Abstract
There is a growing interest both in identifying the neural mechanisms of magnitude estimation and in identifying forms of bias that can explain aspects of behavioral syndromes like unilateral neglect. Magnitude estimation is associated with activation of temporo-parietal cortex in both cerebral hemispheres of normal subjects; however, it is unclear if and how left hemisphere lesions bias magnitude estimation because the infrequency of neglect and the presence of aphasia in these subjects confound examination. In contrast, we examined magnitude estimation using 12 different types of sensory stimuli that spanned five sensory domains in two patients with very different clinical presentations following unilateral left hemisphere stroke. One patient had neglect sub-acutely without aphasia. The other had aphasia chronically after a temporo-parietal lesion but not neglect. The neglect patient was re-examined 48 hours after being treated with modafinil (Provigil) for decreased arousal. Both patients demonstrated bias in magnitude estimation relative to normal subjects (n=83). Alertness improved in the neglect patient after taking modafinil. His neglect also resolved and his magnitude estimates more closely resembled those of normal subjects. This is the first evidence, to our knowledge, that the left hemisphere injury can bias magnitude estimation in a manner similar but not identical to that associated with right hemisphere injury.
Keywords: psychophysics, power law, modafinil (Provigil), stroke, unilateral neglect
1. Introduction
Magnitude estimation is a method of ratio scaling in which subjects use numbers to rate the intensity of supra-threshold sensory stimuli(Stevens, 1975a; Gescheider, 1997). Ratio scaling is the principle behind the Psychophysical Power Law (Stevens, 1975b) which holds that perceived stimulus intensity is a power function of objective intensity. Power functions (log-log relationships) are used to characterize the relationship between subjective estimates of stimulus magnitude and objective measures of stimulus intensity for a given type of sensory stimulus (perceptual continua). Power function transformations may reflect and organizing principle by which the peripheral and central nervous systems interact to construct mental representations of stimulus intensity; predominantly in the right cerebral hemisphere (Mennemeier et al., 2005; MacKay, 1963).
Magnitude estimation has been linked to the inferior parietal cortex of the right hemisphere in both human and animal studies of time, space and quantity perception (Walsh, 2003). Power function relationships are altered by right hemisphere injury, particularly in association with unilateral neglect (Chatterjee, Mennemeier, & Heilman, 1992b; Chatterjee, Dajani, & Gage, 1994; Chatterjee, Mennemeier, & Heilman, 1994; Chatterjee, 1995; Mennemeier, Rapcsak, Dillon, & Vezey, 1998; Mennemeier, Vezey, Lamar, & Jewell, 2002; Mennemeier et al., 2005) which follows damage to a neural system involving frontal, cingulate, temporal, and parietal cortices in both hemispheres (Heilman, Watson, & Valenstein, 1994; Mesulam, 1981; Vallar & Perani, 1987; Karnath, Ferber, & Himmelbach, 2001; Karnath, Himmelbach, & Rorden, 2002). When data from bedside tests for neglect are fit to power functions, a restricted range of perception is revealed in neglect patients signaled by a decrease in the power function exponent (a flatter slope) and an increase in the constant (an elevated y-intercept) relative to normal subjects (Chatterjee et al., 1992b; Chatterjee, Mennemeier, & Heilman, 1992a; Chatterjee et al., 1994; Chatterjee, 1995; Mennemeier et al., 2002; Mennemeier et al., 2005).
A restricted range of perception implies bias in magnitude estimation such that patients underestimate the intensity of greater stimuli and overestimate that of lesser stimuli when judging a range of sensory magnitudes (Cross, 1973; Hollingworth, 1909) In fact, patients with neglect following right hemisphere injury under-draw the lengths of long lines and over-draw short lines (Mennemeier et al., 2002; Mennemeier et al., 2005; Tegner & Levander, 1991; Chatterjee, 1995) and, on reading tasks, they may truncate long words and insert letters into short words (Chatterjee, 1995). More recently, bias in magnitude estimation, in the form or range restriction, has been shown following right hemisphere injury for non-spatial as well as spatial stimuli (Chatterjee, Thompson, & Ricci, 1998; Mennemeier, Murphey, Kretzmer, Jewell, & Nunn, 2003) and in patients with right hemisphere injury independent of neglect status (Mennemeier et al., 2003); Mennemeier, 2005). Neglect appears then to be a marker of both the location and volume of right hemisphere injury associated with bias in magnitude estimation.
No study to our knowledge has reported bias in magnitude estimation in association with left hemisphere injury; however, one functional imaging study of normal subjects found bilateral cortical activation of the intraparietal sulci and precentral and occipitotemporal regions when subjects compared stimuli on number, size and luminance dimensions (Pinel, Piazza, LeBihan, & Dehaene, 2004). While this finding might imply that magnitude estimates of stimulus intensity are constructed in both the left and right hemispheres, studies of magnitude estimation following unilateral brain injury have not found differences between patients with left hemisphere injury and normal control subjects (Mennemeier et al., 2002; Mennemeier et al., 2005; Mennemeier et al., 2003). Several factors could account for the paucity of documented alterations in magnitude estimation following left hemisphere injury. First, the left hemisphere might not construct magnitude estimates of stimulus intensity and the activation observed in fMRI studies during these tasks may only reflect inhibition of homologous regions in the left hemisphere. Second, communication disorders and the infrequency of neglect following left hemisphere injury (Ogden, 1987) may make it hard to detect bias in magnitude estimation following left hemisphere injury. In previous studies(Mennemeier et al., 2002; Mennemeier et al., 2005), patients with left hemisphere injury have had smaller brain lesions than those with right hemisphere injury, none had neglect and all were screened for language disturbances prior to study. Additionally, decreased arousal (hypoarousal) is less common clinically following left than right hemisphere injury. Hypoarousal exacerbates, if not causes, symptoms of neglect (Storrie-Baker, Segalowitz, Black, McLean, & Sullivan, 1996; Heilman, Watson, & Valenstein, 1985; Coslett, Bowers, & Heilman, 1987; Samuelsson, Hjelmquist, Jenson, Ekholm, & Blomstrand, 1998; Robertson, 2001). Therefore, all of the above mentioned factors could reduce the likelihood of detecting bias in magnitude estimation following left hemisphere injury.
We encountered two patients with unilateral strokes of the left hemisphere who provided a unique opportunity to address the above mentioned uncertainties. One patient had hypoarousal and right neglect, without aphasia, when tested in the sub-acute stage of a putaminal hemorrhage. The other had expressive aphasia but not neglect or hypoarousal when tested in the chronic stage of a stroke that involved temporo-parietal cortex. Both patients could complete tests of magnitude estimation. Whereas most studies use line bisection to examine psychophysical scaling and magnitude estimation in brain injured patients (for reviews see (Halligan, 1995; Monaghan & Shillcock, 1998; Mennemeier et al., 2005; Gregson, 2000), we used 12 perceptual continua spanning five sensory domains to examine the extent of bias in magnitude estimation. We sought to determine if 1) magnitude estimation is biased in association with right neglect and 2) if bias is associated with damage to temporo-parietal regions in the left hemisphere. Finally, the first patient was reassessed after treatment with modafinil (Provigil) for hypoarousal to determine if increased arousal is associated with improved neglect and reduced bias in magnitude estimation.
2. Methods
2.1 Participants
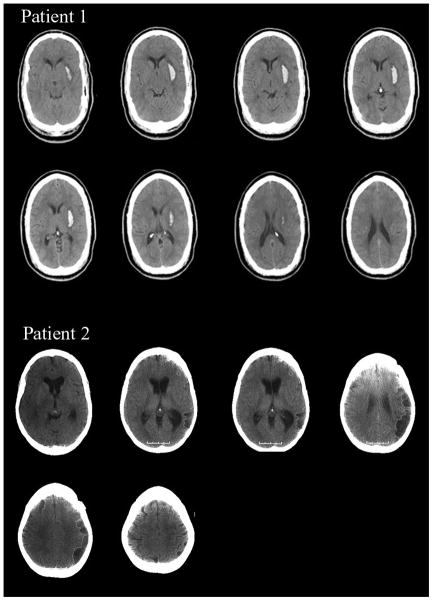
Patient 1 was a 39-year-old right-handed man with thirteen years of formal education who suffered a hemorrhagic stroke one week prior to testing. A CT scan obtained two days post-stroke revealed a hemorrhage in the left putamen (Figure 1, volume = 10.14 cc). Patient 1 suffered mild right hemiparesis but was not aphasic. His Western Aphasia Battery (Kertesz & Poole, 1974) Aphasia Quotient was 95.8 (normal range = 93.8–100). He was oriented to time, person and place but demonstrated acute deficits in arousal, often falling asleep during rehabilitation. Neglect was evident according to the Behavioural Inattention Test (BIT: (Wilson, Cockburn, & Halligan, 1987)) (124/146; neglect < 129), where right-sided omissions were observed on both cancellation and drawing tasks. Right neglect was also evident on line bisection. Patient 1 neglected approximately 10 percent of the line’s total length (20 cm) (Mennemeier et al., 1997). Finally, patient 1 could not complete part B of the Trail Making Test (Army Individual Test Battery, 1944) because he could not alternate between number and letter consistently.
Figure 1.
MRI scans of patient 1 indicating a putaminal hemorrhage in the left hemisphere. CT scans of patient 2 indicating hypodensities involving temporo-parietal cortex in the left hemisphere. Both scans are in radiological format (R=L, L=R).
Patient 2 was a 45-year-old right-handed woman with sixteen years of formal education who suffered a stroke approximately two years and three months prior to testing. A post-stroke CT scan revealed hypodensities in the left hemisphere presumably due to cerebral infarction (Figure 1). Mapping the lesion us ing Damasio templates (Damasio & Damasio, 1989) indicated clear involvement of temporo-parietal cortex, i.e., Brodmann’s Areas 21, 37, 22, 40, & 39. Patient 2 had expressive aphasia (Western Aphasia Battery Aphasia Quotient = 55.7) without hemiparesis. Her ability to follow sequential commands was poor (40/80). However, this appeared to reflect output rather than comprehension, per se, as her fluency was 0/10, repetition was 46/100, object naming was 42/60, and animal naming was 5/20. In contrast, information content was 10/10, auditory word recognition was 54/60 and yes-no responding was 57/60. Though patient 2 had difficulty speaking, she could express herself in writing. She did not evidence neglect on the BIT (142/146) and did not evidence any overt deficits in arousal. She too could not complete part B of the Trail Making Test as she was slow and made errors alternating between letter and number.
2.2. Magnitude estimation methods and procedures
Magnitude estimates were obtained in twelve perceptual continua for patient 1; however, only seven were administered to patient 2. In both cases, magnitude estimates spanned visual, tactile, proprioceptive, thermal, and gustatory sensory domains(see table 1). Three trials of eight stimulus intensities were presented in each perceptual continuum according to a pseudo-random schedule (no stimulus intensities were repeated). Subjects rated stimulus intensity using numbers from 10 (least intense) to 99 (most intense). They were instructed assign a number to each stimulus so that the size of the number matched their impression of stimulus intensity. Patient 2 gave written rather than oral responses due to her expressive aphasia. No standard or modulus for judgment was used. Stimuli were presented in the midsagittal plane within a comfortable viewing and reaching distance. Both patients completed the tests over a 48-hour period. Estimation tasks were not timed, except that temperature estimation was performed with a Medoc TSA-2001 Thermal Sensory Adaptor that administered stimuli for approximately five to ten seconds per trial, during which time subjects must provide a response. Patient 2 could not always write her responses in time and so was not tested using temperature.
Table 1.
Magnitude estimation stimuli
| Sensory Domain | Perceptual Continuum | Stimulus Description | Stimulus Magnitudes |
|---|---|---|---|
| Visual | Line Length | Line lengths on a 9.5 × 56 cm sheet of paper | 1, 3.2, 6, 11.5, 17, 28, 39, 50 cm |
| Area | Squares on a 24 × 24 cm sheet of paper | 1, 2.99, 6.05, 15.37, 28.94, 68.89, 125.8, 200 cm2 | |
| Numerosity | Dots arranged about a 26.5 × 33 cm sheet of paper | 6, 10, 15, 22, 36, 50, 71, 89 dots | |
| Reflectance | Chips of light reflectance material painted on 7.5 × 12.5 cm pieces of paper | 84.2, 63.6, 46.8, 33, 22.19, 13.7, 7.7, 3.8% light reflectance | |
| Tactile | Roughness (R & L) | Textures of sand paper applied to the fingertips | 24, 60, 100, 150, 220, 400, 800, 1200 grit |
| Proprioceptive | Finger Span (R & L) | Blocks of wood with constant pressure placed between the thumb and forefinger | 0.4, 0.8, 1.4, 2.4, 3.4, 4.4, 5.4, 6.3 cm |
| Thermal | Temperature (R & L) | 3.2 × 3.2 cm heated disk presented to the forearm | 36, 38, 40, 42, 44, 46, 48, 50 ° F |
| Gustatory | Sugar | Sugar concentrations diluted in water | 0.15, 0.30, 0.60, 0.70, 0.80, 0.90, 1.05, 1.20 M |
| Salt | Salt concentrations diluted in water | 0.19, 0.25, 0.32, 0.46, 0.60, 0.74, 0.87, 1.00 M |
2.3. Data analysis and statistics
Power functions were derived by log-transforming data and regressing numerical estimates of magnitude on objective measures of intensity. The power function exponent (slope of the regression line), constant (y-intercept) and r2 values for the two patients were compared to ninety-five percent confidence limits derived from a large sample of normal subjects (n = 83; mean age: 42.2 ± 20.9; years of education: 15.5 ± 2.5; 48 female & 35 male) tested in a manner identical to patient 1 (see table 2). The r2 value of the regression equation was evaluated for statistical significance and used to assess variability or how well the data fit a power function.
Table 2.
Magnitude estimation data for patients 1 & 2
| Continua | r2 r2 (95% limit)† |
Exponent Exp (95% limit)† |
Constant Con (95% limit)† |
|---|---|---|---|
| Patient 1 | |||
| Area | 0.93* (.88 – .91) | 0.35 (.35 – .37) | 8.70* (8.9 – 10.1) |
| Length | 0.77* (.88 – .91) | 0.51* (.53 – .57) | 6.47* (8.3 – 9.6) |
| Numerosity | 0.82* (.85 – .89) | 0.71 (.69 – .75) | 2.10* (2.7 – 3.8) |
| Reflect | 0.46* (.73 – .79) | 0.79* (1.0 – 1.1) | 0.76* (.35 – .68) |
| Rough R | 0.37* (.50 – .57) | 0.72* (.94 – 1.0) | 0.18 (−.02 – .37) |
| Rough L | 0.33* (.51 – .57) | 0.76* (.96 – 1.0) | 0.09 (.04 – .09) |
| Finger R | 0.75* (.84 – .88) | 0.65* (.69 – .74) | 2.71* (3.3 – 4.3) |
| Finger L | 0.76* (.84 – .88) | 0.67 (.67 – .72) | 2.78* (3.5 – 4.7) |
| Temp R | 0.53* (.56 – .63) | 4.45* (3.6 – 4.3) | 2.00E-06 (−.15 – .51) |
| Temp L | 0.22* (.56 – .64) | 2.55* (3.7 – 4.4) | 0.002 (−.006 – .07) |
| Sugar | 0.78* (.62 – .70) | 1.17* (.75 – 1.1) | 4.66* (7.6 – 9.4) |
| Salt | 0.64* (.70 – .76) | 1.04 (.97 – 1.1) | 10.1 (8.9 – 11.9) |
| Patient 2 | |||
| Area | 0.88 (.88 – .91) | 0.42* (.35 – .37) | 7.06* (8.9 – 10.1) |
| Length | 0.73* (.88 – .91) | 0.56 (.53 – .57) | 6.36* (8.3 – 9.6) |
| Numerosity | 0.84* (.85 – .89) | 0.79* (.69 – .75) | 1.81* (2.7 – 3.8) |
| Rough R | 0.31* (.50 – .57) | 0.88* (.94 – 1.0) | 0.08 (−.02 – .37) |
| Rough L | 0.40* (.51 – .54) | 0.94* (.96 – 1.0) | 0.44* (.04 – .09) |
| Sugar | 0.29* (.62 – .70) | 0.65* (.75 – 1.1) | 16.1* (7.6 – 9.4) |
| Salt | 0.45* (.70 – .73) | 0.91* (.97 – 1.1) | 15.3* (6.8 – 11.9) |
95% limits established by 83 NCP; age = 45.2 years, ed = 15.5 years;
outside 95% limit
3. Results
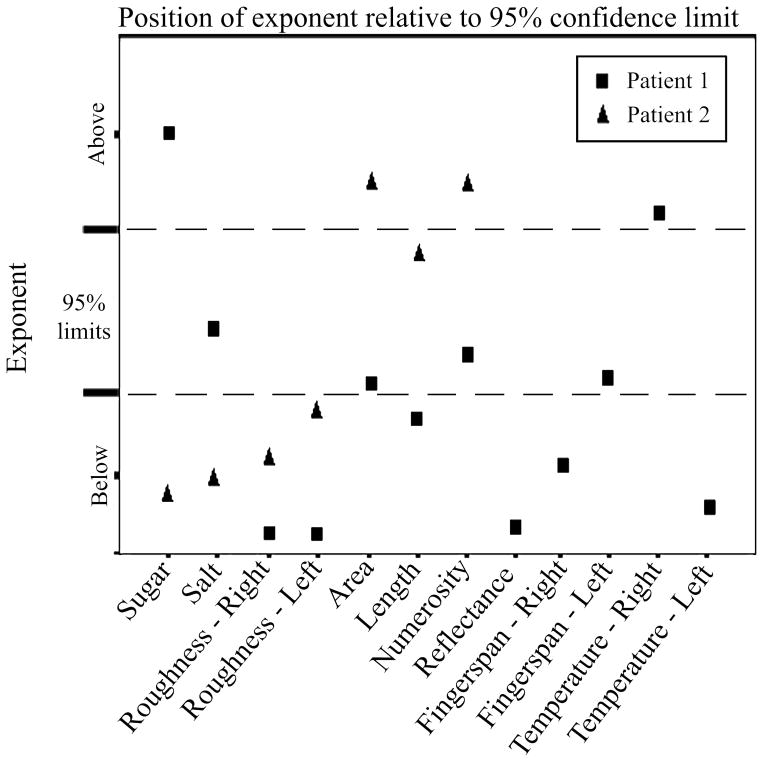
For patient 1, the r2 values of his power functions were statistically significant for all 12 perceptual continua; however, his r2 values were lower than the 95% confidence limits for 11/12 perceptual continua (Figure 2, Table 2). A lower r2 indicates greater variability. The size of patient 1’s exponents also fell below confidence limits for 7/12 perceptual continua but exceeded confidence limits for 2/12 continua (Figure 3, Table 2). The size of his constants fell below confidence limits for 6/12 continua and exceeded confidence limits on one continuum (Table 2).
Figure 2.
r-square values for each patient relative to 95% confidence limits established by normal control subjects (n=83).
Figure 3.
Size of exponent for each patient relative to 95% confidence limits established by normal control subjects (n=83).
For patient 2, the r2 value of her power functions was significant for all seven of the perceptual continua completed but her r2 values fell below confidence limits for normal subjects in 6/7 continua (Figure 2, Table 2). The size of patient 2’s exponents fell below confidence limits on 4/7 continua and exceeded them on 2/7 continua (Figure 3, Table 2). The size of the constants fell below confidence limits in 3/7 continua and exceeded confidence limits in 3/7 continua (Table 2).
3.1. Follow up study in patient 1
The day after patient 1 completed his magnitude estimation tests he was started on modafinil (Provigil) 200mg each morning to treat his hypoarousal. Forty-eight hours following the first dose of modafinil, patient 1 was retested on the BIT (Wilson et al., 1987) and Part B of the Trail Making Test, and he completed 11/12 of the previously administered magnitude estimation tests in a random order. (Judgments of salt concentrations in solution were excluded because patient 1 found them aversive.) The WAB was not re-administered because he performed normally on the first test administration. At the time of testing, patient 1’s family members described a marked improvement in his alertness during the day. His BIT score normalized from 124 pre-treatment to 143 post-treatment (total possible BIT score is 146). He no longer omitted lines on the line cancellation test, he bisected lines closer to true center (1% error), and his star drawing utilized right as well as left hemispace. He remained unable to complete part B of the Trail Making Test, again being unable to alternate between number and letter. Regarding magnitude estimation, patient 1’s r2 values fell below 95% confidence limits on 5/11 perceptual continua (11/12 previously) but exceeded them on 6/11 continua. The size of his exponents fell below 95% confidence limits on 1/11 perceptual continua (7/12 previously) and exceeded them on 6/11 (2/12 previously). The size of the constant was essentially the same, falling below 95% confidence limits on 7/11 perceptual continua (6/12 previously) and exceeded them on 1/11 (also 1/12 previously).
4. Discussion
The two patients reported in this study provide the first evidence to our knowledge that left hemisphere injury can bias magnitude estimates of stimulus intensity. When care is taken to match patients with left and right hemisphere lesions on behavioral characteristics, like neglect, or lesion location, left hemisphere injury altered power function parameters in a manner similar, but not necessarily identical, to that observed following right hemisphere injury.
Patient 1’s data are consistent with that of patients with right hemisphere lesions and left neglect (Mennemeier et al., 2005). His magnitude estimates fit power functions but were more variable than normal as denoted by a decreased r2 value and the size of his power function exponents were different than normal, most often being lower, in most perceptual continua tested (8/12). In contrast to patients with left neglect following right hemisphere injury; however, the size of patient 1’s constants were typically lower than normal rather than higher. While it can not be determined with certainty if a decreased constant is specific to patients with left hemisphere injury; however, this finding is a notable departure from patients with left neglect after right hemisphere injury, who typically have higher constants (Chatterjee, 1995; Chatterjee et al., 1994; Mennemeier et al., 2005; Mennemeier et al., 2003).
Forty-eight hours after starting modafinil, Patient 1’s alertness was reported to improve, his neglect resolved, and both the variability of magnitude estimation and the size of his power function exponents more closely resembled those of normal subjects despite remaining unable to complete part B of the Trail Making Test. It seems unlikely that altered ratio scaling is merely the result of cognitive dysfunction because patients 1’s performance on Trails B did not improve 48 hours after taking modafinil even though his performance on magnitude estimation did improve. Additionally, in a large group study of magnitude estimation (Mennemeier et al., 2003), performance on Trails B (both percentile scores and total time to completion) was negatively correlated with variability in magnitude estimation but not with the size of the power function exponents or constants arguing against the notion that altered ratio scaling is merely an artifact of cognitive disturbance. We can not infer whether patient 1’s improvement is due to modafinil or factors like spontaneous recovery because he was prescribed modafinil for clinical reasons and could not be withdrawn. Practice could account for some improvement; however, in a group study of length estimation (Pierce, Jewell, & Mennemeier, 2003) practice did not altered power function parameters at two week testing intervals. Whatever the reason for patient 1’s improvement, the end result was increased alertness associated with improved neglect and reduced bias in magnitude estimation. Moreover, his improvement suggests that arousal mechanisms may contribute to magnitude estimation and, at the least, it warrants a more controlled investigation of this potentially useful form of treatment..
Patient 2 showed that magnitude estimation is biased following damage to temporo-parietal regions of the left hemisphere even in the absence of behavioral symptoms of neglect. Her data are consistent with previous studies of magnitude estimation in patients without neglect following right hemisphere injury (MacKay, 1963; Mennemeier et al., 1998; Mennemeier et al., 2002; Mennemeier et al., 2005). Patient 2 had an expressive aphasia but we do not think deficits in comprehension or writing account for bias in magnitude estimation because her data fit power functions, indicating response consistency, and her r2 values were not appreciably lower than either patient 1 or patients with right hemisphere injury who do not have aphasia. Like patient 1, the size of her power function exponents were typically lower than normal but the size of her constants were not consistently altered in one direction. Also like patient 1, two of her exponent values were higher than normal which suggest that perceptual ranges might be expanded as well as restricted following brain injury. These patients are just beginning to establish patterns of bias in magnitude estimation across multiple perceptual continua; clearly larger studies are necessary to confirm these patterns.
Substantial differences between patients 1 and 2 in lesion type and chronicity, neglect, aphasia, and arousal make it unlikely that any one factor can account for altered ratio scaling in both patients. Rather what these patients have in common is damage to a fronto-cingulo-temporo-parietal network in the left hemisphere - implicated in patient 1 by the presence of neglect and in patient 2 by lesion involvement. These observations converge with Pinel et al (Pinel et al., 2004) findings of bilateral, temporo-parietal activation during magnitude estimation and extend them to suggest that the left hemisphere is contributing to magnitude estimation. A remaining question for future studies concerns what factors might account for the relatively stronger empirical link between magnitude estimation and the right rather than the left hemisphere. Three possibilities seem reasonable at this juncture: 1) the observation that magnitude estimation is most severely biased in patients with neglect could suggest that attentional deficits, which are more common after right hemisphere injury, alter power function parameters, 2) the right hemisphere may have a greater influence on arousal responses (Heilman, Watson, Valenstein, & Goldberg, 1987) that influence sensory perception (Llinas, Ribary, Contreras, & Pedroarena, 1998) and 3) the left and right hemispheres may construct mental representations of stimulus magnitude equally well, as implied by (Pinel et al., 2004), but communication disorders may obscure bias in magnitude estimation following left hemisphere injury.
Acknowledgments
Supported by the National Institutes of Neurological Disorders and Stroke (NS39348) – Mennemeier, Jewell, Mark, Woods; the National Center for Medical Rehabilitation Research (R03 HD042519-01A1, R03 HD40631, and R01 HD34273-04-05) – Mark, Mennemeier, Woods; the John A. Hartford Foundation/Southeast Center of Excellence in Geriatric Medicine – Mark, Woods; the National Institute on Aging (R03 AG 21256-01) – Mark, Woods; and the National Center for Research Resources (RR020146) – Mennemeier, Woods, Garcia-Rill.
Reference List
- Army Individual Test Battery. Washington, D.C: War Department; Adjunct General’s Office. Army Individual test Battery; 1944. [Google Scholar]
- Chatterjee A. Cross-over, completion and confabulation in unilateral spatial neglect. Brain. 1995;118:455–465. doi: 10.1093/brain/118.2.455. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Dajani BM, Gage RJ. Psychophysical constraints on behavior in unilateral spatial neglect. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1994;7:267–274. [Google Scholar]
- Chatterjee A, Mennemeier M, Heilman KM. A comparison of line bisection and cancellation in unilateral neglect. Journal of Clinical & Experimental Neuropsychology. 1992a;14:85. [Google Scholar]
- Chatterjee A, Mennemeier M, Heilman KM. A stimulus-response relationship in unilateral neglect: the power function. Neuropsychologia. 1992b;30:1101–1108. doi: 10.1016/0028-3932(92)90101-q. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Mennemeier M, Heilman KM. The psychophysical power law and unilateral spatial neglect. Brain and Cognition. 1994;25:92–107. doi: 10.1006/brcg.1994.1025. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Thompson KA, Ricci R. Weigh(t)ing for awareness. Brain and Cognition. 1998;37:477–409. doi: 10.1006/brcg.1998.1009. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Bowers D, Heilman KM. Reduction in cerebral activation after right hemisphere stroke. Neurology. 1987;37:957–962. doi: 10.1212/wnl.37.6.957. [DOI] [PubMed] [Google Scholar]
- Cross DV. Sequential dependencies and regression in psychophysical judgments. Perception and Psychophysics. 1973;14:547–552. [Google Scholar]
- Damasio H, Damasio AR. Lesion Analysis in Neuropsychology. 1. New York: Oxford University Press; 1989. [Google Scholar]
- Gescheider GA. Psychophysics: The Fundamentals. Mahwah: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- Gregson RAM. Magnitude estimation for line bisection under lateral visuo-spatial neglect. Nonlinear Dynamics, Psychology, and Life Sciences. 2000;4:219–233. [Google Scholar]
- Halligan PW. Drawing attention to neglect: the contribution of line bisection. The Psychologist. 1995;8:257–264. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 2. New York: Oxford University Press; 1985. pp. 243–293. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Localization of lesions in neglect and related disorders. In: Kertesz A, editor. Localization and Neuroimaging in Neuropsychology. New York: Academic Press; 1994. pp. 495–524. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Goldberg ME. Attention: behavior and neural mechanisms. In: Plum F, editor. The Nervous System: Higher Functions of the Brain. Bethesda: American Physiological Society; 1987. pp. 461–481. [Google Scholar]
- Hollingworth HL. The indifference point. In: Hollingworth HL, editor. The Inaccuracy of Movement. New York: The Science Press; 1909. pp. 21–39. [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden M. The subcortical anatomy of human spatial neglect; putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philosophical Transactions of the Royal Society of London. 1998:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D. Psychophysics of perceived intensity. Science. 1963;139:1213. [Google Scholar]
- Mennemeier M, Murphey HL, Kretzmer T, Jewell G, Nunn T. Altered psychophysical function in neglect across a dozen perceptual continua. Journal of the International Neuropsychological Society. 2003;9:199. [Google Scholar]
- Mennemeier M, Pierce CA, Chatterjee A, Anderson B, Jewell G, Dowler R, et al. Bias in attentional orientation and magnitude estimation explain crossover: neglect is a disorder of both. Journal of Cognitive Neuroscience. 2005;17:1194–1211. doi: 10.1162/0898929055002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeier M, Rapcsak SZ, Dillon M, Vezey E. A search for the optimal stimulus. Brain and Cognition. 1998;37:439–459. doi: 10.1006/brcg.1998.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeier M, Vezey E, Lamar M, Jewell G. Crossover is not a consequence of neglect. Journal of the International Neuropsychological Society. 2002;8:107–114. [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Shillcock R. The cross-over effect in unilateral neglect. Modeling detailed data in the line-bisection task. Brain. 1998;121:907–921. doi: 10.1093/brain/121.5.907. [DOI] [PubMed] [Google Scholar]
- Ogden JA. The neglected left hemisphere and its contribution to visuo-spatial neglect. In: Jeannerod M, editor. Neurophysiological and neuropsychological aspects of spatial neglect. 1. Amsterdam: Elsevier Science; 1987. pp. 215–234. [Google Scholar]
- Pierce CA, Jewell G, Mennemeier M. Are psychophysical functions derived from line bisection reliable. Journal of the International Neuropsychological Society. 2003;9:72–78. doi: 10.1017/s1355617703910083. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, LeBihan DL, Dehaene S. Distributed and overlapping cerebral representations of number, size and luminance during comparative judgments. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Robertson IH. Do we need the “lateral” in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. Neuroimage. 2001;14:S85–S90. doi: 10.1006/nimg.2001.0838. [DOI] [PubMed] [Google Scholar]
- Samuelsson H, Hjelmquist EK, Jenson C, Ekholm S, Blomstrand C. Nonlateralized attention deficits; an important component behind persisting visuospatial neglect. Journal of Clinical & Experimental Neuropsychology. 1998;20:73–88. doi: 10.1076/jcen.20.1.73.1481. [DOI] [PubMed] [Google Scholar]
- Stevens SS. Psychophysics: introduction to its perceptual, neural, and social prospects. New York: John Wiley & Sons; 1975a. [Google Scholar]
- Stevens SS. The psychophysical law. In: Stevens G, editor. Psychophysics: introduction to its perceptual neural and social prospects. Toronto: John Wiley & Sons; 1975b. pp. 1–36. [Google Scholar]
- Storrie-Baker HJ, Segalowitz SJ, Black SE, McLean JA, Sullivan N. Improvement of hemispatial neglect with cold-water calorics: an electrophysiological test of the arousal hypothesis of neglect. Journal of the International Neuropsychological Society. 1997;3:394–402. [PubMed] [Google Scholar]
- Tegner R, Levander M. The influence of stimulus properties on visual neglect. Journal of Neurology, Neurosurgery, and Psychiatry. 1991;54:882–886. doi: 10.1136/jnnp.54.10.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of spatial neglect in humans. In: Jeannerod M, editor. Neurophysiological and neuropsychological aspects of spatial neglect. Amsterdam: Elsevier Science; 1987. pp. 235–258. [Google Scholar]
- Walsh V. A theory of magnitude; common cortical metrics of time, space and quantity. Trends in Cognitive Neuroscience. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Halligan PW. Behavioral Inattention Test. Titchfield, Tants: Thames Valley Test Co; 1987. [Google Scholar]