Abstract
Monocytes and macrophages play a prominent role in the establishment of HIV-1 infection, virus dissemination and development of viral reservoirs. Like T cells, macrophages display immune polarization that can promote or impair adaptive immunity. We hypothesize that dysregulation of monocyte/macrophage activation and differentiation may promote immune dysfunction and contribute to AIDS pathogenesis. Using flow cytometry, we analyzed the frequency of monocyte subsets in human immunodeficiency virus type 1 (HIV-1) infection relative to seronegative controls, focusing on the CD163+/CD16+ monocyte as a likely precursor of the “alternatively-activated” macrophage. Individuals with detectable HIV-1 infection showed an increase in the frequency of CD163+/CD16+ monocytes (CD14+) when compared to seronegative or HIV-1 infected persons with undetectable viral loads. A positive correlation between increased CD163+/CD16+ monocyte frequency and viral load was revealed that was not seen between viral load and the number of CD4+ T cells or frequency of CD16+ monocytes (without CD163 sub-typing). We also found strong inverse correlations between CD16+ monocytes (r=-0.71, r2=0.5041, p=0.0097) or CD163+/CD16+ monocytes (r=-0.86, r2=0.7396, p=0.0003) and number of CD4+ T cells below 450 cells/μl. An inverse relationship between CD163+/CD16+ and CD163+/CD16− monocytes suggests the expanded CD163+/CD16+ population is derived exclusively from within the “alternatively activated” (MΦ-2) subset. These data suggest a potential role for CD163+/CD16+ monocytes in virus production and disease progression. CD163+/CD16+ monocytes may be a useful biomarker for HIV-1 infection and AIDS progression and a possible target for therapeutic intervention.
Macrophages can exhibit immune polarization analogous to the TH1 versus TH2 cytokine profiles expressed by T cells (for review, see Mantovani, 2005) 1. While the pro-inflammatory, interleukin (IL)-12 producing MΦ-1 are involved in antigen presentation and memory T cell activation, IL-10 producing, alternatively activated macrophages, MΦ-2, are primarily involved in housekeeping functions, i.e. phagocytosis, tissue remodeling and immune suppression. Both populations have their appropriate functions; however polarization to an MΦ-2 phenotype could have important immune consequences in the setting of HIV-1 infection by adversely affecting the ability of the host immune system to adequately respond to HIV-1 infection as well as other opportunistic pathogens.
Expression of CD163, a scavenger receptor for hemoglobin-haptoglobin (hg-hp) complex 2, is reportedly exclusive to a subset of monocytes and specific tissue macrophages in humans and has been used to phenotypically identify MΦ-2 3–6. In rhesus macaques infected with SIV, a recent study identified increased numbers of CD163+ macrophages in the heart, correlating positively with SIV infected cells and negatively with inflammatory infiltration 7. These results suggest an immunosuppressive effect of CD163+ macrophages and a potential imbalance in classical versus non-classical macrophage activation. CD163+ macrophages have also been shown to accumulate in CNS tissue in HIV-1 and SIV encephalopathy 8, 9. These findings, together with the observed accumulation in the heart in SIV infected animals might suggest a systemic abnormality in monocyte/macrophage differentiation in HIV-1 infection, possibly contributing to immune suppression and increased virus dissemination.
While virtually all monocytes express CD14, a lipopolysaccharide (LPS) receptor 10, the less frequent CD16+ monocyte subset is considered more mature, with characteristics similar to mature macrophages 11. We, therefore, sought to determine if there was an alteration in the frequency of CD163+/CD16+ monocytes in HIV-1 infection as an indication of altered differentiation toward the alternatively activated MΦ-2 subset. Such alterations would suggest the importance of macrophage polarization in the pathogenesis of HIV-1 infection. In the studies presented here, we performed flow cytometric analysis on peripheral blood mononuclear cells (PBMC) from seronegative and HIV-1 seropositive volunteers, some on antiretroviral therapy, where viral load and CD4 counts were available from clinical records (Table 1).
Table 1.
HIV-1 infected donors. ARV=anti-retroviral therapy. HIV grouping refers to categorization of HIV-1 infected donors for analyses comparing patients with and without detectable plasma viremia. D=detectable plasma viremia. U=undetectable plasma viremia.
| Patient ID | HIV grouping | Viral load (copies/ml) | CD4 T cell count (cells/μl) | ARV | Naïve to ARV |
|---|---|---|---|---|---|
| 002 | D | 3652 | 638 | N | Y |
| 004 | U | 34788 | 399 | N | N |
| 005 | D | 3748 | 358 | N | Y |
| 006 | D | 558 | 268 | Y | N |
| 007 | D | 3232 | 202 | N | N |
| 009 | D | 16383 | 267 | N | N |
| 011 | U | <50 | 960 | Y | N |
| 013 | D | 722 | 693 | N | N |
| 014 | U | <400 | 263 | Y | N |
| 018 | U | <50 | 414 | Y | N |
| 019 | U | <50 | 280 | Y | N |
| 020 | U | <50 | 628 | Y | N |
| 021 | U | <50 | 437 | Y | N |
| 022 | D | 525941 | 493 | N | Y |
| 023 | D | 476269 | 74 | N | N |
| 025 | U | <50 | 895 | Y | N |
| 026 | U | <50 | 711 | Y | N |
| 027 | U | <400 | 359 | Y | N |
| 028 | D | 268606 | 263 | N | Y |
Flow cytometric analysis was performed on PBMC isolated by Ficoll separation from heparinized whole blood specimens from 25 volunteers (6 HIV-1- and 19 HIV-1+) using fluorescent tagged monoclonal antibodies against CD14-PerCP-Cy5.5 (clone M5E2, BD), CD16-Pacific Blue (clone 3G8, BD), and CD163-FITC (clone Mac2-158, Trillium Diagnostics). PBMC were isolated from whole blood within four hours of collection. Isotype controls and fluorescence minus one (FMO) tests were performed for antibody specificity and accuracy of gating, respectively.
For each sample, 20,000 events were collected on a FACSAria (BD Biosciences) within a broad monocyte gate, defined by forward and side scatter properties, and analyze using FlowJo version 7.1.3 software, (TreeStar). The broad monocyte gate was refined by plotting side scatter against CD14, an LPS receptor expressed by the majority of monocytes. CD16 and CD163 expression were analyzed on the total CD14+ monocyte population. Gates were set using FMO controls with less than 0.3% background present in all positive gates.
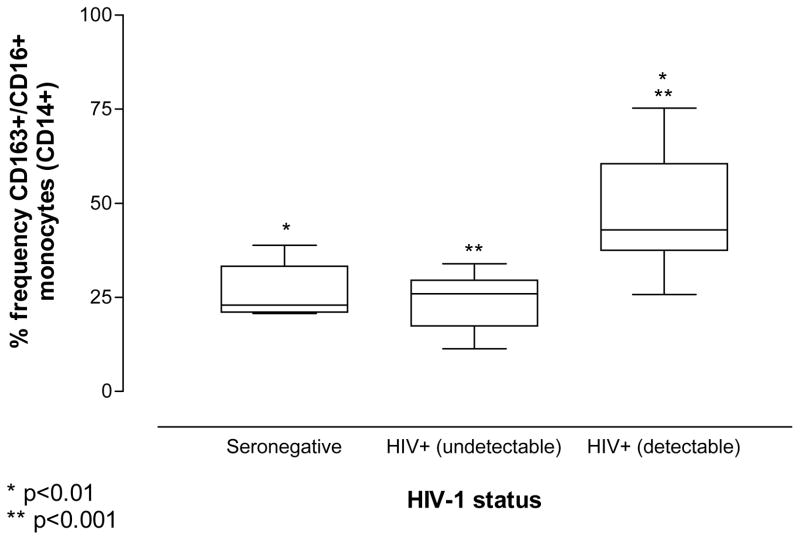
The HIV-1+ volunteers were divided into two groups: 9 volunteers successfully treated with anti-retroviral therapy (ARV) with an undetectable viral load and 10 with detectable plasma viremia. All subjects without detectable plasma viremia were on ARV, while only a single donor with detectable virus was on ARV (Table 1). One-way analysis of variance (ANOVA) revealed that HIV-1 infected donors with detectable plasma viral loads have a significant increase in the frequency of CD163+/CD16+ monocytes compared to seronegative donors (p<0.01; Figure 1). Interestingly, individuals with an undetectable viral load show a small decrease in the frequency of CD163+/CD16+ monocytes from that observed in seronegative donors, however, this difference is not significant. Among all HIV-1 infected individuals, the percent frequency of CD163+/CD16+ monocytes was significantly higher in volunteers with, versus those without, detectable viral loads (p<0.001; Figure 1). This observation is consistent with earlier reports demonstrating reduced frequency of CD16+ monocytes among HIV-1 infected individuals undergoing successful pharmacologic intervention 12, 13.
Figure 1. The frequency of CD163+/CD16+ monocytes is greater in patients with detectable viral loads.
The frequency of CD163+/CD16+ monocytes (CD14+) is significantly increased in HIV-1 infected individuals with detectable plasma viremia when compared to seronegative donors or patients with HIV-1 infection who are successfully controlling virus with pharmaceutical intervention. A slight reduction in the number of CD163+/CD16+ monocytes is seen with virus suppression as compared to seronegative individuals, however this is not statistically significant. All “undetectable” HIV-1 infected subjects were on ARV. Only a single donor within the “detectable” group was on ARV.
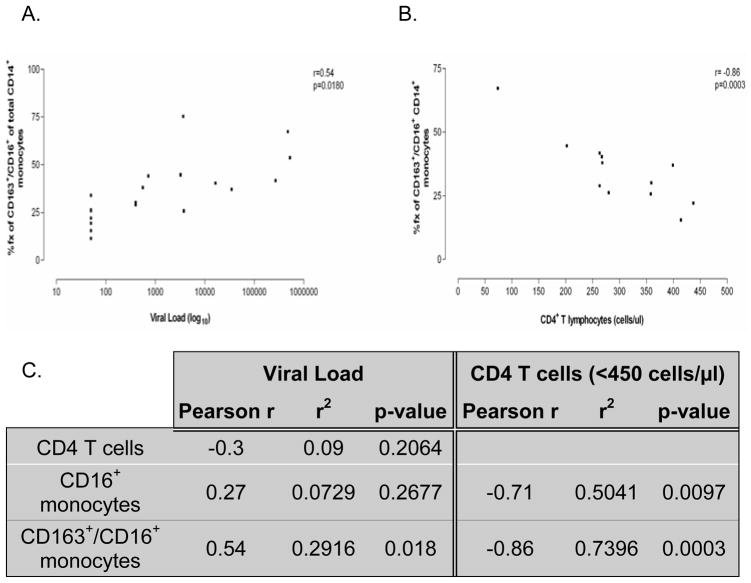
Pearson correlation statistics were carried out on flow data obtained from HIV-1 infected individuals to determine the relationship between absolute number of CD4+ T cells, plasma viremia and percent frequency of CD16+ and CD163+/CD16+ monocytes. A reduction in CD4+ T cells with increased viral load was observed, however, this correlation did not reach significance (Figure 2, Panel C). Conversely, the percent frequency of CD16+ monocytes appears to increase with increased viral burden, however, like CD4+ T cells, this relationship did not reach statistical significance (Figure 2, Panel C). Sub-fractionating the analysis of total monocytes for CD163 expression in addition to CD16 expression demonstrated a correlation between the frequency of CD163+/CD16+ monocytes and plasma viremia (r=0.54, p=0.018; Figure 2, Panels A and C), suggesting a relationship between virus production and the expansion of this monocyte subset.
Figure 2. CD163+/CD16+ monocyte expansion correlates positively with viral load and inversely with CD4+ T cell number in HIV-1 infected individuals.
Pearson correlation statistics demonstrate a significant correlation between expansion of the CD163+/CD16+ monocyte subset with increasing viral burden (Panel A). Further, in HIV-1 infected individuals with CD4+ T cell counts below 450 cells/μl (12 of 19 subjects), an inverse correlation was seen between absolute number of CD4+ T cells and CD16+ or CD163+/CD16+ monocyte frequency, with greater significance seen with CD163+/CD16+ monocyte (r2 = 74%) (Panels B and C) than CD16+ monocyte frequency (r2 = 50%) (Panel C). Data analyses were performed using the original viral load values. Viral loads were log transformed after analyses for data presentation.
Correlation statistics were also performed to determine the relationship between absolute number of CD4+ T cells and percent frequency of CD16+ and CD163+/CD16+ monocytes. A trend toward a decrease in the absolute number of CD4+ T cells with increased CD16+ and CD163+/CD16+ monocyte frequencies was observed in all HIV-1 infected individuals, however this was not significant for either monocyte subset (data not shown). Interestingly, patients with CD4+ T cell counts below 450 cells/μl show a significant inverse correlation between CD4+ T cells and CD163+/CD16+ (r=-0.86, p=0.0003) or CD16+ (r=-0.71, p=0.0097) monocytes (Figure 2, Panels B and C). CD16+ and CD163+/CD16+ monocyte subset expansion correlate with CD4+ T cell loss in HIV-1 infected persons with counts less than 450 cells/μl. The r2 values, however, demonstrate that a greater number of subjects fall within the correlation between CD163+/CD16+ monocyte subset expansion and CD4+ T cell loss (r2=0.7396) (Figure 2, Panels A and C) than the correlation between CD16+ monocyte subset expansion and CD4+ T cell loss (r2=0.5041) (Figure 2, Panel C). The percent frequency of CD163+/CD16+ monocytes also correlates inversely with the number of CD4+ T cells at the clinically relevant value of 350 cells/μl and below (r=-0.92, r2=0.8464, p=0.0033). Our observation that expansion of the CD163+/CD16+ monocyte subset correlates inversely with CD4+ T cell count in patients with less than 450 CD4+ T cells/μl, however, may provide an earlier indicator of AIDS development/progression and a potential opportunity for therapeutic intervention.
To begin to understand the observed expansion of the CD163+/CD16+ monocyte subset in HIV-1 infection, we investigated differences in all four CD163±/CD16± monocyte populations for divergences in the frequencies of any of these populations among the seronegative and HIV-1 infected (with or without virus detection) groups. CD163−/CD16+ and CD163−/CD16− populations were not significantly altered in any of the groups examined (data not shown). In contrast, the frequency of CD163+/CD16− monocytes is greater in seronegative individuals and those with virus suppression than HIV-1 infected donors with detectable plasma viremia (p<0.05). Additionally, the frequency of CD163+/CD16− monocytes shows a strong inverse correlation with the frequency of CD163+/CD16+ monocytes (r=-0.9195, r2=0.8455, p<0.0001) suggesting that these two subsets are inversely associated; the CD163+/CD16+ monocyte subset may be derived from CD163+/CD16− monocytes. A strong trend toward a decrease of the CD163+/CD16− monocyte subset with increased viral load (p=0.07) was observed among all HIV-1 infected individuals enrolled in this study. The absence of a significant relationship appears to be due to the presence of a single outlier, where the removal of this outlier reveals a highly significant inverse correlation, with r=-0.66 and p=0.003. We also observe a positive trend toward a greater number of CD4+ T cells with increased CD163+/CD16− monocyte frequency that is not statistically significant until CD4+ T cells drop below 450 cells/μl (r=0.65, r2=0.4225, p=0.022).
In view of the positive correlation with viral load and negative correlation with CD4+ T cell decline below 450 cells/μl, it is tempting to speculate that T cell loss and/or macrophage infection may contribute to the expansion of the CD163+/CD16+ subset during the course of HIV-1 infection. In fact, CD16+ monocytes may be an important reservoir of HIV-1 infection. A recent report shows CD16+ monocytes express higher levels of CCR5, are preferentially HIV-1 infected relative to CD16− monocytes, and harbor HIV-1 infection in vivo 14. Furthermore, CD16+ monocytes produce high levels of virus upon differentiation into macrophages and T cell interaction 15. CD16+ monocytes may also play an important role in AIDS pathogeneses. In earlier work, we demonstrate that CD16+ monocytes/macrophages accumulate in the CNS in HIVE, many of which harbor productive HIV-1 infection 16. Interestingly, we have recently observed that the CD163+ cells reported by others to accumulate in the CNS in HIVE 8 are largely CD16+ (unpublished observations).
It is unclear how CD163+/CD16+ monocytes are expanded in HIV-1 infection. Macrophage colony stimulating factor (M-CSF) production is up-regulated in HIV-1 infected monocyte-derived macrophages (MDM) 17 and enhances the susceptibility of macrophages to HIV-1 infection 18. Further, this increased susceptibility is suggested to occur through alterations in monocyte differentiation/activation by M-CSF rather than any effect M-CSF might have on the virus, itself 17–19. Interestingly, in vitro treatment of primary monocytes promotes differentiation to CD163+ macrophages 20 and has also been shown to up-regulate CD16 expression 21, 22. Additionally, macrophages stimulated with M-CSF produce high levels of IL-10 but do not produce IL-12 23 suggesting a role for M-CSF in reducing inflammation and potentially promoting a MΦ-2 phenotype 24. It is worth noting that M-CSF levels in plasma and cerebral spinal fluid (CSF) have been correlated with shortened time to death in a cohort of patients with advanced HIV-1 infection 25.
Longitudinal studies evaluating alterations of CD163+/CD16+ monocyte frequency with plasma viremia and CD4+ T cell decline will be required to determine if expansion of this monocyte subset is predictive of disease progression. Additional studies will also be necessary to further characterize the cytokine polarization of this monocyte/MΦ subset, and determine its role in HIV-1 pathogenesis. The CD163+/CD16+ monocyte subset may prove to be a predictive as well as associative, biomarker and may possibly serve as an effective target for HIV-1 therapeutics.
Acknowledgments
This work was supported by NIH NINDS grant R01 NS047031 to JR. TFS is supported by a training grant (T32-DA07237) from NIH/NIDA.
Footnotes
The authors declare no conflict of interest or financial interests.
References
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Hogger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1883–1890. [PubMed] [Google Scholar]
- Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- Sulahian TH, Hogger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, Natkunam Y. Expression of CD163 (Hemoglobin Scavenger Receptor) in Normal Tissues, Lymphomas, Carcinomas, and Sarcomas Is Largely Restricted to the Monocyte/Macrophage Lineage. Am J Surg Pathol. 2005;29:617–624. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]
- Yearley JH, Pearson C, Shannon RP, Mansfield KG. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retroviruses. 2007;23:515–524. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Masliah E, Fox HS. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE) J Neuropathol Exp Neurol. 2004;63:1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- Kim WK, Alvarez X, Fisher J, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Passlick B, Flieger D. The monoclonal antimonocyte antibody My4 stains B lymphocytes and two distinct monocyte subsets in human peripheral blood. Hybridoma. 1988;7:521–527. doi: 10.1089/hyb.1988.7.521. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Fingerle G, Strobel M, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–2058. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- Amirayan-Chevillard N, Tissot-Dupont H, Capo C, et al. Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clin Exp Immunol. 2000;120:107–112. doi: 10.1046/j.1365-2249.2000.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A, Ellery P, Maslin CL, et al. Normal CD16 expression and phagocytosis of Mycobacterium avium complex by monocytes from a current cohort of HIV-1-infected patients. J Infect Dis. 2006;193:693–697. doi: 10.1086/500367. [DOI] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Kunstman KJ, Autissier P, et al. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Gruber MF, Weih KA, Boone EJ, Smith PD, Clouse KA. Endogenous macrophage CSF production is associated with viral replication in HIV-1-infected human monocyte-derived macrophages. J Immunol. 1995;154:5528–5535. [PubMed] [Google Scholar]
- Kalter DC, Nakamura M, Turpin JA, et al. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J Immunol. 1991;146:298–306. [PubMed] [Google Scholar]
- Bergamini A, Perno CF, Dini L, et al. Macrophage colony-stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood. 1994;84:3405–3412. [PubMed] [Google Scholar]
- Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- Munn DH, Garnick MB, Cheung NK. Effects of parenteral recombinant human macrophage colony-stimulating factor on monocyte number, phenotype, and antitumor cytotoxicity in nonhuman primates. Blood. 1990;75:2042–2048. [PubMed] [Google Scholar]
- Young DA, Lowe LD, Clark SC. Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J Immunol. 1990;145:607–615. [PubMed] [Google Scholar]
- Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage colony-stimulating factor are deficient in IL-12 production. Eur J Immunol. 1998;28:2498–2507. doi: 10.1002/(SICI)1521-4141(199808)28:08<2498::AID-IMMU2498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ritter M, Buechler C, Langmann T, Orso E, Klucken J, Schmitz G. The scavenger receptor CD163: regulation, promoter structure and genomic organization. Pathobiology. 1999;67:257–261. doi: 10.1159/000028105. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol. 2007;64:97–102. doi: 10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]