Abstract
Sjögren’s syndrome (SS) is a complex polygenic autoimmune disorder. A few major genetic effects have been identified. Historically, HLA and non-HLA genetic associations have been reported. Recently, the HLA region continued to reveal association findings. A new susceptibility region has been suggested by a study of a D6S349 microsatellite marker. Among non-HLA studies, recent association of immunoglobulin κ chain allotype KM1 with anti-La autoantibodies in primary Sjögren’s syndrome confirms findings in a study from two decades ago. Meanwhile, mouse models have been employed to study the genetic contribution to salivary lymphadenitis or dry eyes and mouth. Gene transfer exploration in mouse models shows promise. The authors review the HLA and non-HLA association studies and the mouse model work that has been reported. Newly developed genomic capacity will provide, in the future, a much closer approximation of the true picture of the genetic architecture of Sjögren’s syndrome.
Keywords: Sjögren’s syndrome, Genetics, HLA, Histocompatibility
Introduction
The technical capacity to explore the human genome in detail has arrived. We can evaluate almost any of the approximate 3 billion human DNA base pairs or estimated 10 million common Homo sapiens DNA polymorphisms. The genetic revolution has already generated billions of data points in attempts to identify genetic risk for those common disease phenotypes with high mortality, such as breast cancer, type 1 diabetes, and heart disease. Sjögren’s syndrome (SS) and a large number of more rare phenotypes have been slower to benefit from new technical capacities. The present progress trajectory hopefully will envelop these phenotypes of less compelling public interest, resulting in gene discoveries that can redefine their pathogenic mechanisms.
What are the prospects for genetic discovery in Sjögren’s syndrome? Certainly, no easily identified Mendelian pattern has been reported percolating through SS patient families. In fact, there is no population-based study of SS familial aggregation patterns or comparison of concordance between monozygotic and dizygotic twins, either of which would be very helpful to estimate the genetic contribution to disease causation.
Modern geneticists characterize the SS phenotype that consists of suspected, yet undiscovered, interplay of various genes, multiple genetic mechanisms, and environmental triggers, as “complex”. Sjögren’s syndrome features genetic complexities that are compounded by clinical setting presentations with diverse combinations of organ specific, systemic, exocrine and/or nonexocrine manifestations.
Diagnosis ambiguity and prognosis variability present another layer of complexity for Sjögren’s study. No specific diagnostic criteria, exclusively or conclusively, establish Sjögren’s syndrome. Likewise, no specific set of criteria is uniformly employed by all current clinical investigators, albeit a recently adopted European–American Consensus Criteria [1] has been added to the panoply of choices.
Genetic etiology in autoimmune disorders is traditionally surmised by recognition of familial aggregation. Genetic predisposition in SS development was first recognized in a 1937 study of three-generation kindred [2]. Since that time, genetic contributions to particular disease symptoms in SS are only beginning to be understood. To date, the best family study in primary SS for establishing a tendency for familial aggregation evaluated six multiplex kindreds. Segregation analysis suggested that a Mendelian dominant genetic effect for autoimmunity was present in these pedigrees. This genetic effect was not apparently linked to HLA or heavy chain immunoglobulin genes. These authors made the still valid conclusion that the genetic origin of SS results from the interaction of several genes [3].
Although only a relatively few genetic studies of Sjögren’s syndrome have been performed, a few of the major genetic effects have been identified. Two convincing associations dominate identified genetic effects:
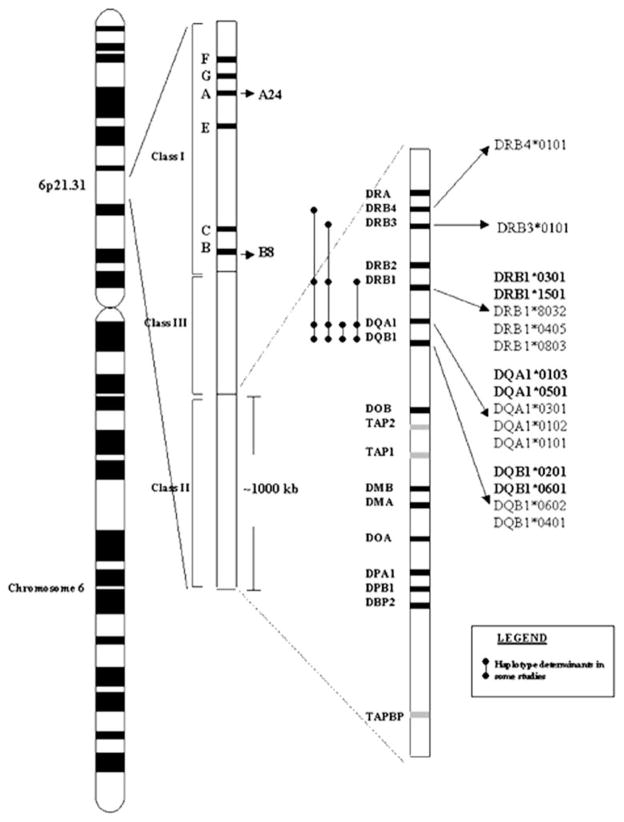
Association with the locus called human leukocyte antigen (HLA) locus, located on human chromosome 6p21.3 (Fig. 1)
Confirmed association of immunoglobulin κ chain allotype KM1 with anti-La autoantibodies in primary Sjögren’s syndrome [4, 5] (Table 1).
Fig. 1.
The human leukocyte antigen (HLA) allele reported to have associations with primary Sjögren’s syndrome. Confirmed associations are in bold type
Table 1.
Reported Sjögren’s syndrome genetic associations outside the human leukocyte antigen (HLA) region
| Gene | Polymorphism site | Allele or genotype | Association found | Study (case/control) | Association not found (case/control) |
|---|---|---|---|---|---|
| ApoE | ApoE ε4 | Early onset Primary SS | Pertovaara et al. [28] (63/64) | ||
| CCR5 | CCR53Δ2 | Lower in Primary SS | Petrek et al. [22] (39/76) | ||
| Fas | Nt–671 | G/G | Primary SS | Bolstad et al. [26] (70/72) | Mullighan et al. [27] (108/101) |
| IVS2nt176 | SNP C/T | ||||
| IVS5nt82 | SNP C/G | ||||
| GSTM1 | Homozygous null genotype | Primary SS | Wang et al. [29] (106/143) | ||
| HA-1 | Nt+500/504* | 168His | Lower in Primary SS | Harangi et al. [30] (88 /371) | |
| IL-1RA | Intron-2 | IL1rn*2 | Primary SS | Perrier et al. [24] (36/100) | Petrek et al. [22] (39/76) |
| IL-4Rα | Q576R | Primary SS | Youn et al. et al. [31] (45/74) | ||
| Ramos-Casals et al. [25] (48/98) | |||||
| Wang et al. [29], | |||||
| IL-10 | Promoter -1082 | SNP G/A | Primary SS | Hulkkonen et al. [23] (62/400) | Rischmueller et al. [34], (108/165) |
| Promoter -819 | SNP C/T | ||||
| Promoter -592 | SNP C/A | ||||
| Promoter (-1,082, -819, -592) | ATA/ATA | Origuchi et al. [32], (47/107) | |||
| Promoter (-1,082, -819, -592) | GCC/ATA | Font et al. [33], (63/150) | |||
| G9 | SS w/ cutaneous vasculitis | Gomez et al. [17], (39/15) | |||
| Ig KM 26/1204 anti-La in 1 SS [4] | KM1 | Anti-La in Primary SS | Whittingham et al. [4], (26 /1204) Pertovaara et al. [28], (6/26) |
||
| MBL | Codon 54 | Wild-type allele | Primary SS | Wang et al. [29], (104/143) | Mullighan et al. [27], (97/106) |
| Homozygous mutant allele | Lupus, RA, SS | Tsusumi et al. [35], (266/129) | Aittoniemi et al. [36], (62/400) | ||
| PTPN22 | Nt. 1858 | SNP C/T | Primary SS | Gomez et al. [17], (70/308) | Ittah et al. [21], (183/172) |
| Criswell et al. [9], (16/2064) | |||||
| Ro52 | Intron 1 (nt. 7216) | SNP C/T | Anti-Ro in SS | Nakken et al. [37], (97/72) | |
| Intron-3 (137 from exon4) | SNP A/G | Anti-Ro52 in Primary SS | Imanishi et al. [38] (111/97) | ||
| TCRBV | Deletion/Deletion | Primary SS | Lawson et al. [39], (61/121) | ||
| TGFβ1 | Codon 10 | SNP C/T | Primary SS with anti-La | Gottenberg [10], (92/254) |
Two nucleotide polymorphism
Susceptibility Genes
Susceptibility gene identification provides diagnostic and prognostic tools. Susceptibility genes define SS disease risk variation within the human species. Each gene discovered to contribute to disease risk establishes part of disease causation. Genes that carry risk are causation genes that define the mechanism of disease and are critical to basic clinical and scientific comprehension of pathogenesis. Identification and inventorying susceptibility genes is the first step towards clinical applications of such knowledge.
Susceptibility gene identification appears to be only now barely possible in complex diseases, such as Sjögren’s syndrome. Once identified, how quickly we achieve a subsequent understanding of how they operate is completely unpredictable. Some genes will be like HLA-B27 in ankylosing spondylitis where the mechanism is not yet agreed three decades after discovery [6], while others will be like sickle cell anemia, where the discovery of the structural changes and oxygen-binding behavior of the mutant form clearly implies mechanism [7].
With only ~25,000 genes in the human genome, the task looks less daunting than a few years ago when estimates were that humans consisted of >150,000 genes. Even so, no effort has been made in Sjögren’s syndrome to evaluate all of these genes and the intervening sequence simultaneously in what is commonly called a “genome scan”. No doubt, the ink will hardly be dry on this review before this more global approach will be accomplished for Sjögren’s syndrome, as well. This discussion is therefore historically restricted to candidate gene results from association and linkage studies.
Before being able to understand how candidate genes interplay to increase disease risk, we need to identify the players. The specter of false positive results is always a risk in association studies. Some have estimated that only about a third of published association results are replicated and, thereby, sufficiently confirmed to conclude that they are robust to a degree of true-positive associations [8]. Replication is a minimum, although powerful requirement of the scientific method, and is the major problem for most genetic associations now alleged.
Working List of Candidate Genes
HLA Region
Recent findings from larger samples are consistent with older studies supporting a genetic predisposition from HLA class II marker alleles [9]. Except for a strong association of anti-Ro(SS-A) and anti-La(SS-B) autoantibodies to the HLA region (Fig. 1), there is, as yet, only modest support for disease severity or other clinical subsets being associated with the HLA region, despite reports to the contrary that have not been replicated. Indeed, some show that without these autoantibodies, they find no HLA association with Sjögren’s syndrome, whatsoever [10].
The first reported HLA association with Sjögren’s syndrome was in 1976 with B8, the HLA Class I allele I of HLA-B that is on the HLA-HR3 haplotype [11]. In 1975, HLA-DR3 had been shown to be associated with serologically defined Dw3 by Tom Chused and colleagues. This was the first evidence of the now well-known and many times replicated HLA-DR3 association with Sjögren’s syndrome [12]. DR3 and B8 are in disequilibrium, being found together more frequently than predicted by chance. As the B8 association vanishes when DR3 is considered but controlling for B8 does not entirely remove the DR3 effect [13], the associations with B8 and DR3 are probably measuring the same association, and furthermore, the primary association is with DR3. This means that the HLA-DR locus is closer to the causative gene than is HLA-B. The existing data are not sufficiently discriminating to determine whether the causative allele is DR3 itself, or not.
There are a number of important immune system genes in the HLA region in addition to the members of HLA Class I and II, including TNF, LTA, LTB, TAP, and LMP genes, some of which have also been associated. Of these, neither of the associations between anti-Ro positive primary Sjögren’s and TNFα [14] or TAP2 [15] were replicated in the subsequent study by Jean et al. [16].
An interesting example of association in the HLA region, but 2 Mb away from the HLA-DR, is the D6S349 microsatellite marker. Located in a region of otherwise undistinguished DNA, it is associated and may identify a new susceptibility region for primary Sjögren’s syndrome [17]. As of this writing, no known genes reside in the haplotype block containing D6S439.
The extent of disequilibrium has been estimated for some haplotypes in HLA [e.g. DR3) to be as large as 3.5 Mb, complicating the interpretation of any association result, as the example demonstrates of DR3 and B8, which are both on this haplotype and presented above.
Dissecting the interplay between various effects of multiple alleles of a gene and multiple genes remains a daunting and largely unsolved problem. Both systemic lupus erythematosus and Sjögren’s syndrome are associated with DR3 and DR2 and illustrate this issue. A large association study done by Graham and colleagues [18] shows that disequilibrium around the DR2 association disintegrates relatively rapidly, while that around DR3 does not, and hence, the 3.5 Mb haplotype. This is the same as appreciating that the DR2 containing haplotypes responsible for the observed association tend to be much smaller than the associated DR3 containing haplotype.
There is strong evidence that some haplotypes operate together. Indeed, gene complementation at HLA-DQ was described two decades ago with the combination of the DQw1 and DQw2 allelic serotypes producing an odds ratio >17 for high levels of anti-Ro and anti-La in primary Sjögren’s syndrome patients [19], suggesting a synergistic interaction between alleles.
PA Correa and colleagues present the interesting observation that a TNFα allele and haplotype increases risk for primary Sjögren’s syndrome, while the same allele appears to protect from tuberculosis [20]. Perhaps, what we currently view as “HLA susceptibility genes” should be thought of as an incredibly complicated equation that influences the survival past so many problems potentially faced in life and certainly not just susceptibility genes unique for Sjögren’s syndrome. Meanwhile, the TNFα -308A allele is thought to be associated secondarily with Sjögren’s syndrome through DRB1*03 [21], raising a question on whether tuberculosis risk and primary Sjogren’s syndrome are operating at different loci and whether their opposing risks are related only through linkage disequilibrium.
Genes Outside the HLA Region
Genes outside the HLA Region have also been associated with primary SS, a form of secondary SS or its autoanti-bodies (Table 1). These include chemokine and cytokine polymorphisms for CCR5 [22], IL-10 [23], IL-1 Ra [24], IL-6 [23], TGFβ1 [21] and TNF-α [14]. The IL-4 receptor polymorphism is a promising candidate [25]. Fas and Fas ligand gene polymorphisms have also been investigated as possible candidate susceptibility genes [26, 27], perpetuating themes of relatively dysfunctional apoptotic genes being important autoimmunity risk factors.
Lack of replication results is a serious problem with non-HLA genetic associations. Only primary Sjögren’s syndrome with the association of anti-La autoantibodies in immunoglobulin light chain allotype KM1 has been confirmed. The confirmation effort required 20 years, a reflection of minimal interest levels in Sjögren’s syndrome genetics within academic research communities. No other alleged non-HLA region genetic associations are considered confirmed; possible associations of many are obviously controversial. Perhaps closest to being repudiated is mannose binding ligand associations (Table 1).
There are many other possible genes based both on knowledge of inflammatory response physiology and upon other literature related to SS. CXCL13 and CXCR5 attract B cells to target organs and are expressed in Sjögren’s [40]. The known and alleged Sjögren’s syndrome autoantigens are obvious candidate genes, including Ro, La, immunoglobulin, α-fodrin [41], β-fodrin [42], SS-56 [43], ABCA1 [44], P230 trans-Golgi Network protein [45], 97 kD protein associated with Golgi complex [46], α-amylase [47], and muscarinic-3 receptor [48]. Polymorphisms in the RO52 (SSA1) gene have been associated with anti-Ro52 kD autoantibodies in primary Sjögren’s (Table 1). Perhaps, findings that different RO52 (SSA1) gene polymorphisms have been related to anti-Ro52 autoantibodies and to anti-Ro in Sjögren’s syndrome are interrelated.
Protein expression changes have been found in Sjögren’s syndrome including the cysteine-rich secretory protein 3 (CRISP3) [49], aquaporin 5 [50], and aquaporin 1 [51], COX-1 [52], that also suggest candidate genes. There are unpublished studies that will greatly expand the gene expression data available for Sjögren’s syndrome.
The genes responsible for effects in the NOD mouse model of Sjögren’s on mouse chromosomes 1 [53], 3 [54], and 4 [55] will become candidates when they are identified. Other findings in the NOD, such as the LGP10 autoantigen [56], high levels of matrix metalloproteases [57] and cysteine proteases [58], also suggest candidate genes.
The technical capacity to evaluate large numbers of polymorphisms relatively quickly should lead to an evaluation of all of these candidates and the entire complement of human genes that is now accessible for evaluation.
Mouse Models and Gene Transfer
Meanwhile, genetic contributions toward understanding the salivary lymphadenitis or dry eyes and mouth of Sjögren’s syndrome are available from work in mouse models.
The non-obese diabetic (NOD) mouse is an inbred strain that has been used for many years as a model of Type 1 diabetes. More recently, lymphocytic infiltrates of the salivary glands and reduced salivary and lacrimal gland function have been noted in these animals [59]. Strikingly, the NOD mouse is the only previously reported model of SS, which shows exocrine glandular dysfunction. There are circulating antibodies that bind the surface and the nucleus of salivary gland cells. NOD mice make antibodies against alpha-fodrin [60], but there is little evidence that anti-Ro or anti-La are present in these mice. There is, however, evidence that antibodies are critical to disease in this model. NOD mice without functional B cells (NOD IgMnull) have salivary infiltrates consisting of T cells but have normal salivary gland function. Infusion of IgG from NOD mice or from humans with SS induces a decrement of salivary gland function in the NOD IgMnull mouse, though [61]. Antibodies against muscarinic receptors may be important in this regard.
Like some other reported animals with SS, the NOD mouse may be considered a model of secondary SS as another autoimmune disease is present—namely, type 1 diabetes. The diabetes in these animals is dependent on the MHC haplotype. Studies with NOD congenic for H-2b demonstrate that while diabetes does not develop, salivary gland infiltrates do develop [62]. Thus, these congenic animals appear to be a model of primary SS. Because the cellular infiltrate, cell surface binding antibodies, and glandular dysfunction are present, the NOD mouse and its variants will no doubt prove useful in deciphering the pathogenesis of SS. Nonetheless, while replicating the disease in several ways, this model does not have appreciable anti-Ro and anti-La, a near universal finding in human disease.
The MRL-lpr/lpr mouse has a genetic defect in the fas gene, and therefore, a defect in the apoptotic death of lymphocytes. There is massive accumulation of lymphocytes and development of a disease similar to human systemic lupus erythematosus. This animal has been used extensively to study lupus autoimmunity [63]. The salivary glands of MRL-lpr/lpr mice are infiltrated with activated T lymphocytes. After transfer of these cells into SCID mice (no functional T or B cells), salivary and lacrimal lesions develop. Anti-CD4 or anti-Vβ8 monoclonal antibodies prevent development of lesions in the SCID recipients [64].
Unfortunately, there are inherent problems with the MRL-lpr/lpr mouse as a model of SS. Clearly, this is a model of secondary, not primary disease, as these mice also have a lupus-like illness. Second, there is no decreased function of the salivary glands [65]. Third, these animals do not develop anti-Ro or anti-La in a manner similar to human patients. In one study, 6 of 17 mice had antibodies to 52 kD Ro, but the titer was high in only 3. Meanwhile 1 of 17 was reported to have anti-60 kD Ro, and no titer or inhibition data were given [66].
Other animal models of SS have been reported (and are discussed elsewhere in this journal), each of them with significant problems. An acute sialoadenitis occurs in a graft-vs-host transplant model [67]. Other than a pathological description, no other data have been reported. Mice transgenic for the envelope protein of the hepatitis C virus develop salivary lesions resembling SS [68], and humans with hepatitis C can develop a sicca illness [69]. The NFS/ sld mouse bears a mutation resulting in sublingual gland differentiation arrest. After neonatal thymectomy, these mice develop pathology in the other salivary and lacrimal glands that resembles that found in SS, with females affected more often than males [64]. The infiltrate was predominantly composed of CD8-positive lymphocytes, and while there were anti-salivary antibodies in the sera, no anti-Ro or anti-La was detected [64]. Mice homozygous for the alymphoplasia (aly) mutation lack lymph nodes and Peyer’s patches. These animals develop inflammatory infiltrates (CD4 lymphocyte rich) of the salivary glands, lungs, pancreas, and lacrimal glands. The mechanism by which this defect gives the absent lymph node and absent Peyer’s patches phenotype is not known [70].
Of particular interest, Fleck and colleagues reported infection of C57BL/6-lpr/lpr mice with murine cytomegalovirus with resultant acute and chronic sialoadenitis [71]. This inflammation persisted after clearance of the virus. High levels of anti-Ro, anti-La, rheumatoid factor, and anti-dsDNA were produced. Thus, this is the only previously reported mouse model of SS with convincingly high levels of anti-Ro and anti-La, although whether the anti-Ro is anti-60 kD Ro or anti-52 kD Ro, or both, is not clear. Pathology of other organs is not given, but the presence of anti-dsDNA, which is usually considered specific to lupus, implies that this is also a model of secondary SS [71].
Therapeutic genetic transfer exploration in mouse salivary glands shows great promise as models for gene expression. These experiments are being done to explore the potential advantages of salivary glands as vehicles for gene expression. This approach toward improving salivary gland functioning or toward inhibiting immune mediated gland destruction in Sjögren’s syndrome has been pursued, particularly in the laboratory led by Bruce Baum at the National Institute of Dental and Craniofacial Research (NIDCR) at the National Institutes of Health [72].
Salivary tissue can be accessed noninvasively, as a site for gene transfers. For example, a recombinant adenovirus-associated virus vector (AAV) transfer of interleukin-10 cDNA into the non-obese diabetic (NOD) murine model has been reported to suppress inflammatory salivary disease [72].
Efficacy of a transgenic gastrointestinal hormone vaso-active intestinal peptide (VIP) in prevention of NOD sialadenitis was also recently reported [72]. The immunomodulatory properties of VIP increase IL-10 expression [73], while inhibiting lymphocyte migration, some macrophage functions, T cell proliferation, and expression of certain inflammatory mediators [74—76]. VIP may have the potential to improve the management of several autoimmune disorders. Interestingly, there is sufficient homology between mouse and human VIP that transgenic human VIP (hVIP) acts through mouse VIP receptors in transgenic mice [72].
Transfer by retrograde ductal infusion into the NOD mouse salivary gland of recombinant serotype 5 adeno-associate virus, encoded as a vector for human VIP cDNA (rAd5CMVhVIP or as rhVIP below) had astonishing consequences [72]. The mice were treated before onset of disease. Relative to either empty vector controls or uninfected controls, these animals had twice the salivary flow of control animals. Perplexingly, there was no difference in the inflammatory changes in the salivary gland between the rhVIP NOD mice and their controls. Levels of IL-2, IL-10, IL-12, and TNFα were much lower in the mice expressing rhVIP, while levels of IL-4, IL-6, INFγ, and RANTES did not differ from controls.
VIP gene transfer showed local disease modifying and immunosuppressive effects in the NOD model. These results suggest that this approach may be useful in the prevention of Sjögren’s syndrome salivary manifestations [72] and leaving us to wonder whether there might be susceptibility genes in this pathway influencing the human disease.
Future Horizons and Perspectives
Clearly, we know very little about the genetics of Sjögren’s syndrome. Other than the strong association with alleles of the HLA Class II genes, HLA-DR and HLA-DQ, there is not much else that is at present sufficiently convincing to be considered conclusive. As in virtually all of the HLA-Class II associations, the molecular mechanism explaining why these alleles are susceptibility genes is not known. To know how susceptibility creates these associations would be an important advance in our understanding, indeed. The prospects are that the genes now being found in other related autoimmune diseases, especially rheumatoid arthritis and systemic lupus erythematosus will be evaluated in Sjögren’s syndrome patients and controls. This work is likely to greatly expand the list of genes associated with Sjögren’s syndrome.
Previously, the impediment to progress was the inability to evaluate genes directly and rapidly. Recent technical progress has removed this problem. The evaluation of hundreds of thousands of allelic differences between human chromosomes is within our grasp and would accompany suitable patient and appropriate control materials and databases. In addition, the newly developed capacity to evaluate the entire genome will provide, in the future, a much closer approximation of the genetic architecture of Sjögren’s syndrome.
Acknowledgments
We appreciate support of the NIH grants AR42460, AR12253, AI24717, AI31584, AR049084, RR020143, AR048940, and DE015223 (JBH), NIH grant number P20-RR015577 from the National Center for Research Resources (AHS), and US Department of Veteran Affairs (CC103) for our work.
Contributor Information
Pamela H. Williams, Arthritis and Immunology Program, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104, USA
Beth L. Cobb, Arthritis and Immunology Program, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104, USA
Bahram Namjou, Arthritis and Immunology Program, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104, USA.
R. Hal Scofield, Arthritis and Immunology Program, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104, USA, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA, US Department of Veterans Affairs Medical Center, Oklahoma City, OK, USA.
Amr H. Sawalha, Arthritis and Immunology Program, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104, USA, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA, US Department of Veterans Affairs Medical Center, Oklahoma City, OK, USA
John B. Harley, Email: john-harley@omrf.ouhsc.edu, Arthritis and Immunology Program, Oklahoma Medical Research Foundation, 825 NE 13th Street, Oklahoma City, OK 73104, USA, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA, US Department of Veterans Affairs Medical Center, Oklahoma City, OK, USA
References
- 1.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH European Study Group on Classification Criteria for Sjogren’s S. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisch K. Uber hereditarisches vorkommen des mit keratoconjuctivitis sicca verbunden Sjogrenschen symptomen-komplexes. Arch Augenheilkd. 1937;110:357. [Google Scholar]
- 3.Reveille JD, Wilson RW, Provost TT, Bias WB, Arnett FC. Primary Sjogren’s syndrome and other autoimmune diseases in families. Prevalence and immunogenetic studies in six kindreds. Ann Intern Med. 1984;101:748–756. doi: 10.7326/0003-4819-101-6-748. [DOI] [PubMed] [Google Scholar]
- 4.Whittingham S, Propert DN, Mackay IR. A strong Xassociation between the antinuclear antibody anti-La (SS-B) and the kappa chain allotype Km(1) Immunogenetics. 1984;19:295–299. doi: 10.1007/BF00345402. [DOI] [PubMed] [Google Scholar]
- 5.Pertovaara M, Hurme M, Antonen J, Pasternack A, Pandey JP. Immunoglobulin KM and GM gene polymorphisms modify the clinical presentation of primary Sjogren’s syndrome. J Rheumatol. 2004;31:2175–2180. [PubMed] [Google Scholar]
- 6.Kim T-H, Uhm W-S, Inman RD. Pathogenesis of ankylosing spondylitis and reactive arthritis. Curr Opin Rheumatol. 2005;17:400–405. doi: 10.1097/01.bor.0000163447.44037.c4. [DOI] [PubMed] [Google Scholar]
- 7.Pauling L, Itano H, Singer S, Wells I. Sickle cell anemia, a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies [see comment] Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 9.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, Moser KL, Begovich AB, Carlton VEH, Li W, Lee AT, Ortmann W, Behrens TW, Gregersen PK. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottenberg J-E, Busson M, Loiseau P, Cohen-Solal J, Lepage V, Charron D, Sibilia J, Mariette X. In primary Sjogren’s syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum. 2003;48:2240–2245. doi: 10.1002/art.11103. [DOI] [PubMed] [Google Scholar]
- 11.Fye KH, Terasaki PI, Moutsopoulos H, Daniels TE, Michalski JP, Talal N. Association of Sjogren’s syndrome with HLA-B8. Arthritis Rheum. 1976;19:883–886. doi: 10.1002/art.1780190508. [DOI] [PubMed] [Google Scholar]
- 12.Gershwin ME, Terasaki I, Graw R, Chused TM. Increased frequency of HL-A8 in Sjogren’s syndrome. Tissue Antigens. 1975;6:342–346. doi: 10.1111/j.1399-0039.1975.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 13.Chused TM, Kassan SS, Opelz G, Moutsopoulos HM, Terasaki PI. Sjogren’s syndrome association with HLA-Dw3. N Engl J Med. 1977;296:895–897. doi: 10.1056/NEJM197704212961602. [DOI] [PubMed] [Google Scholar]
- 14.Guggenbuhl P, Veillard E, Quelvenec E, Jego P, Semana G, Jean S, Meadeb J, Chales G, Perdriger A. Analysis of TNFalpha microsatellites in 35 patients with primary Sjogren’s syndrome. Joint, Bone, Spine: Revue du Rhumatisme. 2000;67:290–295. [PubMed] [Google Scholar]
- 15.Kumagai S, Kanagawa S, Morinobu A, Takada M, Nakamura K, Sugai S, Maruya E, Saji H. Association of a new allele of the TAP2 gene, TAP2*Bky2 (Val577), with susceptibility to Sjogren’s syndrome. Arthritis Rheum. 1997;40:1685–1692. doi: 10.1002/art.1780400919. [DOI] [PubMed] [Google Scholar]
- 16.Jean S, Quelvennec E, Alizadeh M, Guggenbuhl P, Birebent B, Perdriger A, Grosbois B, Pawlotsky PY, Semana G. DRB1*15 and DRB1*03 extended haplotype interaction in primary Sjogren’s syndrome genetic susceptibility. Clin Exp Rheumatol. 1998;16:725–728. [PubMed] [Google Scholar]
- 17.Gomez LM, Anaya JM, Gonzalez CI, Pineda-Tamayo R, Otero W, Arango A, Martin J. PTPN22 C1858T polymorphism in Colombian patients with autoimmune diseases. Genes Immun. 2005;6:628–631. doi: 10.1038/sj.gene.6364261. [DOI] [PubMed] [Google Scholar]
- 18.Graham RR, Ortmann WA, Langefeld CD, Jawaheer D, Selby SA, Rodine PR, Baechler EC, Rohlf KE, Shark KB, Espe KJ, Green LE, Nair RP, Stuart PE, Elder JT, King RA, Moser KL, Gaffney PM, Bugawan TL, Erlich HA, Rich SS, Gregersen PK, Behrens TW. Visualizing human leukocyte antigen class II risk haplotypes in human systemic lupus erythematosus. Am J Hum Genet. 2002;71:543–553. doi: 10.1086/342290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harley JB, Reichlin M, Arnett FC, Alexander EL, Bias WB, Provost TT. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjogren’s syndrome. Science. 1986;232:1145–1147. doi: 10.1126/science.3458307. [DOI] [PubMed] [Google Scholar]
- 20.Correa PA, Gomez LM, Cadena J, Anaya JM. Autoimmunity and tuberculosis. Opposite association with TNF polymorphism. J Rheumatol. 2005;32:219–224. [PubMed] [Google Scholar]
- 21.Ittah M, Gottenberg JE, Proust A, Hachulla E, Puechal X, Loiseau P, Mariette X, Miceli-Richard C. No evidence for association between 1858 C≠T single-nucleotide polymorphism of PTPN22 gene and primary Sjogren’s syndrome. Genes Immun. 2005;6:457–458. doi: 10.1038/sj.gene.6364229. [DOI] [PubMed] [Google Scholar]
- 22.Petrek M, Cermakova Z, Hutyrova B, Micekova D, Drabek J, Rovensky J, Bosak V. CC ckemokine receptor 5 and interleukin-1 receptor antagonist gene polymorphisms in patients with primary Sjogren’s syndrome. Clin Exp Rheumatol. 2002;20:701–703. [PubMed] [Google Scholar]
- 23.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren’s syndrome and correlate with the clinical manifestations of the disease. Rheumatology. 2001;40:656–661. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 24.Perrier S, Coussediere C, Dubost JJ, Albuisson E, Sauvezie B. IL-1 receptor antagonist (IL-1RA) gene polymorphism in Sjogren’s syndrome and rheumatoid arthritis. Clin Immunol Immunopathol. 1998;87:309–313. doi: 10.1006/clin.1998.4520. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Casals M, Font J, Brito-Zeron P, Trejo O, Garcia-Carrasco M, Lozano F. Interleukin-4 receptor alpha polymorphisms in primary Sjogren’s syndrome. Clin Exp Rheumatol. 2004;22:374. [PubMed] [Google Scholar]
- 26.Bolstad AI, Wargelius A, Nakken B, et al. Fas and Fas Ligand gene polymorphisms in priary Sjogren’s syndrome. J Rheumatol. 2000;27:2397–2405. [PubMed] [Google Scholar]
- 27.Mullighan CG, Heatley S, Bardy PG, Lester S, Rischmueller M, Gordon TP. Lack of association between mannose-binding lectin gene polymorphisms and primary Sjogren’s syndrome. Arthritis Rheum. 2000;43:2851–2852. doi: 10.1002/1529-0131(200012)43:12<2851::AID-ANR28>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Pertovaara M, Lehtimaki T, Rontu R, Antonen J, Pasternack A, Hurme M. Presence of apolipoprotein E epsilon 4 allele predisposes to early onset of primary Sjogren’s syndrome. Rheumatology. 2004;43:1484–1487. doi: 10.1093/rheumatology/keh383. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZY, Morinobu A, Kanagawa S, Kumagai S. Polymorphisms of the mannose binding lectin gene in patients with Sjogren’s syndrome. Ann Rheum Dis. 2001;60:483–486. doi: 10.1136/ard.60.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harangi M, Kaminski WE, Fleck M, Orso E, Zeher M, Kiss E, Szekanecz Z, Zilahi E, Marienhagen J, Aslanidis C, Paragh G, Bolstad AI, Jonsson R, Schmitz G. Homozygosity for the 168His variant of the minor histocompatibility antigen HA-1 is associated with reduced risk of primary Sjogren's syndrome. Eur J Immunol. 2005;35:305–317. doi: 10.1002/eji.200425406. [DOI] [PubMed] [Google Scholar]
- 31.Youn J, Hwang SH, Cho CS, Min JK, Kim WU, Park SH, Kim HY. Association of the interleukin-4 receptor alpha variant Q576R with Th1/Th2 imbalance in connective tissue disease. Immunogenetics. 2000;51:743–746. doi: 10.1007/s002510000196. [DOI] [PubMed] [Google Scholar]
- 32.Origuchi T, Kawasaki E, Ide A, Kamachi M, Tanaka F, Ida H, Kawakami A, Migita K, Eguchi K. Correlation between interleukin 10 gene promoter region polymorphisms and clinical manifestations in Japanese patients with Sjogren's syndrome. Ann Rheum Dis. 2003;62:1117–1118. doi: 10.1136/ard.62.11.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Font J, Garcia-Carrasco M, Ramos-Casals M, Aldea AI, Cervera R, Ingelmo M, Vives J, Yague J. The role of interleukin-10 promoter polymorphisms in the clinical expression of primary Sjogren's syndrome. Rheumatology. 2002;41:1025–1030. doi: 10.1093/rheumatology/41.9.1025. [DOI] [PubMed] [Google Scholar]
- 34.Rischmueller M, Limaye V, Lester S, et al. Polymorphisms of the interleukin 10 gene promoter are not associated with anti-Ro autoantibodies in primary Sjogren's syndrome. J Rheumatol. 2000;27:2945–2946. [PubMed] [Google Scholar]
- 35.Tsutsumi A, Sasaki K, Wakamiya N, Ichikawa K, Atsumi T, Ohtani K, Suzuki Y, Koike T, Sumida T. Mannose-binding lectin gene: polymorphisms in Japanese patients with systemic lupus erythematosus, rheumatoid arthritis and Sjogren's syndrome. Genes Immun. 2001;2:99–104. doi: 10.1038/sj.gene.6363744. [DOI] [PubMed] [Google Scholar]
- 36.Aittoniemi J, Pertovaara M, Hulkkonen J, Pasternack A, Hurme M, Laippala P, Antonen J. The significance of mannan-binding lectin gene alleles in patients with primary Sjogren's syndrome. Scand J Rheumatol. 2002;31:362–365. doi: 10.1080/030097402320817095. [DOI] [PubMed] [Google Scholar]
- 37.Nakken B, Jonsson R, Bolstad AI. Polymorphisms of the Ro52 Gene Associated with Anti-Ro 52-kd Autoantibodies in Patients With Primary Sjogren's Syndrome. Arthritis Rheum. 2001;44:636–638. doi: 10.1002/1529-0131(200103)44:3<638::AID-ANR112>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Imanishi T, Morinobu A, Hayashi N, Kanagawa S, Koshiba M, Kondo S, Kumagai S. A novel polymorphism of the SSA1 gene is associated with anti-SS-A/Ro52 autoantibody in Japanese patients with primary Sjogren's syndrome. Clin Exp Rheumatol. 2005;23:521–524. [PubMed] [Google Scholar]
- 39.Lawson CA, Donaldson IJ, Bowman SJ, Shefta J, Morgan AW, Gough A, Isaacs JD, Griffiths B, Emery P, Pease CT, Boylston AW. Analysis of the insertion/deletion related polymorphism within T cell antigen receptor beta variable genes in primary Sjogren’s syndrome. Ann Rheum Dis. 2005;64:468–470. doi: 10.1136/ard.2003.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoan-tibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren’s syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 41.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, Sugino H, Hayashi Y. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276:604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 42.Kuwana M, Okano T, Ogawa Y, Kaburaki J, Kawakami Y. Autoantibodies to the amino-terminal fragment of beta-fodrin expressed in glandular epithelial cells in patients with Sjogren’s syndrome. J Immunol. 2001;167:5449–5456. doi: 10.4049/jimmunol.167.9.5449. [DOI] [PubMed] [Google Scholar]
- 43.Billaut-Mulot O, Cocude C, Kolesnitchenko V, Truong MJ, Chan EK, Hachula E, de la Tribonniere X, Capron A, Bahr GM. SS-56, a novel cellular target of autoantibody responses in Sjogren’s syndrome and systemic lupus erythematosus. J Clin Invest. 2001;108:861–869. doi: 10.1172/JCI13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka AR, Ikeda Y, Abe-Dohmae S, Arakawa R, Sadanami K, Kidera A, Nakagawa S, Nagase T, Aoki R, Kioka N, Amachi T, Yokoyama S, Ueda K. Human ABCA1 contains a large amino-terminal extracellular domain homologous to an epitope of Sjogren’s syndrome. Biochem Biophys Res Commun. 2001;283:1019–1025. doi: 10.1006/bbrc.2001.4891. [DOI] [PubMed] [Google Scholar]
- 45.Tsukada Y, Ichikawa H, Chai Z, Lai FP, Dunster K, Sentry JW, Toh BH. Novel variant of p230 trans-Golgi network protein identified by serum from Sjogren’s syndrome patient. Eur J Cell Biol. 2000;79:790–794. doi: 10.1078/0171-9335-00114. [DOI] [PubMed] [Google Scholar]
- 46.Griffith KJ, Chan EK, Lung CC, Hamel JC, Guo X, Miyachi K, Fritzler MJ. Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjogren’s syndrome. Arthritis Rheum. 1997;40:1693–1702. doi: 10.1002/art.1780400920. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto I, Maeda T, Takemoto Y, Hashimoto Y, Kimura F, Iwamoto I, Saito Y, Nishioka K, Sumida T. Alpha-amylase functions as a salivary gland-specific self T cell epitope in patients with Sjogren’s syndrome. Int J Mol Med. 1999;3:485–490. doi: 10.3892/ijmm.3.5.485. [DOI] [PubMed] [Google Scholar]
- 48.Beroukas D, Goodfellow R, Hiscock J, Jonsson R, Gordon TP, Waterman SA. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjogren’s syndrome. Lab Invest. 2002;82:203–210. doi: 10.1038/labinvest.3780412. [DOI] [PubMed] [Google Scholar]
- 49.Tapinos NI, Polihronis M, Thyphronitis G, Moutsopoulos HM. Characterization of the cysteine-rich secretory protein 3 gene as an early-transcribed gene with a putative role in the pathophysiology of Sjogren’s syndrome. Arthritis Rheum. 2002;46:215–222. doi: 10.1002/1529-0131(200201)46:1<215::AID-ART10024>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 50.Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjogren's syndrome patients. Lab Invest. 2001;81:143–148. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 51.Cavill D, Waterman SA, Gordon TP. Failure to detect antibodies to extracellular loop peptides of the muscarinic M3 receptor in primary Sjogren's syndrome. J Rheumatol. 2002;29:1342–1344. [PubMed] [Google Scholar]
- 52.Tominaga M, Migita K, Sano H, Fukui W, Kohno M, Tsubouchi Y, Honda S, Fukuda T, Nakamura H, Yamasaki S, Kawabe Y, Kawakami A, Eguchi K. Expression of cyclooxygenase-1 (COX-1) in labial salivary glands of Sjogren's syndrome. Clin Exp Immunol. 2000;122:459–463. doi: 10.1046/j.1365-2249.2000.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulard O, Fluteau G, Eloy L, Damotte D, Bedossa P, Garchon H-J. Genetic analysis of autoimmune sialadenitis in nonobese diabetic mice: a major susceptibility region on chromosome 1. J Immunol. 2002;168:4192–4201. doi: 10.4049/jimmunol.168.8.4192. [DOI] [PubMed] [Google Scholar]
- 54.Brayer J, Lowry J, Cha S, Robinson CP, Yamachika S, Peck AB, Humphreys-Beher MG. Alleles from chromosomes 1 and 3 of NOD mice combine to influence Sjogren's syndrome-like autoimmune exocrinopathy. J Rheumatol. 2000;27:1896–1904. [PubMed] [Google Scholar]
- 55.Johansson ACM, Nakken B, Sundler M, Lindqvist A-KB, Johannesson M, Alarcon-Riquelme M, Bolstad AI, Humphreys-Beher MG, Jonsson R, Skarstein K, Holmdahl R. The genetic control of sialadenitis versus arthritis in a NOD.QxB10.Q F2 cross. Eur J Immunol. 2002;32:243–250. doi: 10.1002/1521-4141(200201)32:1<243::AID-IMMU243>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 56.Esch TR, Poveromo JD, Aikins MC, Levanos VA. A novel lacrimal gland autoantigen in the NOD mouse model of Sjogren's syndrome. Scand J Immunol. 2002;55:304–310. doi: 10.1046/j.1365-3083.2002.01042.x. [DOI] [PubMed] [Google Scholar]
- 57.Yamachika S, Nanni JM, Nguyen KH, Garces L, Lowry JM, Robinson CP, Brayer J, Oxford GE, da Silveira A, Kerr M, Peck AB, Humphreys-Beher MG. Excessive synthesis of matrix metalloproteinases in exocrine tissues of NOD mouse models for Sjogren's syndrome. J Rheumatol. 1998;25:2371–2380. [PubMed] [Google Scholar]
- 58.Robinson CP, Yamachika S, Alford CE, Cooper C, Pichardo EL, Shah N, Peck AB, Humphreys-Beher MG. Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjogren syndrome. Proc Natl Acad Sci U S A. 1997;94:5767–5771. doi: 10.1073/pnas.94.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humphreys-Beher MG. Animal models for autoimmune disease-associated xerostomia and xerophthalmia. Adv Dent Res. 1996;10:73–75. doi: 10.1177/08959374960100011501. [DOI] [PubMed] [Google Scholar]
- 60.Yanagi K, Ishimaru N, Haneji N, Saegusa K, Saito I, Hayashi Y. Anti-120-kDa alpha-fodrin immune response with Th1-cytokine profile in the NOD mouse model of Sjogren's syndrome. Eur J Immunol. 1998;28:3336–33450. doi: 10.1002/(SICI)1521-4141(199810)28:10<3336::AID-IMMU3336>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 61.Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, Peen E, Jonsson R, Humphreys-Beher MG. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren's syndrome. Proc Natl Acad Sci U S A. 1998;95:7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson CP, Yamachika S, Bounous DI, Brayer J, Jonsson R, Holmdahl R, Peck AB, Humphreys-Beher MG. A novel NOD-derived murine model of primary Sjogren's syndrome. Arthritis Rheum. 1998;41:150–156. doi: 10.1002/1529-0131(199801)41:1<150::AID-ART18>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 63.Singh AK. Lupus in the Fas lane? J R Coll Physicians Lond. 1995;29:475–478. [PMC free article] [PubMed] [Google Scholar]
- 64.Haneji N, Hamano H, Yanagi K, Hayashi Y. A new animal model for primary Sjogren's syndrome in NFS/sld mutant mice. J Immunol. 1994;153:2769–2777. [PubMed] [Google Scholar]
- 65.Fujita H, Fujihara T, Takeuchi T, Saito I, Tsubota K. Lacrimation and salivation are not related to lymphocytic infiltration in lacrimal and salivary glands in MRL lpr/lpr mice. Adv Exp Med Biol. 1998;438:941–948. doi: 10.1007/978-1-4615-5359-5_133. [DOI] [PubMed] [Google Scholar]
- 66.Wahren M, Skarstein K, Blange I, Pettersson I, Jonsson R. MRL/lpr mice produce anti-Ro 52,000 MW antibodies: detection, analysis of specificity and site of production. Immunology. 1994;83:9–15. [PMC free article] [PubMed] [Google Scholar]
- 67.Kimura T, Suzuki K, Inada S, Hayashi A, Saito H, Miyai T, Ohsugi Y, Matsuzaki Y, Tanaka N, Osuga T, et al. Induction of autoimmune disease by graft-versus-host reaction across MHC class II difference: modification of the lesions in IL-6 transgenic mice. Clin Exp Immunol. 1994;95:525–529. doi: 10.1111/j.1365-2249.1994.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, Kurokawa K, Matsuura Y, Miyamura T. Sialadenitis histologically resembling Sjogren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci U S A. 1997;94:233–236. doi: 10.1073/pnas.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramos-Casals M, Garcia-Carrasco M, Cervera R, Font J. Sjogren's syndrome and hepatitis C virus. Clin Rheumatol. 1999;18:93–100. doi: 10.1007/s100670050064. [DOI] [PubMed] [Google Scholar]
- 70.Tsubata R, Tsubata T, Hiai H, Shinkura R, Matsumura R, Sumida T, Miyawaki S, Ishida H, Kumagai S, Nakao K, Honjo T. Autoimmune disease of exocrine organs in immunodeficient alymphoplasia mice: a spontaneous model for Sjogren's syndrome. Eur J Immunol. 1996;26:2742–2748. doi: 10.1002/eji.1830261129. [DOI] [PubMed] [Google Scholar]
- 71.Fleck M, Kern ER, Zhou T, Lang B, Mountz JD. Murine cytomegalovirus induces a Sjogren's syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis Rheum. 1998;41:2175–2184. doi: 10.1002/1529-0131(199812)41:12<2175::AID-ART12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 72.Lodde BM, Mineshiba F, Wang J, Cotrim AP, Afione S, Tak PP, Baum BJ. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome. Ann Rheum Dis. 2006;65:195–200. doi: 10.1136/ard.2005.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999;162:1707–1716. [PubMed] [Google Scholar]
- 74.Delgado M, Abad C, Martinez C, Juarranz MG, Arranz A, Gomariz RP, Leceta J. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med. 2002;80:16–24. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- 75.Pozo D, Delgado M, Martinez M, Guerrero JM, Leceta J, Gomariz RP, Calvo JR. Immunobiology of vasoactive intestinal peptide (VIP)[erratum appears in Immunol Today 2000 Apr;21 (4):191] Immunol Today. 2000;21:7–11. doi: 10.1016/s0167-5699(99)01525-x. [DOI] [PubMed] [Google Scholar]
- 76.Grimm MC, Newman R, Hassim Z, Cuan N, Connor SJ, Le Y, Wang JM, Oppenheim JJ, Lloyd AR. Cutting edge: vasoactive intestinal peptide acts as a potent suppressor of inflammation in vivo by trans-deactivating chemokine receptors. J Immunol. 2003;171:4990–4994. doi: 10.4049/jimmunol.171.10.4990. [DOI] [PubMed] [Google Scholar]