowdhury SM, Du X, Tolic N, Wu S, Moore RJ, Mayer MU, Smith RD, Adkins JN. Anal Chem. 2009;81:5524–5532. doi: 10.1021/ac900853k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lead reference: Finn MG, Fokin VV. Chem Soc Rev. 2010;39:1231–1232. doi: 10.1039/c003740k.
- 13.Murguia MC, Vaillard SE, Grau RJ. Synthesis-S. 2001;7:1093–1097. [Google Scholar]
- 14.Although yield ranges were reported for many transformations to reflect our experience with the sequence, the overall yields were calculated based on the specific examples written up in the experimental section.
- 15.Ma Y. Heteroatom Chem. 2002;13:307–309. [Google Scholar]
- 16.Tsunoda T, Suzuki M, Noyori R. Tetrahedron Lett. 1980;21:1357–1358. [Google Scholar]
- 17.Leonard NM, Brunckova J. J Org Chem. 2011;76:9169–9174. doi: 10.1021/jo201686e. [DOI] [PubMed] [Google Scholar]
- 18.Vellucci D, Kao A, Kaake RM, Rychnovsky SD, Huang L. J Am Soc Mass Spectrom. 2010;21:1432–1445. doi: 10.1016/j.jasms.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.