Abstract
Importance
Cerebral white matter (WM) damage has been reported in childhood obesity and in metabolic syndrome (MetS) but mechanisms remain unclear.
Objectives
To ascertain whether adolescents with MetS have retinal vessel alterations and if the anticipated reductions in retinal arteriolar diameter are associated with diminished cerebral WM microstructural integrity and to test a model for vascular etiology of the WM abnormalities.
Design, Setting, and Participants
Cross-sectional study of the brain correlates of obesity and related metabolic disease in youths. This study was conducted at the Brain, Obesity, and Diabetes Laboratory, New York University School of Medicine, New York. Thirty-nine obese adolescents with MetS and 51 matched adolescents without MetS received comprehensive endocrine, neuropsychological, retinal vessel, and diffusion tensor imaging—based cerebral WM evaluations.
Main Outcomes and Measures
Retinal arteriolar diameter, cerebral WM microstructural integrity, waist circumference, and insulin resistance.
Results
Obese adolescents with MetS had significant reductions in retinal arteriolar diameter relative to adolescents without MetS (mean [SD] central retinal arteriolar equivalent, 182.35 [16.10] vs 198.62 [19.03] μm, respectively; P < .001). The greater the number of MetS criteria present, the greater the reduction was in retinal arteriolar diameter (β = −8.61; Δr2 = 0.335; ΔF1,83 = 70.79; P < .001). We found that abdominal obesity (waist circumference) was the strongest MetS component related to reductions in retinal arteriolar diameter (rp[85] = −0.661; P < .001), and importantly, for the first time to our knowledge, we demonstrated that its effect was partially mediated by comorbid insulin resistance (indirect effect = −0.1355 [95% CI, −0.2471 to −0.0593]; Z = −2.56; P = .01). Consistent with our prior report of nondiabetic adolescents with MetS, we also uncovered cerebral WM microstructural damage. These subtle WM changes were associated with reductions in retinal arteriolar diameter, a proxy for cerebral microvascular health (3150 voxels or 3.15 cm3; P < .001). Importantly, some of the WM regions showing lower microstructural integrity also demonstrated associations with retinal arteriolar diameter, suggesting that the observed WM pathology is likely vascular in nature.
Conclusions and Relevance
We document, for the first time to our knowledge, the associations between retinal vessel alterations and subclinical WM pathology among obese adolescents with MetS. This suggests that the subtle WM pathology in adolescents with MetS may have a vascular origin. Future work should include direct assessments of cerebral microvascular health.
Keywords: Obesity, Metabolic Syndrome, insulin resistance, adolescence, retinal vessel, diffusion tensor imaging
Introduction
Metabolic syndrome (MetS) is a group of co-occurring risk factors that increase the risk for coronary artery disease1 and stroke.2 Owing to the childhood obesity epidemic, the prevalence of MetS has already reached 9.4% among US children and adolescents.3
In adults, MetS has been associated with increased incidences of intracranial arteriosclerosis,4 silent brain infarction,5 and white matter (WM) hyperintensities and subcortical lesions.6 There are few data documenting the effect of MetS on the pediatric brain. We have reported increased global brain atrophy, reduced hippocampal volume, and diffuse WM microstructural damage among obese adolescents with MetS7 and with type 2 diabetes mellitus (DM).8 We have also demonstrated that insulin resistance (IR), which is central to MetS, is the most robust predictor of these brain volume changes.7 The underlying mechanisms for the brain damage remain unclear.
Cerebral WM damage in adults is likely vascular in nature.9 In contrast to adults, children with MetS do not have clear atherosclerosis or gross WM pathologies. However, obese adolescents show evidence of endothelial dysfunction,10 increased carotid stiffness,11 and intima-media thickness12 similar to those reported in adults with MetS.13,14 Direct evaluation of cerebral vasculature can be quite expensive and invasive. Given that retinal and cerebral microvasculatures share developmental and physiological controls, retinal arteriolar diameter measured using nonmydriatic retinal photography15 is a potential biomarker for cerebral arteriolar health.16 Obese adolescents have reductions in retinal arteriolar diameter, which is associated with increased cerebrospinal fluid and smaller hippocampal volumes.17 We have also demonstrated an association between subclinical WM damage and reductions in retinal arteriolar diameter among adults with hypertension.9
In this study, we ascertain whether obese adolescents with MetS have reductions in retinal arteriolar diameter, explore how those reductions are associated with the MetS components, and assess whether IR plays a mediating role in the relationship between obesity and retinal arterial health. Lastly, we evaluate whether the anticipated reductions in retinal arteriolar diameter are associated with the changes in cerebral WM microstructure also expected in this group.
Methods
Study Participants
The study was conducted at the Brain, Obesity, and Diabetes Laboratory, New York University School of Medicine, New York. The study protocol was approved by the New York University School of Medicine Institutional Review Board. Participants were recruited through online advertisements or were relatives or friends of other participants. All participants (and if <18 years of age, one of their parents) signed informed consent and were compensated for their participation. Given that the prevalence of impaired fasting glucose levels is very low in nondiabetic youths,18 we defined MetS with a modification of the standard definition by using IR measured by the quantitative insulin sensitivity check index (QUICKI), 1/[log(fasting insulin) + log(fasting glucose in milligrams per deciliter)],19 rather than fasting hyperglycemia as one of our MetS components (see the article by Yau et al7 for the MetS definition). The QUICKI has been validated in nondiabetic children and adolescents.20
Exclusion criteria included significant medical conditions (other than diabetes, IR, polycystic ovary syndrome, dyslipidemia, and hypertension), use of psychoactive medications, a diagnosis of psychiatric disorder, a history of significant learning disability, and pregnancy. Of the 149 adolescents screened, 46 were excluded (1 did not meet entry criteria, 9 were receiving psychoactive medication, 2 had clinical magnetic resonance imaging [MRI] abnormalities, 2 were diagnosed as having type 2 DM but were not obese, 6 had insufficient data for MetS determination, and 26 did not have retinal vessel measurements and/or diffusion tensor imaging [DTI] data). Thirteen were further excluded from either group, blinded to their brain data, so as to carefully balance groups on demographic variables. This resulted in a final set of 39 adolescents with MetS (7 with type 2 DM) and 51 without MetS.
All participants underwent a comprehensive 7-hour battery of medical, endocrine, psychiatric, and brain MRI assessments completed over 2 separate days. A diagnosis of depression was exclusionary, but given the evidence linking childhood obesity and depression21,22 and their associations with increased cardiovascular risk,23 we adjusted for potential subclinical symptoms using the Beck Depression Inventory (BDI)24 in our analyses. Additionally, we measured sleep apnea with a 20-item questionnaire25 and adjusted for its potential effect. The International Physical Activity Questionnaire evaluated level of physical activity.26
MRI Acquisition
All participants underwent MRI of the brain on the same 1.5-T Siemens Avanto MRI System using a standard protocol. The DTI echo planar sequence (repetition time, 6100 milliseconds; echo time, 75 milliseconds; delay in repetition time, 0 milliseconds; b values: 0, 1000 seconds/mm2; 6 directions; field of view, 210 × 210; 4 averages and 1 concatenation; 50 axial slices; voxel size, 1.64 × 1.64 × 3 mm3) was used to derive the fractional anisotropy (FA) maps for WM assessment. We reformatted the T1-weighted magnetization-prepared rapid acquisition gradient echo sequence (MPRAGE; repetition time, 1300 milliseconds; echo time, 4.38 milliseconds; T1, 800 milliseconds; field of view, 250 × 250; 196 coronal slices; slice thickness, 1.2 mm; number of excitations, 1; flip angle, 15°) and T2-weighted sequence (repetition time, 9000 milliseconds; echo time, 94 milliseconds; T1, 2000 milliseconds; field of view, 210 × 210; 50 axial slices; slice thickness, 3 mm) with adequate gray-white contrast to act as structural guides to correct spatial distortions on DTI. The fluid-attenuated inversion recovery image (repetition time, 9000 milliseconds; echo time, 97 milliseconds; field of view, 210 × 210; 1 average and 2 concatenations; flip angle, 145°; slice thickness, 3 mm; matrix size, 256 × 256; 50 axial slices) was used with the MPRAGE image to rule out primary neurological abnormalities. The DTI and T2-weighted images were acquired using the same image properties to optimize registration. All scans were evaluated clinically, blinded for all other clinical data by a neuroradiologist.
DTI-Based WM Microstructural Assessment
We used the Automatic Registration Toolbox27 to prepare the FA maps for voxelwise comparisons in Talaraich space.28 First, we normalized the skull-stripped native MPRAGE image to the standard Montreal Neurological Institute brain template using a 3-dimensional nonlinear warping algorithm. A rigid-body linear transformation optimized the registration between T2-weighted and MPRAGE images by iteratively correcting for subject motion. With a nonlinear 2-dimensional warping algorithm, the non—diffusion-weighted b0 image was iteratively warped to correct for spatial distortions inherent in echo planar acquisitions using the skull-stripped T2-weighted image as an anatomical guide. Lastly, we applied the transformation parameters from previous steps and applied them to spatially correct and normalize the native FA maps to Talaraich space to reduce interpolation errors. These procedures have been previously described in more detail.7
Retinal Vessel Caliber Measurements
Retinal vessel photographs were obtained using a nonmydriatic 45° fundus camera (Canon CR4-45NM, Canon EOS Rebel 6.1MPix). All procedures were performed by a physician trained in ophthalmology (A.T.). Before the photography, participants were seated in a dimly lit room for 5 to 10 minutes to allow pupillary dilatation. The photographs were centered on the optic disc for each eye. Using semiautomated in-house–developed software, we measured the diameters of the 6 largest arterioles and 6 largest venules coursing through a standard area 0.5 to 1.0 disc diameter from the optic disc margin, and using standardized procedures15 we computed the central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE). To estimate the absolute measurements of the vessels and to account for refractive errors, we adjusted all vessel measurements to a standardized disc diameter of 1.850 mm.15 In our analyses, we used the averaged CRAE and CRVE of both eyes. Our method is well validated15 with high interrater reliability.29
Statistical Analysis
Dependent on the group size, the Kolmogorov-Smirnov test or Shapiro-Wilk test was used to evaluate data normality of continuous variables. Normally distributed continuous variables were tested with 2-tailed independent samples t tests (effect size expressed by Cohen d); otherwise, the Mann-Whitney U test (effect size expressed by r) was used. C-reactive protein (CRP) levels higher than 10 mg/L (to convert to nanomoles per liter, multiply by 9.524) may indicate acute inflammation and were thus excluded casewise from analyses involving CRP. Scores more than 3 SDs from the respective group means were excluded.
Multiple regression analysis assessed the differences in CRAE among adolescents who had 0, 1, 2, 3, or 4 or more MetS components after accounting for age and CRVE. Partial correlation analyses evaluated the associations between CRAE and the MetS components independent of age and CRVE. We chose systolic blood pressure (BP) as a continuous measure of hypertension as it is more frequently linked to microvascular abnormalities than diastolic BP.30 In these analyses, triglycerides data were transformed with natural log to correct for data skewness. Systolic BP remained nonnormally distributed after log transformation; therefore, raw values were used. A follow-up mediation analysis ascertained whether insulin sensitivity, as estimated by the QUICKI score, mediated the effects of obesity (waist circumference) when predicting CRAE, independent of age and CRVE. Although only 7 participants with MetS and 3 without MetS reached the threshold for hypertension, to account for subclinical BP elevations, we also controlled for systolic BP in the mediation analysis. The indirect effect (after accounting for the QUICKI score) was evaluated with the Sobel test31 and the bootstrapping method with 5000 bootstrapped samples at 95% confidence interval.32
Two-tailed voxelwise analyses examined the group differences in WM FA controlling for age. A subsequent 2-tailed voxelwise correlation analysis evaluated associations between WM FA and CRAE also controlling for age and venular diameter. A mask created from the average MPRAGE image of all participants restricted voxelwise analyses to WM. To reduce the likelihood of making type I errors and to adjust for multiple comparisons, we chose a false discovery rate33 of 1% and limited the accepted voxels showing statistical significance to those having at least 100 contiguous significant voxels (equivalent to at least 0.1 cm3 in volume) in the same direction.
Results
Demographic and Endocrine Data
The groups were demographically well matched (Table). Adolescents with MetS compared with those without MetS had a significantly larger waist circumference (mean [SD], 115.70 [15.00] vs 87.55 [16.39] cm, respectively; P < .001) and body mass index (calculated as weight in kilograms divided by height in meters squared; mean [SD], 38.88 [5.91] vs 26.52 [7.63], respectively; P < .001), worse glycemic control (mean [SD] QUICKI score, 0.31 [0.03] vs 0.36 [0.04], respectively; P < .001), elevated BP (mean [SD] systolic, 116.87 [12.51] vs 104.51 [10.28] mm Hg, respectively; P < .001; mean [SD] diastolic, 71.42 [10.18] vs 63.18 [7.17] mm Hg, respectively; P < .001), and a poorer lipid profile (mean [SD] high-density lipoprotein cholesterol, 40.18 [7.57] vs 51.22 [11.54] mg/dL, respectively [to convert to millimoles per liter, multiply by 0.0259]; P < .001; mean [SD] triglycerides, 106.59 [47.49] vs 69.31 [25.85] mg/dL, respectively [to convert to millimoles per liter, multiply by 0.0113]; P < .001). Adolescents with MetS compared with those without MetS also had significantly higher levels of plasma fibrinogen (mean [SD], 356.83 [94.23] vs 293.67 [51.98] mg/dL, respectively [to convert to micromoles per liter, multiply by 0.0294]; P = .004) and CRP (mean [SD], 7.15 [7.90] vs 2.29 [4.71] mg/L, respectively; P < .001) and had significantly higher self-ratings of subclinical depression on the BDI (mean [SD] BDI total score, 11.50 [9.43] vs 7.44 [6.63], respectively; P = .04). The groups did not differ on self-ratings of sleep apnea (mean [SD] score, 0.24 [0.17] vs 0.19 [0.13], respectively; P = .17) or level of physical activity (mean [SD] International Physical Activity Questionnaire total score, 4324.75 [3201.71] vs 4518.70 [3231.88] metabolic equivalent task minutes per week, respectively; P = .74).
Table. Descriptive and Endocrine Data.
| Characteristic | MetS (n = 39) |
No MetS (n = 51) |
P Value | Effect Size |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 17.82 (1.67) | 17.39 (1.61) | 0.23 | 0.26 |
| Range | 14.29-21.61 | 14.28-20.76 | ||
| Sex, No.a | ||||
| Female | 24 | 28 | 0.53 | |
| Male | 15 | 23 | ||
| School grade, mean (SD) | 11.78 (1.92) | 11.60 (1.76) | 0.66 | 0.10 |
| Socioeconomic status, mean (SD) | 2.14 (1.09) | 2.36 (1.37) | 0.63 | 0.05 |
| Race/ethnicity, No.a | ||||
| White | 11 | 12 | 0.94 | |
| Hispanic | 14 | 21 | ||
| African American | 9 | 11 | ||
| Asian | 5 | 7 | ||
| Meets criterion, No. (%)a | ||||
| IR | 39 (100) | 18 (35) | <.001 | |
| Waist circumference | 39 (100) | 15 (29) | <.001 | |
| HDL-C | 32 (82) | 14 (27) | <.001 | |
| Triglycerides | 15 (39) | 3 (6) | <.001 | |
| Hypertension | 7 (18) | 3 (6) | .07 | |
| BMI, mean (SD)b | 38.88 (5.91) | 26.52 (7.63) | <.001 | 0.67 |
| Waist circumference, mean (SD), cmb | 115.7 (15.00) | 87.55 (16.39) | <.001 | 0.66 |
| QUICKI score, mean (SD) | 0.31 (0.03) | 0.36 (0.04) | <.001 | 1.64 |
| Glucose, mean (SD), mg/dLb | 99.64 (66.48) | 75.57 (7.36) | .13 | -0.16 |
| Insulin, mean (SD), µIU/mLb | 23.54 (14.03) | 9.82 (7.72) | <.001 | -0.63 |
| Hemoglobin A1C, mean (SD), % of total hemoglobinb | 6.21 (2.30) | 5.25 (0.31) | .004 | -0.30 |
| HDL-C, mean (SD), mg/dL | 40.18 (7.57) | 51.22 (11.54) | <.001 | 1.10 |
| Triglycerides, mean (SD), mg/dLb,c | 106.59 (47.49) | 69.31 (25.85) | <.001 | 0.43 |
| Blood pressure, mean (SD), mm Hgb | ||||
| Systolic | 116.87 (12.51) | 104.51 (10.28) | <.001 | 0.49 |
| Diastolic | 71.42 (10.18) | 63.18 (7.17) | <.001 | 0.43 |
| Fibrinogen, mean (SD), mg/dLb | 356.83 (94.23) | 293.67 (51.98) | .004 | 0.37 |
| CRP, mean (SD), mg/Lb,d | 7.15 (7.90) | 2.29 (4.71) | <.001 | 0.45 |
| CRAE, mean (SD), µme | 182.35 (16.10) | 198.62 (19.03) | <.001 | 0.40 |
| CRVE, mean (SD), µme | 292.19 (27.63) | 279.68 (28.91) | <.001 | 0.31 |
| BDI total score, mean (SD)b | 11.5 (9.43) | 7.44 (6.63) | .04 | 0.22 |
| Sleep apnea score, mean (SD)b,f | 0.24 (0.17) | 0.19 (0.13) | .17 | 0.15 |
| IPAQ total score, mean (SD), MET-min/wkb | 4324.75 (3201.71) | 4518.7 (3231.88) | .74 | 0.04 |
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRAE, central retinal arteriolar equivalent; CRP, C-reactive protein; CRVE, central retinal venular equivalent; HDL-C, high-density lipoprotein cholesterol; IPAQ, International Physical Activity Questionnaire; IR, insulin resistance; MET, metabolic equivalent task; QUICKI, quantitative insulin sensitivity check index.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 8 0.0555; to convert insulin to picomoles per liter, multiply by 6.945; too 9 convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01; 10 to convert HDL-C to millimoles per liter, multiply by 0.0259; to convert triglycerides to millimoles per liter, multiply by 0.0113; to convert fibrinogen to micromoles per liter, multiply by 0.0294; and to convert CRP to nanomoles per liter, multiply by 9.524.
The χ2 test was used for categorical variables.
The Mann-Whitney U test was used for nonnormally distributed variables. Effect sizes are expressed as r.
One triglycerides outlier by more than 3 SDs was excluded from analysis.
Cases with a CRP level greater than 10 mg/L were excluded from analysis.
Raw vessel diameter values are presented. Group comparison was evaluated using univariate analysis controlling for CRVE when evaluating CRAE, and vice versa. Effect sizes are expressed as partial η2.
Based on 20-item questionnaire.
Effects of MetS Criteria on Retinal Vessel Diameter
As hypothesized, adolescents with MetS compared with those without MetS had significantly smaller CRAE (mean [SD], 182.35 [16.10] vs 198.62 [19.03] μm, respectively; P < .001) and larger CRVE (mean [SD], 292.19 [27.63] vs 279.68 [28.91] μm, respectively; P < .001), both with very large effect sizes (0.40 and 0.31, respectively) (Table). We confirmed with a univariate analysis of variance that the group difference in CRAE remained significant even after also accounting for the BDI score (data not shown). Linear regression analysis revealed that with increasing number of MetS criteria met, there were progressive reductions in CRAE (β = −8.61; Δr2 = 0.335; ΔF1,83 = 70.79; P < .001) (Figure 1A), independent of age and CRVE (βage = −0.75; βCRVE = 0.435; Δr2 = 0.272; ΔF2,84 = 15.71; P < .001). Two obese adolescents with MetS were excluded from the analysis because they were missing data for at least 1 of the criteria, leaving a total of 88 participants for the analysis; no participants met all 5 criteria.
Figure 1. Relationships Between Central Retinal Arteriolar Equivalent (CRAE) and Metabolic Syndrome (MetS) Components.
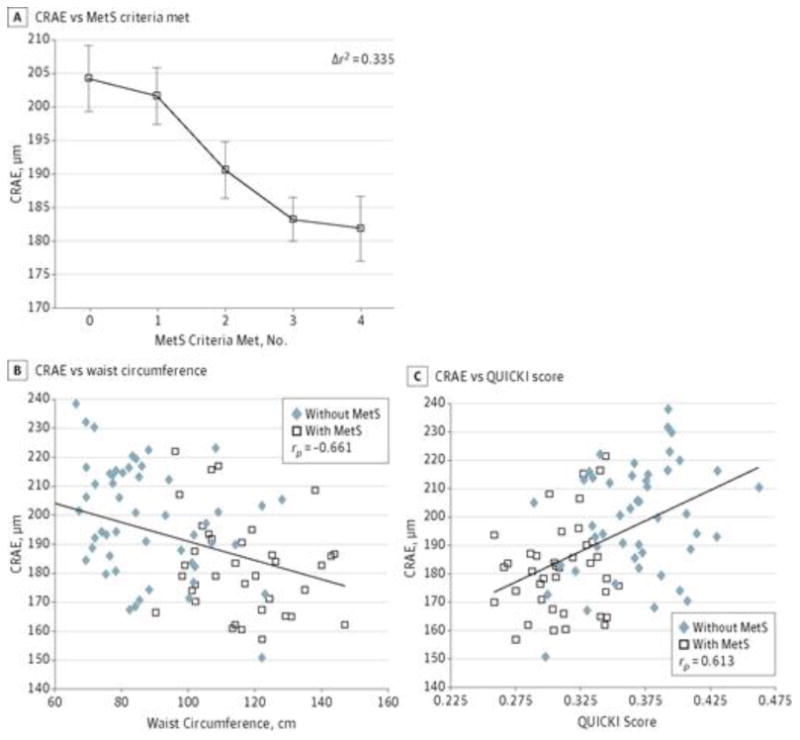
A, Smaller retinal diameter with increasing number of MetS components present for individuals who met 0 criteria (n = 16), 1 criterion (n = 17), 2 criteria (n = 18), 3 criteria (n = 25), or 4 criteria (n = 12). Data presented are raw retinal arteriolar diameters (CRAE values) expressed as mean (SEM [error bars]), but the statistics were done controlling for age, central retinal venular equivalent, and systolic blood pressure. B, Smaller CRAE is associated with larger waist circumference after accounting for age and central retinal venular equivalent. C, Smaller CRAE is associated with more insulin resistance (lower quantitative insulin sensitivity check index [QUICKI] score) after accounting for age and central retinal venular equivalent. B and C, The CRAE values presented are raw values; the analysis was performed controlling for age and central retinal venular equivalent.
Imaging Results: There were no clinically relevant WM hyperintensities on any of the fluid-attenuated inversion recovery images. The voxelwise analysis of covariance revealed a total of 11 clusters (2654 voxels or 2.654 cm3 in volume; P < .0005; the 6 largest clusters are displayed in order of size in Figure 2), all showing significant reductions in WM FA in adolescents with MetS. The clusters were located in major WM fibers including the optic radiation (right hemisphere, 565 voxels), left internal capsule (left hemisphere, 385 voxels), extreme capsule (left hemisphere, 359 voxels), superior cerebellar peduncle (left hemisphere, 314 voxels; right hemisphere, 161 voxels), corpus callosum (body, 286 voxels), cerebral peduncle (left hemisphere, 138 voxels; right hemisphere, 107 voxels), middle cerebellar peduncle (left hemisphere, 102 voxels; right hemisphere, 129 voxels), and corona radiata (left hemisphere, 108 voxels). The largest 4 clusters remained significant at the next more conservative P value threshold of .0001, and at this extremely conservative statistical threshold, they still totaled 579 voxels (Figure 2). Given the known association between depression and cerebral WM, we repeated the analysis adding the BDI score as a covariate and confirmed that the FA differences remained largely unchanged.
Retinal Arterial Diameter and MetS Components
Partial correlation analyses revealed that after accounting for age and CRVE, CRAE correlated most strongly with waist circumference (rp[85] = −0.661; P < .001) (Figure 1B), QUICKI score (rp[86] = 0.613; P < .001) (Figure 1C), and systolic BP (rp[85] = −0.622; P < .001), followed by triglycerides (rp[84] = −0.405; P < .001) and high-density lipoprotein cholesterol (rp[85] = 0.355; P < .001).
Given the high correlation between the QUICKI score and waist circumference (r[89] = −0.642; P < .001), we conducted a subsequent mediation analysis confirming that the QUICKI score partially mediated the negative association between CRAE and waist circumference independent of systolic BP. The direct effect of waist circumference on CRAE after accounting for QUICKI score was statistically significant (effect = −0.2452 [95% CI, −0.4084 to −0.0820]; t = −2.98; P < .004), as was the indirect effect of waist circumference (Sobel test, Z = −2.56; P = .01; bootstrapping, effect = −0.1355 [95% CI, −0.2471 to −0.0593]; SE = 0.0473).
Discussion
To our knowledge, this is the first report demonstrating reductions in CRAE in obese adolescents with MetS relative to matched controls. We also found progressive reductions in CRAE with increasing numbers of MetS components present. Importantly, we report for the first time that the retinal vessel alteration present in obese adolescents with MetS was most strongly related to increased waist circumference and that this relationship was partially mediated by IR and independent of subclinical elevations in BP. Our findings of reduced cerebral WM microstructural integrity are consistent with our prior reports in adolescents.7,8,34 More importantly, many of the cerebral WM regions showing significant FA reduction in MetS were the same regions showing robust associations with CRAE. Given that retinal vessels are a surrogate biomarker for cerebral arterioles,16 our robust findings of reduced retinal arteriolar diameter in MetS in adolescents strongly suggest that the observed subtle WM abnormalities may be related to cerebral microvascular damage.
Our finding of compromised retinal vessel health in obese adolescents with MetS is congruent with prior reports in obese children35 and adolescents17 and in adults with MetS,36 hypertension, and type 2 DM.9 Along with the finding of progressive reductions of retinal arteriolar diameter with increasing numbers of MetS components present, these data emphasize that retinal arterial diameter, a surrogate measure of cerebral arteriolar integrity, is particularly sensitive to metabolic disturbance. Unlike our prior report that suggests IR as the MetS component most strongly related to brain volume changes in obese nondiabetic adolescents with MetS,7 herein we report that central obesity (waist circumference) was the most prominent contributor to retinal vessel alteration and that its effect was partially mediated by comorbid IR. Although insulin normally has a dilatory effect on retinal arterioles, it is possible that insulin dysfunction (resistance) leads to vasoconstriction,37 which may in part contribute to the retinal observations. We have previously reported similar retinal vessel alterations among hypertensive adults.9 In the current study, the fact that BP was not a major contributor to the subclinical retinal pathology is likely due to the fact that very few of our adolescents (7 with MetS and 3 without) reached the threshold for hypertension; we have also confirmed that the observed retinal vessel alterations were not restricted to adolescents with elevated BP.
As expected, we uncovered widespread WM microstructural abnormalities in major fiber tracts in obese adolescents with MetS, similar to those reported previously.7 Of conceptual importance, many of the WM regions demonstrating FA reduction in MetS also showed associations with reductions in CRAE. Hypertension is a known risk factor for WM disease, and we have previously described associations between CRAE and WM FA in hypertensive adults.9 In an exploratory analysis, we confirmed that the associations remained largely unchanged after controlling for systolic BP (data not shown). Although we do not expect obese adolescents to have significant occlusive vascular disease, our data suggest that cerebral WM may be particularly sensitive to subclinical cerebral microvascular pathology.
Obesity, when coupled with IR, increases inflammation and oxidative stress,38 which in turn contribute to endothelial dysfunction and subsequently impair vascular reactivity and diminish blood supply crucial for maintaining cerebral WM integrity and function. It is possible that these mechanisms mediate some of the adverse effects of MetS on the brain. We have proposed this endothelial dysfunction to be at least partly responsible for these negative brain effects.39,40 Further, the obesity-inflammation link is well established, and as expected, our participants with MetS had marked elevation in both plasma fibrinogen and CRP levels. Future work should include detailed evaluation of endothelial and inflammatory markers as well as direct brain perfusion and cerebral vascular reactivity measures.
This study has several strengths. Our retinal measurement method is highly reliable and sensitive and the mediation effect of IR on the association between obesity and CRAE is strong and robust. In addition, our WM assessment method is unbiased and well documented and we used very conservative statistical thresholds. Our WM findings are consistent with prior reports in adolescents with obesity,34 MetS,7 and type 2 DM.8 The groups were moderate in size and were carefully matched demographically. Given our prior robust findings (medium to large effect sizes) using the same MRI and retinal evaluations,7,17 we felt that the current sample size was adequate. Further, our study participants are a nonclinical population and represent a real-life sample, with the control group not being totally metabolically healthy (eg, 35% of controls had IR and 29% were obese; they did not meet 3 of 5 criteria for MetS but may have met 1 or 2). Importantly, our results remained strong even after excluding those 7 adolescents with type 2 DM. As in our prior report,7 we used IR rather than hyperglycemia (only 5 participants, all with type 2 DM, had a fasting glucose level ≥100 mg/dL [to convert to millimoles per liter, multiply by 0.0555]) as one of our MetS components. One of the possible weaknesses of this study is the fact that given this definition of MetS, the results presented herein may not be directly comparable to those of the studies using impaired fasting glycemia (fasting glucose level ≥100 mg/dL). However, the use of IR instead of impaired fasting glucose level allows the study of adolescents in whom the presence of glucose abnormalities in the absence of diabetes is rare, even in the presence of significant obesity-associated IR.18 Our prior work in adolescents suggests a possible dose effect with more severe metabolic dysregulation giving rise to greater brain abnormalities.7,8,34 Future prospective studies with an expanded sample should track changes in the vascular and metabolic risk factors and explore possible interactions in their effects on brain and vascular health. In sum, this study suggests that retinal vessel imaging is a promising, noninvasive screening tool for early detection of cerebral microvascular complications, which is potentially important for early interventions.
Conclusion
We have previously reported subclinical brain white matter abnormalities in adolescents with MetS and type 2 DM. Herein, we show that reductions in retinal arteriolar width, a biomarker for cerebral microvascular integrity, are associated with central obesity and IR. Most importantly, we show clear evidence linking reductions in retinal arteriolar width with subclinical white matter abnormalities. These data suggest that subclinical brain WM abnormalities present in obesity and MetS may be microvascular in origin.
Figure 2. Six Largest Clusters of Significant Cerebral White Matter Fractional Anisotropy Reduction.
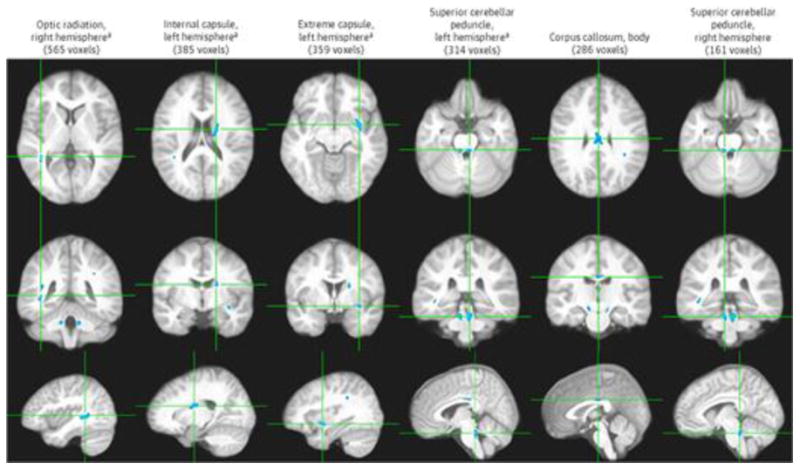
Six largest clusters of significant cerebral white matter fractional anisotropy reduction (P < .0005). Each column shows 3 orthogonal orientations of the average normalized structural image illustrating a significant cluster (blue; minimum cluster size of 100 contiguous voxels) with the axes passing through the centroid of the cluster.
aSignificant at P < .0001.
When we performed the voxelwise correlation analyses between WM FA and CRAE, we identified a total of 13 clusters (3150 voxels or 3.150 cm3; P < .001; the 6 largest clusters are displayed in Figure 3) of significant correlation independent of age and CRVE. All but 1 of these clusters showed positive correlation. The significant clusters were found in the optic radiation (left hemisphere, 439 voxels, rp2 = 0.149), middle cerebellar peduncle (right hemisphere, 422 voxels, rp2 = 0.157), corona radiata (left hemisphere, 354 voxels,rp2 = 0.143), corpus callosum (splenium, 301 voxels, rp2 = 0.131; genu, 279 voxels, rp2 = 0.139; body, 194 voxels, rp2 = 0.144), cerebral peduncle (left hemisphere, 283 voxels, rp2 = 0.147; right hemisphere, 277 voxels, rp2 = 0.147), superior cerebellar peduncle (left hemisphere, 143 voxels, rp2 = 0.135), extreme capsule (left hemisphere, 134 voxels, rp2 = 0.144), internal capsule (left hemisphere, 102 voxels, rp2 = 0.138), and cingulum (left hemisphere, 101 voxels, rp2 = 0.142). The single cluster that showed negative correlation was in the superior longitudinal fasciculus and was among the smallest (121 voxels, rp2 = 0.136). Of these 13 clusters, the 7 largest clusters, all showing positive association between CRAE and FA, remained significant at the next more conservative P value threshold of .0005; at this very conservative threshold, they still totaled 1458 voxels (Figure 3).
Figure 3. Six Largest White Matter Clusters All Demonstrating Significant Positive Association Between Fractional Anisotropy and Central Retinal Arteriolar Equivalent Independent of Age and After Controlling for Central Retinal Venular Equivalent.
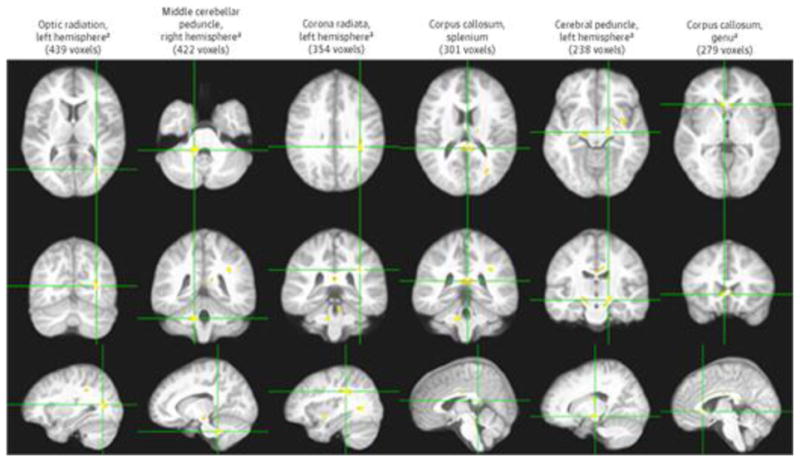
Six largest white matter clusters all demonstrating significant positive association between fractional anisotropy and central retinal arteriolar equivalent independent of age and after controlling for central retinal venular equivalent (P < .001). Each column shows 3 orthogonal orientations of the average normalized structural image illustrating a significant cluster (orange and yellow; minimum cluster size of 100 contiguous voxels) with the axes passing through the centroid of the cluster.
aSignificant at P < .0005.
Very importantly, 8 of the clusters showing significant WM FA reduction in MetS also demonstrated positive associations between FA and CRAE (Figure 4), suggesting that the subtle WM damage demonstrated in the FA analyses was likely vascular in nature.
Figure 4. Eight White Matter Regions Demonstrating Both Fractional Anisotropy Reductions and Positive Correlation With Central Retinal Arteriolar Equivalent.
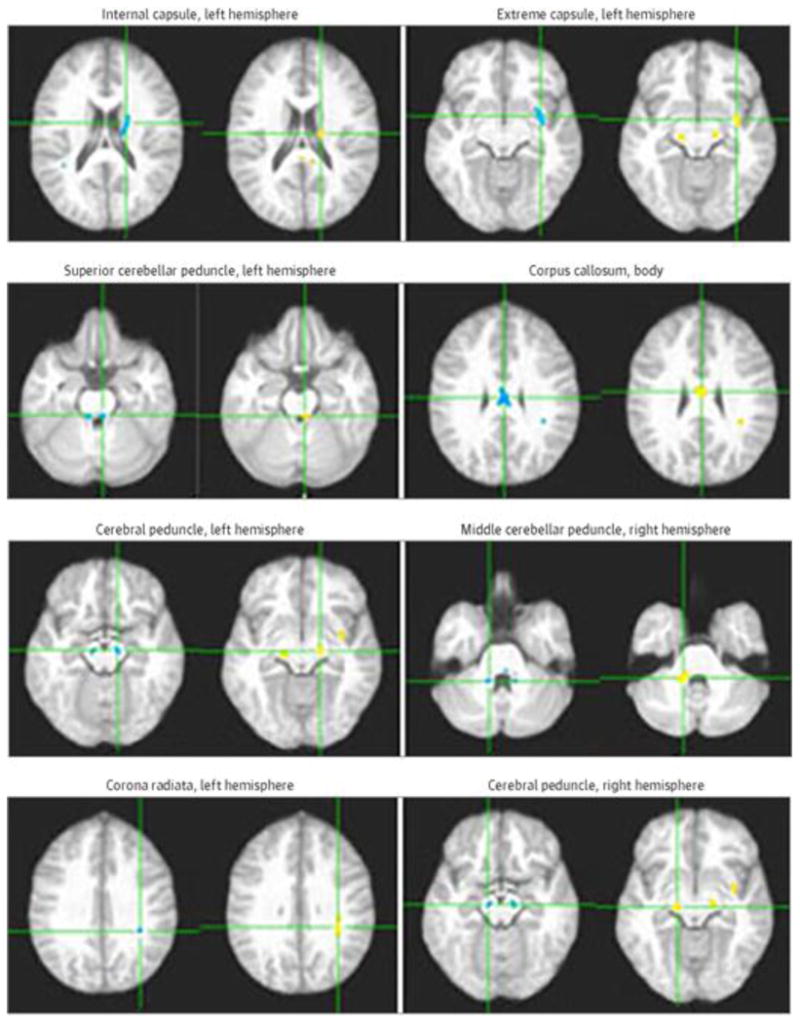
Eight white matter regions demonstrating both fractional anisotropy reductions in metabolic syndrome (blue clusters) and positive correlation between fractional anisotropy and central retinal arteriolar equivalent independent of age and central retinal venular equivalent (orange and yellow clusters) in axial view. Each panel shows the axes passing through the centroid of the cluster.
Acknowledgments
Funding/Support: This work was supported by grants DK 083537 from the National Institutes of Health and 1UL1RR029893 from the National Center for Research Resources.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Yau and Mr Kim contributed equally to this article. Dr Convit had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Convit.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Yau, Kim, Convit.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Yau, Kim, Convit.
Obtained funding: Convit.
Administrative, technical, or material support: Yau, Kim, Tirsi.
Study supervision: Yau, Kim, Convit.
Conflict of Interest Disclosures: None reported.
References
- 1.Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III) National Cholesterol Education Program (NCEP) NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 2.Kurl S, Laukkanen JA, Niskanen L, et al. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke. 2006;37(3):806–811. doi: 10.1161/01.STR.0000204354.06965.44. [DOI] [PubMed] [Google Scholar]
- 3.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr. 2008;152(2):165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Park HY, Kyeong-Ho, Park DS, et al. Correlation between insulin resistance and intracranial atherosclerosis in patients with ischemic stroke without diabetes. J Stroke Cerebrovasc Dis. 2008;17(6):401–405. doi: 10.1016/j.jstrokecerebrovasdis.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Kwon HM, Kim BJ, Park JH, et al. Significant association of metabolic syndrome with silent brain infarction in elderly people. J Neurol. 2009;256(11):1825–1831. doi: 10.1007/s00415-009-5201-8. [DOI] [PubMed] [Google Scholar]
- 6.Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39(5):1607–1609. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- 7.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53(11):2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yau PL, Hempel R, Tirsi A, Convit A. Cerebral white matter and retinal arterial health in hypertension and type 2 diabetes mellitus. Int J Hypertens. 2013;2013:329602. doi: 10.1155/2013/329602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mimoun E, Aggoun Y, Pousset M, et al. Association of arterial stiffness and endothelial dysfunction with metabolic syndrome in obese children. J Pediatr. 2008;153(1):65–70. doi: 10.1016/j.jpeds.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 11.Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23(10):1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 12.Huang K, Zou CC, Yang XZ, Chen XQ, Liang L. Carotid intima-media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Arch Pediatr Adolesc Med. 2010;164(9):846–851. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 13.Sipilä K, Moilanen L, Nieminen T, et al. Metabolic syndrome and carotid intima media thickness in the Health 2000 Survey. Atherosclerosis. 2009;204(1):276–281. doi: 10.1016/j.atherosclerosis.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Koivistoinen T, Hutri-Kähönen N, Juonala M, et al. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk in Young Finns Study. Ann Med. 2011;43(4):312–319. doi: 10.3109/07853890.2010.549145. [DOI] [PubMed] [Google Scholar]
- 15.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 16.Kwa VIH, van der Sande JJ, Stam J, Tijmes N, Vrooland JL, Amsterdam Vascular Medicine Group Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59(10):1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- 17.Tirsi A, Duong M, Tsui W, Lee C, Convit A. Retinal vessel abnormalities as a possible biomarker of brain volume loss in obese adolescents. Obesity (Silver Spring) 2013;21(12):E577–E585. doi: 10.1002/oby.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turchiano M, Sweat V, Fierman A, Convit A. Obesity, metabolic syndrome, and insulin resistance in urban high school students of minority race/ethnicity. Arch Pediatr Adolesc Med. 2012;166(11):1030–1036. doi: 10.1001/archpediatrics.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 20.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 21.Reeves GM, Postolache TT, Snitker S. Childhood obesity and depression: connection between these growing problems in growing children. Int J Child Health Hum Dev. 2008;1(2):103–114. [PMC free article] [PubMed] [Google Scholar]
- 22.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 23.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 24.Beck A, Steer RA, Brown GK. Manual for Beck Depression Inventory–II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 25.Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142(1):67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirsi A, Bruehl H, Sweat V, et al. Retinal vessel abnormalities are associated with elevated fasting insulin levels and cerebral atrophy in nondiabetic individuals. Ophthalmology. 2009;116(6):1175–1181. doi: 10.1016/j.ophtha.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 32.Hayes AF, Preacher KJ. Quantifying and testing indirect effects in simple mediation models when the constituent paths are nonlinear. Multivariate Behav Res. 2010;45(4):627–660. doi: 10.1080/00273171.2010.498290. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 34.Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22(8):1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanssen H, Siegrist M, Neidig M, et al. Retinal vessel diameter, obesity and metabolic risk factors in school children (JuvenTUM 3) Atherosclerosis. 2012;221(1):242–248. doi: 10.1016/j.atherosclerosis.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk in Communities study. Invest Ophthalmol Vis Sci. 2004;45(9):2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- 37.Siegrist M, Hanssen H, Neidig M, et al. Association of leptin and insulin with childhood obesity and retinal vessel diameters. Int J Obes (Lond) 2014;38(9):1241–1247. doi: 10.1038/ijo.2013.226. [DOI] [PubMed] [Google Scholar]
- 38.Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: markers and effect of lifestyle intervention. Obes Rev. 2012;13(5):441–455. doi: 10.1111/j.1467-789X.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- 39.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(1 suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32(9):2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]


