Abstract
Increasing use of predictive genetic testing to gauge hereditary cancer risk has been paralleled by rising cost-sharing practices. Little is known about how demographic and psychosocial factors may influence individuals’ willingness-to-pay for genetic testing. The Gastrointestinal Tumor Risk Assessment Program Registry includes individuals presenting for genetic risk assessment based on personal/family cancer history. Participants complete a baseline survey assessing cancer history and psychosocial items. Willingness-to-pay items include intention for: genetic testing only if paid by insurance; testing with self-pay; and amount willing-to-pay ($25–$2000). Multivariable models examined predictors of willingness-to-pay out-of-pocket (versus only if paid by insurance) and willingness-to-pay a smaller versus larger sum (≤200 vs. ≥$500). All statistical tests are two-sided (α=0.05). Of 385 evaluable participants, a minority (42%) had a personal cancer history, while 56% had ≥1 first-degree relative with colorectal cancer. Overall, 21.3% were willing to have testing only if paid by insurance, and 78.7% were willing-to-pay. Predictors of willingness-to-pay were: 1) concern for positive result; 2) confidence to control cancer risk; 3) fewer perceived barriers to colorectal cancer screening; 4) benefit of testing to guide screening (all p<0.05). Subjects willing-to-pay a higher amount were male, more educated, had greater cancer worry, fewer relatives with colorectal cancer, and more positive attitudes toward genetic testing (all p<0.05). Individuals seeking risk assessment are willing-to-pay out-of-pocket for genetic testing, and anticipate benefits to reducing cancer risk. Identifying factors associated with willingness-to-pay for genetic services is increasingly important as testing is integrated into routine cancer care.
Keywords: Willingness-to-pay, genetic testing, gastrointestinal, risk, colorectal cancer
INTRODUCTION
Genetic risk assessment and germ-line genetic testing are increasingly integral to cancer prevention efforts in light of research demonstrating benefits afforded by risk reducing surgeries, chemoprevention, and intensive screening among high-risk mutation carriers.(Burn et al., 2011; Järvinen et al., 2009; Mecklin et al., 2007) As greater numbers of cancer-driving gene mutations are identified in the post-Human Genome Project era, the use of predictive genetic testing will continue to expand and diversify in cancer care. Rapid growth in uptake of predictive genetic testing for cancer risk has been paralleled by introduction of patient-targeted cost-sharing strategies by payors aimed at controlling costs(Hudson et al., 2006; Japsen, 2013). Indeed, coverage of genetic testing for hereditary cancer risk by private and federal insurers is neither uniform nor complete—patients frequently face co-payments in the range of 10–20% or $400–$1000 or more. Other insurance policies may not cover any genetic services at all, or may only cover testing but not genetic counseling (e.g. Medicare).(Powell, Chandrasekharan, & Cook-Deegan, 2010)
Individuals meeting clinical high-risk criteria for a hereditary cancer syndrome and considering GT may be faced with high out-of-pocket costs. Little research has examined what characteristics may impact a high-risk individual’s willingness-to-pay for genetic testing for cancer risk. In line with the Health Belief Model and Andersen’s behavioral model of health services utilization, past research has demonstrated that individuals who seek cancer risk assessment have strong intentions to undergo genetic testing, and that intention is associated with perceived risk of developing cancer, benefits of preventive screening, and control over cancer risks.(M. R. Andersen, Smith, Meischke, Bowen, & Urban, 2003; Bottorff et al., 2002; Jacobsen, Valdimarsdottier, Brown, & Offit, 1997; Lerman, Daly, Masny, & Balshem, 1994; Lerman et al., 1997; Lerman et al., 1996; Lerman, Seay, Balshem, & Audrain, 1995; Leventhal, 1970; Leventhal, Safer, & Panagis, 1983; Rosenstock, 1990; Schwartz, Lerman, Miller, Daly, & Masny, 1995) High cost is a significant barrier to screening in multiple cancer types, including breast, colorectal and cervical (Burak & Meyer, 1997; Miller & Champion, 1997; Thomas & Clarke, 1998; Urban, Anderson, & Peacock, 1994; Vernon, 1997), however the influence of out-of-pocket costs on individual perceptions and behaviors in the setting of increased hereditary cancer risk has been less studied. In a study of average-risk mammography patients, 96% of 464 women would have BRCA1/2 gene mutation testing if it was free, but only 68% were willing-to-pay more than $25 for the test (Chaliki et al., 1995). The authors did not evaluate the association between psychosocial factors and amount patients were willing-to-pay. Given the substantially larger out-of-pocket costs that may be incurred during clinical genetic testing, this past research leaves unanswered questions about how cost-sharing practices may impact testing behaviors in high-risk individuals.
It is important to understand how demographic and psychosocial factors modulate the amount of cost burden that participants are willing to accept when assessing their hereditary cancer risk. High-risk individuals may be vulnerable to taking on substantial debt due to high perceived risk of cancer and high cancer worry, while cost-sharing practices may serve to dissuade other high-risk patients with limited financial resources or negative perceptions of cancer screening and genetic testing from pursuing testing despite the benefits to their family. In the current study, we sought to examine how demographic, family history, and psychosocial factors assessed prior to undergoing genetic counseling may impact high-risk individuals’ willingness-to-pay out-of-pocket for genetic testing, and how much these individuals are willing-to-pay. We hypothesized that indicators of higher socioeconomic status (as evidenced by higher education and/or income levels) would be associated with willingness-to-pay for testing. Additionally, we explored whether psychosocial factors associated with intention to undergo genetic testing would be associated with willingness-to-pay for testing and the amount individuals would be willing-to-pay.
METHODS
Participant Selection and Data Collection
The Gastrointestinal (GI) Tumor Risk Assessment Program (TRAP) Registry at Fox Chase Cancer Center (FCCC), a National Cancer Institute-designated comprehensive cancer center, is a prospective database that includes individuals evaluated for genetic cancer risk between 1999 and 2009. Participants are adults aged 18 years and older with and without a prior diagnosis of cancer but with a suspected increased hereditary risk of cancer due to: 1) personal history of early onset GI cancer or a rare cancer associated with hereditary risk (e.g. small bowel); 2) personal history of multiple cancers including GI cancer or; 3) family history suggestive of hereditary cancer risk due to fulfillment of established criteria such as the Amsterdam Criteria or Revised Bethesda Guidelines. At the time of enrollment, participants complete a baseline questionnaire assessing demographic characteristics, personal and family history of cancer, environmental exposures, cancer screening history, and psychosocial and behavioral measures. Selection of measures for the baseline survey was guided by several health behavioral models including the Health Belief Model, the Transtheoretical Model of Behavior Change, and the Andersen model of health services utilization, and was influenced by relevant literature examining hereditary cancer risk. (Leventhal, 1970; Leventhal et al., 1983; Rosenstock, 1990) Surveys were completed before genetic counseling. Approximately 15% of participants were members of the same family; however, questionnaires were completed individually. All study procedures were approved by the Institutional Review Board at FCCC.
Multivariable Model Measures
Demographics
Demographic information included age, ethnicity, education, income, work and marital status. Categories are shown in Table 1.
Table 1.
Participant characteristics
| N | Percent | |
|---|---|---|
| Gender | ||
| Male | 104 | 27.0 |
| Female | 281 | 73.0 |
|
| ||
| Age (years) | ||
| 18–44 | 130 | 33.8 |
| 45–54 | 139 | 36.1 |
| 55–64 | 86 | 22.3 |
| 65–82 | 30 | 7.8 |
|
| ||
| Race | ||
| White | 353 | 91.7 |
| Other | 32 | 8.3 |
|
| ||
| Education | ||
| Less than high school | 63 | 16.4 |
| Some College | 118 | 30.6 |
| College | 118 | 30.6 |
| Graduate School | 80 | 20.8 |
| No response | 6 | 1.6 |
|
| ||
| Employment | ||
| Full Time | 189 | 49.1 |
| Part Time | 49 | 12.7 |
| Other | 111 | 28.8 |
| No response | 36 | 9.4 |
|
| ||
| Income | ||
| <$30K | 82 | 21.3 |
| $30–74K | 141 | 36.6 |
| ≥$75K | 86 | 22.3 |
| No response | 76 | 19.7 |
|
| ||
| Income by Gender | ||
| Female | ||
| <$30K | 66 | 23.5 |
| $30–74K | 108 | 38.4 |
| >$75K | 49 | 17.4 |
| No response | 58 | 20.6 |
| Male | ||
| <$30K | 16 | 15.4 |
| $30–74K | 33 | 31.7 |
| >$75K | 37 | 35.6 |
| No response | 18 | 17.3 |
|
| ||
| Marital Status | ||
| Married | 279 | 72.5 |
| Never married, Divorced, Separated, or Widowed | 101 | 26.2 |
| No response | 5 | 1.3 |
|
| ||
| Personal History of GI cancer | ||
| No | 312 | 81.0 |
| Yes | 73 | 19.0 |
|
| ||
| Personal History of any cancer | ||
| No | 280 | 72.7 |
| Yes | 105 | 27.3 |
|
| ||
| First Degree Relative with Colon Cancer | ||
| 0 relatives | 170 | 44.2 |
| 1 relative | 171 | 44.4 |
| 2 + relatives | 43 | 11.2 |
| No response | 1 | 0.3 |
History of Cancer
Personal history of cancer was assessed by asking respondents about a prior GI cancer or other type of cancer (Yes/No). Family history was assessed by number of first-degree relatives with a GI cancer or other type of cancer.
Willingness-to-pay for genetic testing
Intention to pay out-of-pocket for genetic testing was evaluated with three items. Participants were first asked if they planned to have genetic testing for GI cancer risk only if their insurance would cover the cost (item 1), or if they would be willing-to-pay out-of-pocket for testing (item 2). Responses were measured on a five-point Likert-type scale dichotomized to willing-to-pay versus unwilling-to-pay unless paid by insurance or unsure. Participants willing-to-pay in item 2 were asked to indicate an out-of-pocket amount in dollars with 7 choices offered: $25, $50, $100, $200, (grouped for analyses as “willing-to-pay less”) and $500, $1000, or $2000 (grouped as “willing-to-pay more”) (item 3). The selected groupings were influenced by current practice by commercial laboratories, which use $300–$400 cut-off as the threshold limit of co-payment above which patients are given the opportunity to refuse an ordered test.
Psychosocial variables
Knowledge and awareness
Knowledge and awareness about GI cancer risk testing and cancer genetics was assessed with 12 face-valid true-false items developed by a multi-disciplinary research team. Responses were analyzed as correct versus incorrect/don’t know. Knowledge scale items are provided in Figure 1. The standardized Cronbach’s alpha for this scale was 0.68.
Figure 1.
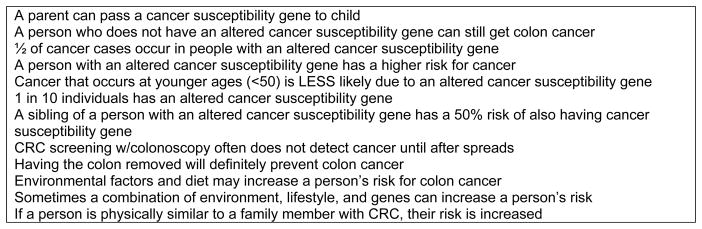
Knowledge questions (answers are True, False or Don’t Know).
Perceived control of cancer risk
One question assessed perception of one’s ability to manage genetic cancer risk: “How much control do you feel you have over whether you develop colon cancer?” (Azjen, 1985) Responses were on a 4-point Likert-type scale (‘none’ to ‘a lot’). For this question, a larger scale had been truncated to a single item by the study team (which included a senior behavioral scientist at Fox Chase) to limit the item burden.
Colorectal cancer worry
Colorectal cancer (CRC) worry was assessed by a two 4-point Likert items (‘not at all’ to ‘a lot):1) “During the past month, how often have you thought about GI cancer?” and 2) During the past month, how often have thoughts about your chances of getting colon cancer affected your mood?” (M. R. Andersen et al., 2003).
Comparative risk
Participants estimated their relative cancer risk on a 5-item Likert scale (“much lower” to “much higher”): “Compared to others your age, what are your chances of getting cancer?”
Benefits of and barriers to cancer screening
Perceived benefits and barriers to screening for GI cancers were measured with 18 five-point Likert-type items adapted from prior research (7 benefits, 11 barriers).(Lerman et al., 1997) Responses ranged from ‘strongly agree’ to ‘strongly disagree’ and were dichotomized (strongly agree or agree vs. other) and combined into a composite score; a high score indicated the participant expected more benefits and fewer barriers and a low score indicated more barriers were expected. A sample benefit statement included ‘Colon cancer screening tests can find pre-cancer.’ Barrier statements included, ‘I cannot afford to have a colon cancer test.’ The standardized Cronbach’s alpha was 0.67 for the benefits score and 0.73 for the barriers score.
Reasons for wanting or not wanting genetic testing to assess cancer risk
Items were adapted from Lerman et al, to assess participants’ reasons for wanting genetic testing (11 items) and reasons for not wanting genetic testing (10 items).(Lerman et al., 1995). An example item for wanting genetic testing included, “To learn if my children are at risk,” while an example for not wanting genetic testing included, “I am not sure the test is accurate.” The number of “yes” responses were summed into a composite score; ““No” and “Don’t know” responses were combined for analyses. The standardized Cronbach’s alpha was 0.62 for reasons for wanting genetic testing and 0.69 for reasons for not wanting genetic testing. In addition, we examined individual reasons for wanting genetic testing to identify areas that were specific to willingness to pay.
Reasons for Not Screening Checklist
Participants indicated reasons for not having a CRC screening test, including lack of MD recommendation, low risk of getting CRC, not understanding the test, not sure if screening is effective, not believing that CRC is curable, discomfort/pain from the test, transportation or financial reasons, and worry about a positive result. The number of reasons indicated was determined for each person (0 to 8 possible) and categorized as 0, 1 or 2+ reasons. In addition, specific items related to willingness to pay were considered separately..
Statistical Analysis
Univariate associations between the outcomes and demographic, cancer history, and psychosocial predictors were tabulated and tested using two-sided chi-square tests (categorical variables) and t-tests (continuous variables). To ascertain associations with specific concerns, selected items were analyzed individually as well as within a total summary score. Two multivariable logistic regression models were developed to examine the association between willingness-to-pay out-of-pocket versus willingness to have testing only if covered by insurance (model 1) and, for those who indicated willingness-to-pay, higher versus lower sum out-of-pocket in dollars (model 2). For each outcome, demographic and psychosocial predictors demonstrating significant (p<0.05) or borderline significant (0.5≤p<0.10) univariate associations were included in the multivariable model, excluding participants with missing responses to any of the selected variables. An exception to this was the income variable, where those who refused to respond or were missing were included as a separate category. A type I error of 0.05 was considered significant in the multivariable models. All statistical analyses were performed using SAS version 9.2.
RESULTS
Baseline data were available for 406 participants. Twenty-one individuals who did not respond to the WTP items were excluded, for a final analytic cohort that included 385 participants. Participant characteristics are outlined in Table 1. The majority were women (73%), White (92%), married or living as married (73%), and aged 45–64 (58%). About half (52%) had at least a four-year college degree and were employed full time (49%). Reported incomes were modest—household income <$75,000 a year was reported by 58% of participants. In total, 19% reported a current or past personal history of GI cancer, and 55% reported at least one first degree relative with CRC.
Eighty two participants (21%) were willing to have genetic testing only if paid by insurance and 303 (79%) were willing-to-pay out-of-pocket. Potential demographic and psychosocial predictors of willingness-to-pay out-of-pocket (Model 1) were assessed and those with statistically significant or borderline significant associations are presented in Table 2. Household income, greater perceived control, greater perceived benefits to CRC screening, and fewer perceived barriers to screening were associated with willingness-to-pay out-of-pocket if testing was not fully covered by insurance; younger age trended towards an association with willingness-to-pay. In multivariable analyses, independent predictors of willingness-to-pay out-of-pocket included greater perceived control of cancer risk and fewer total perceived barriers to CRC screening, with younger age again trending towards significance. Among specific items examining reasons for wanting genetic testing, “To know whether additional cancer screening is necessary” was significant (p<0.03). Among specific items examining reasons for not wanting genetic testing, “Fear of a positive result” was also significant (p=0.011). Notably, indicating more perceived barriers to CRC screening was predictive of wanting genetic testing only if covered by insurance (p<0.01), while reporting more perceived benefits was not significant (p=0.23).
Table 2.
Univariable and multivariable associations of demographics and psychosocial measures and willingness-to-pay, Model 1: Genetic testing only if insurance pays v. Willing-to-pay out-of-pocket
| Willing to pay out of pocket (n=303) | Insurance only (n=82) | Univariable logistic regression results, each predictor in a separate model | Multivariable logistic regression, n=359 with complete responses, one model with all predictors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Demographic variables | Category | N | Percent | N | Percent | OR | 95% CI | p-value* | OR | 95% CI | p-value* |
| Age (years) | |||||||||||
| 18–44 | 110 | 36.3 | 20 | 24.4 | 1.00 | Reference | 1.00 | Reference | |||
| 45–54 | 102 | 33.7 | 37 | 45.1 | 0.50 | 0.27–0.92 | 0.026 | 0.48 | 0.24–0.99 | 0.048 | |
| 55–64 | 72 | 23.8 | 14 | 17.1 | 0.94 | 0.44–1.97 | 0.86 | 0.85 | 0.36–2.02 | 0.72 | |
| 65–82 | 19 | 6.3 | 11 | 13.4 | 0.31 | 0.13–0.76 | 0.010 | 0.42 | 0.14–1.26 | 0.12 | |
| Income | |||||||||||
| <$30,000 | 55 | 18.2 | 27 | 32.9 | 1.00 | Reference | 1.00 | Reference | |||
| $30–74,000 | 116 | 38.3 | 25 | 30.5 | 2.28 | 1.21–4.28 | 0.011 | 1.44 | 0.66–3.04 | 0.37 | |
| $75,000+ | 74 | 24.4 | 12 | 14.6 | 3.03 | 1.41–6.50 | 0.0045 | 2.02 | 0.82–4.94 | 0.13 | |
| Refused/missing | 58 | 19.1 | 18 | 22.0 | 0.98 | 0.37–2.58 | 0.97 | 1.23 | 0.53–2.88 | 0.63 | |
|
| |||||||||||
| Psychosocial measures, continuous variables | Range | N | Mean (SD) | N | Mean (SD) | OR | 95% CI | p-value | OR | 95% CI | p-value |
|
| |||||||||||
| Knowledge, genetic risk & CRC | [0–12] | 299 | 6.6 (2.0) | 82 | 6.1 (2.3) | 1.11 | 0.99–1.24 | 0.084 | 1.02 | 0.89–1.18 | 0.73 |
| Perceived control of cancer risk | [1–4] | 295 | 2.6 (1.0) | 79 | 2.3 (1.0) | 1.39 | 1.07–1.80 | 0.014 | 1.36 | 1.01–1.84 | 0.042 |
| Benefits to CRC screening | [7–35] | 296 | 31.4 (3.1) | 78 | 30.0 (3.6) | 1.13 | 1.05–1.21 | 0.0017 | 1.06 | 0.96–1.17 | 0.23 |
| Barriers to CRC screening | [11–55] | 296 | 20.8 (5.8) | 75 | 23.9 (5.9) | 0.92 | 0.88–0.96 | <0.0001 | 0.93 | 0.88–0.98 | 0.0096 |
| Indicated Yes | Indicated Yes | ||||||||||
|
| |||||||||||
| Items from Reasons for wanting genetic testing | N | Percent | N | Percent | OR | 95% CI | p-value | OR | 95% CI | p-value | |
|
| |||||||||||
| To plan for the future | Checked vs. not | 207 | 68.3 | 47 | 57.3 | 1.61 | 0.97–2.65 | 0.063 | 1.03 | 0.56–1.92 | 0.92 |
| To guide decisions about children | Checked vs. not | 18 | 5.9 | 1 | 1.2 | 5.12 | 0.67–38.90 | 0.090 | 3.23 | 0.38–27.36 | 0.28 |
| To know about need for additional screening | Checked vs. not | 286 | 94.4 | 70 | 85.4 | 2.88 | 1.32–6.32 | 0.0081 | 3.31 | 1.15–9.52 | 0.027 |
|
| |||||||||||
| Items from Reasons for Not Screening Checklist | |||||||||||
|
| |||||||||||
| Financial/transportation reasons | Checked vs. not | 35 | 11.6 | 19 | 23.2 | 0.43 | 0.23–0.81 | 0.0084 | 0.59 | 0.28–1.25 | 0.17 |
| Worried about a positive result | Checked vs. not | 43 | 14.2 | 3 | 3.7 | 4.36 | 1.32–14.42 | 0.0091 | 5.34 | 1.48–19.33 | 0.011 |
Abbreviations: CI, confidence interval; CRC, colorectal cancer; OR, odds ratio; SD, standard deviation
p-values are pairwise comparisons versus reference group, and from logistic regression models, Wald Chi-square tests
Missing values, excluded from tests: genetic risk and CRC knowledge, n=4; perceived control, n=11; benefits to CRC screening, n=11; barriers to CRC, n=14;
Of those willing-to-pay out-of-pocket, 255 (85%) selected a dollar amount they would pay. One hundred ten (43%) were willing-to-pay a higher amount ($500–$2000) for genetic testing, and 145 (57%) were willing-to-pay a lower amount ($200 or less). Male sex, higher educational attainment, and fewer FDR with cancer (0 versus 2) were associated with willingness-to-pay a higher amount (Table 3). In multivariable analyses, subjects willing-to-pay a higher sum out-of-pocket ($500–$2000) were more likely male (p<0.01), more educated (p<0.02 for college versus high school), had fewer first degree relatives with CRC (0 versus 2; p=0.011) and more worry that affected mood (p=0.003 for sometimes versus never). A summary score for not wanting genetic testing was predictive of willingness-to-pay a lower sum (p<0.05), while reasons for wanting genetic testing was not significant (p=0.09).
Table 3.
Univariable and multivariable associations of demographics and psychosocial measures and willingness-to-pay, Model 2: Willing-to-Pay High ($500–$2000) v. Low ($25–$200)
| Willing to pay $500–$2000 for GT (n=115) | Willing to pay $25–$200 for GT (n=156) | Univariable, Willing to Pay High vs. Low | Multivariable, n=255 with complete responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Range | N | Percent | N | Percent | OR | 95% CI | p-value* | OR | 95% CI | p-value* | |
| Gender | Female | 75 | 65.2 | 122 | 78.2 | 1.00 | Reference | 1.00 | Reference | 0.0095 | |
| Male | 40 | 34.8 | 34 | 21.8 | 1.91 | 1.12–3.28 | 0.019 | 2.31 | 1.23–4.36 | ||
|
| |||||||||||
| Education | <High School | 9 | 8.0 | 26 | 16.8 | 1.00 | Reference | 1.00 | Reference | ||
| Some College | 28 | 25.0 | 53 | 34.2 | 1.53 | 0.63–3.70 | 0.35 | 1.99 | 0.71–5.57 | 0.19 | |
| College | 43 | 38.4 | 45 | 29.0 | 2.76 | 1.16–6.56 | 0.022 | 3.43 | 1.27–9.30 | 0.015 | |
| Graduate School | 32 | 28.6 | 31 | 20.0 | 2.98 | 1.21–7.37 | 0.018 | 2.6 | 0.92–7.37 | 0.071 | |
|
| |||||||||||
| First degree relatives with colorectal cancer | 0 | 53 | 46.1 | 59 | 38.1 | 1.00 | Reference | 1.00 | Reference | ||
| 1 | 56 | 48.7 | 71 | 45.8 | 0.88 | 0.53–1.46 | 0.62 | 0.74 | 0.41–1.34 | 0.31 | |
| 2+ | 6 | 5.2 | 25 | 16.1 | 0.27 | 0.10–0.70 | 0.0074 | 0.23 | 0.07–0.71 | 0.011 | |
|
| |||||||||||
| Reasons for Not Screening Checklist Score | 0 | 60 | 52.2 | 81 | 51.9 | 0.76 | 0.44–1.31 | 0.32 | 0.85 | 0.45–1.59 | 0.60 |
| 1 | 41 | 35.7 | 42 | 26.9 | 1.00 | Reference | 1.00 | Reference | |||
| 2+ | 14 | 12.2 | 33 | 21.2 | 0.44 | 0.20–0.93 | 0.031 | 0.5 | 0.21–1.20 | 0.12 | |
|
| |||||||||||
| Psychosocial Measures, continuous variables | N* | Mean (SD) | N* | Mean (SD) | OR | 95% CI | p-value | OR | 95% CI | p-value | |
|
| |||||||||||
| Reasons for wanting GT | [0–11] | 115 | 6.5 (2.0) | 156 | 6.0 (1.9) | 1.14 | 1.01–1.30 | 0.043 | 1.15 | 0.98–1.35 | 0.088 |
| Reasons for not wanting GT | [0–10] | 115 | 0.9 (1.6) | 156 | 1.4 (1.9) | 0.84 | 0.72–0.98 | 0.027 | 0.82 | 0.68–0.99 | 0.043 |
| Barriers to CRC screening | [11–55] | 115 | 20.0 (5.3) | 152 | 21.4 (6.0) | 0.96 | 0.92–1.00 | 0.061 | 1.01 | 0.92–1.11 | 0.88 |
| N | Percent | N | Percent | OR | 95% CI | p-value | OR | 95% CI | p-value | ||
|
| |||||||||||
| Worry about developing a gastrointestinal cancer | Never | 25 | 219 | 47 | 30.9 | 1.00 | Reference | 1.00 | Reference | ||
| Sometimes | 53 | 46.5 | 60 | 39.5 | 1.66 | 0.90–3.06 | 0.100 | 1.21 | 0.58–2.56 | 0.61 | |
| Often | 16 | 14 | 30 | 19.7 | 1.00 | 0.46–2.18 | 0.99 | 0.45 | 1.15–9.52 | 0.13 | |
| A lot | 20 | 17.5 | 15 | 9.9 | 2.51 | 1.10–5.73 | 0.029 | 0.9 | 0.26–3.04 | 0.86 | |
|
| |||||||||||
| Worry affects your mood | Never | 56 | 49.6 | 107 | 70.9 | 1.00 | Reference | 1.00 | Reference | ||
| Sometimes | 40 | 35.4 | 30 | 19.9 | 2.55 | 1.44–4.52 | 0.0014 | 3.26 | 1.51–7.02 | 0.0025 | |
| Often | 10 | 8.9 | 10 | 6.6 | 1.91 | 0.75–4.86 | 0.17 | 2.40 | 0.72–7.94 | 0.153 | |
| A lot | 7 | 6.2 | 4 | 2.7 | 3.34 | 0.94–11.91 | 0.063 | 3.64 | 0.64–20.73 | 0.146 | |
Abbreviations: CI, confidence interval; CRC, colorectal cancer; GT, genetic testing; OR, odds ratio; SD, standard deviation
p-values are pairwise comparisons versus reference group, and from logistic regression models, Wald Chi-square tests
Missing values, excluded from tests: Education, n=4; First degree relatives, n=1; Worry develop cancer, n=5; worry affect mood, n=7; barriers to CRC screening, n=4
DISCUSSION
At baseline and before counseling, participants in our high-risk registry reported willingness-to-pay some out-of-pocket costs for genetic testing. However, willingness-to-pay was not uniform, with demographic and psychosocial factors associated with variation in level of and in amount willing to pay. Specifically, we found that willingness-to-pay over $500 out-of-pocket for genetic testing was associated with male sex, higher education, having fewer first degree relatives with CRC, and reporting more reasons for wanting genetic testing. These findings are significant, as individuals seeking clinical risk assessment for a possible hereditary risk of cancer represent those individuals for whom genetic testing may be predicted to have the greatest impact, and thus are a group where the potential negative impact of out-of-pocket costs must be better understood. Genetic assessment in high-risk individuals offers the greatest cancer prevention and cost-savings benefits when viewed from a societal perspective(Gudgeon et al., 2011; G. Wang, Kuppermann, Kim, Phillips, & Ladabaum, 2012; Yang, Caughey, Little, Cheung, & Chen, 2011); efforts to better understand how individuals value genetic testing and the barriers they may experience to uptake of genetic testing in the setting of rising healthcare costs and out-of-pocket expenses are needed. Understanding how cost barriers may affect patient perceptions of genetic testing offers insight to guide the development of future interventions to improve patient access to clinical genetics services including testing.
These results suggest that individuals of higher socioeconomic status (SES) are willing-to-pay more for genetic testing when co-payments are present; this potentially could translate into disparities in access to care based on SES. However, even after controlling for income and education, psychosocial and demographic factors also emerged as contributors to participants’ decisions regarding willingness-to-pay out-of-pocket for elective genetic services. Interestingly, younger respondents generally, although not absolutely, were more willing-to-pay for testing if not covered by insurance. This may reflect shifting norms among younger versus older individuals regarding the acceptability of cost-sharing practices for healthcare services. Interestingly, women, who represented 73% of our sample, were also less willing-to-pay a higher dollar amount for genetic testing despite comprising the majority of participants in our high-risk registry. The inverse association of number of first degree relatives with CRC with willingness-to-pay for genetic testing was unexpected. Possible explanations for this finding include that participants with stronger family history assume the test will be positive and thus are willing to pay less, or that participants with strong family histories have become more familiar with what steps are needed to manage risk due to the family burden of disease and thus may feel testing would not change the approach to disease prevention. These individuals are an important group of patients of whom genetic counselors should be aware. While many patients with strong family histories may be more knowledgeable and familiar with the recommended screening, genetic counselors should nonetheless ensure that their information and risk assessment is accurate in order to individually tailor recommendations and therapy.
Because much of the literature focuses on BRCA1/2 testing among average and high-risk women, between-sex differences in perceptions of genetic risk and the relationship between interest in testing versus willingness-to-pay out-of-pocket may have been less apparent in previous studies.(Bottorff et al., 2002; Chaliki et al., 1995; Graves, Peshkin, Luta, Tuong, & Schwartz, 2011; Jacobsen et al., 1997; Lerman et al., 1994; Lerman et al., 1997; Lerman et al., 1995) Only 50% of the women in our study had a full-time job, and thus may have had less expendable personal income for elective healthcare needs despite overall adequate household finances. We anticipated women would be willing-to-pay higher amounts because of their greater tendency (versus men) to communicate about genetic risk within the family.(Wiseman, Dancyger, & Michie, 2010) Lerman et al found that 76% of women with a first degree relative with ovarian cancer rated “to learn about one’s children’s’ risks” as very important in their motivation to get genetic testing.(Lerman et al., 1994) Studies of families with known BRCA1/2 mutations have shown that men have lower rates of genetic testing completion, and that women are more likely to disclose test results to other women in the family than to men.(Finlay et al., 2008; Patenaude et al., 2006) While women may have high awareness and strong motivation, they were willing-to-pay less compared to men in our study. Studies evaluating uptake of genetic testing among high-risk individuals usually include mostly female participants, therefore offering a one-sided view of the population who could most benefit from risk assessment. Nonetheless there are limitations to comparing BRCA1/2 testing to gastrointestinal cancer risk assessment, primarily that with BRCA1/2 mutations, the impact on women with respect to cancer risk is much greater than on men, as the risk of breast or ovarian cancer can range from 20% to as high as80%(Antoniou et al., 2003; King, Marks, & Mandell, 2003; Mavaddat et al., 2013). Variability in cancer risks by gender is less prominent in the GI risk assessment population, with gynecologic risks in Lynch syndrome being the exception.
One possible explanation for the differences in willingness-to-pay between men and women in our study is differences in income levels. Of participants who indicated an income level, women tended to report lower incomes than men (p=0.0022, X2) (see Table 1). However, the survey questionnaire specifically queried “household income,” not personal income. Therefore, we cannot conclude that women in our sample earn less than men, but only that it is possible women seeking genetic counseling came from households with less overall income compared to men seeking counseling. Further study is needed to examine how men and women differ in their perceptions of risk and interest in and willingness-to-pay for genetic testing, including single women and women in families where they are the primary source of income.
Other investigators have found that individuals with more positive attitudes towards genetic testing and cancer screening will spend more for genetic testing.(Bosompra, Ashikaga, Flynn, Worden, & Solomon, 2001) Several attitudinal factors influence likelihood of undergoing genetic testing, including perceiving fewer barriers to cancer screening, perceiving greater benefits, and having a generally pessimistic outlook on life. (Bosompra et al., 2001) In our study, perceived barriers score and having more reasons for not wanting genetic testing were negatively associated with willingness-to-pay while perceived benefits score was not. In Bosompra et al’s average-risk population, benefits and barriers to CRC screening had an almost equal, yet opposite, effect on likelihood of undergoing genetic testing.(R. M. Andersen, 1995; Bosompra et al., 2000) The results of our study suggest that among higher-risk populations, perceived barriers to genetic testing and downstream screening may be more influential than benefits when individuals assess the value of elective testing to their health. This insight could be clinically useful to genetic service providers; reluctance to pursue testing among high-risk individuals may be rooted in specific barriers and unresolved decisional conflict that, if more thoroughly explored, may lead to improved shared decision-making and identification of solutions.
Prior studies have discredited the commonly held notion that physicians and patients have an “anything at all costs” attitude toward cancer treatment.(Wong et al., 2010) Wong et al showed that cancer patients may not be willing to accept higher out-of-pocket costs for cancer treatments that have only modest benefit.(Wong et al., 2010) These findings suggest that high copayments have the potential to reduce patient willingness to pursue other medical therapies, including predictive genetic testing, that might have a delayed, rather than immediate, benefit. Participants in our study who indicated more reasons for not wanting genetic testing may not have fully understood the value of testing. Alternatively, if individuals decline because they feel they simply cannot afford the test, helping them understand the potential value of knowing the result may lead to greater uptake of genetic testing and a willingness to share some of the cost burden. Small studies have demonstrated that offering genetic testing and counseling to appropriate (i.e. high risk) individuals can be cost effective.(Chikhaoui, Gélinas, Joseph, & Lance, 2002; V. W. Wang, Koh, Chow, & Lim, 2012) The cost effectiveness of genetic testing, in conjunction with our results that some participants may be dissuaded from undergoing genetic testing because of high out-of-pocket costs, suggests that insurers may have a financial incentive to offer genetic testing and enable their customers to obtain potentially life-saving (and cost-saving) genetic information.
Our study should be interpreted with several important limitations in mind. Our sample was primarily drawn from a population living near or directly associated with FCCC for personal cancer care or care of a family member. Additionally, our population was predominantly female, with limited minority group representation and an average income greater than the general US population. Therefore, our findings may not be generalizable to all populations. Nonetheless, our results demonstrate that individuals participating in the GI-TRAP registry are diverse both in perceptions of genetic testing and cancer screening, and by SES. Finally, our registry does not include data on whether respondents are self-referred or referred by a provider. We do not know whether participants in the study ultimately decided to pursue testing, their likelihood of having Lynch mutation, or their actual level of cancer risk. We also acknowledge that internal consistency scores in the low-acceptable range and the use of single items versus full scale for certain questionnaire items, does limit the conclusions we are able to make.
In conclusion, many individuals at high risk of developing cancer are willing-to-pay some out-of-pocket costs for genetic testing. Individuals willing-to-pay are fearful of a positive result and anticipate benefits to control of cancer risk afforded by genetic testing. Among individuals at increased hereditary risk for cancer, perceived barriers to CRC screening and genetic testing may drive cost-based decision-making more so than perceived benefits. Participants’ decisions are influenced by the immediate financial burden of co-payments for genetic services, with less consideration of the potential risk of malignancy in the future. Our findings have implications for guiding genetic counselors to better understand and address individual expectations before a genetic test is performed. More broadly, they may also help researchers and clinicians better understand how cost-related perceptions and concerns impact whether patients ever present for genetic evaluation in the setting of a strong personal or family history of cancer. A better understanding of patient-level factors, and in particular barriers to genetic testing, associated with willingness-to-pay for genetic services will be increasingly important as genetic and genomic testing become more integrated into routine cancer care.
Acknowledgments
Funding:
This research was supported by the Fox Chase Cancer Center Core Grant (P30-006927-45). Dr. Hall is a recipient of a Mentored Research Scholar Grant from the American Cancer Society (MRSG-07-232-01-CPHPS).
Footnotes
Conflicts of Interest:
None of the authors have relevant financial disclosures or conflicts of interest.
References
- Andersen MR, Smith R, Meischke H, Bowen D, Urban N. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiology Biomarkers & Prevention. 2003;12(4):314–320. [PubMed] [Google Scholar]
- Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? Journal of health and social behavior. 1995:1–10. [PubMed] [Google Scholar]
- Antoniou A, Pharoah P, Narod S, Risch HA, Eyfjord JE, Hopper J, Borg Å. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. The American Journal of Human Genetics. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azjen I. From intentions to actions: A theory of planned behavior. In: Beckman JKJ, editor. Action-control: From cognition to behavior. Heidelberg, Germany: Springer; 1985. pp. 11–39. [Google Scholar]
- Bosompra K, Ashikaga T, Flynn BS, Worden JK, Solomon LJ. Psychosocial factors associated with the public’s willingness to pay for genetic testing for cancer risk: a structural equations model. Health Education Research. 2001;16(2):157–172. doi: 10.1093/her/16.2.157. [DOI] [PubMed] [Google Scholar]
- Bosompra K, Flynn BS, Ashikaga T, Rairikar CJ, Worden JK, Solomon LJ. Likelihood of undergoing genetic testing for cancer risk: a population-based study. Preventive medicine. 2000;30(2):155–166. doi: 10.1006/pmed.1999.0610. [DOI] [PubMed] [Google Scholar]
- Bottorff JL, Ratner PA, Balneaves LG, Richardson CG, McCullum M, Hack T, Buxton J. Women’s interest in genetic testing for breast cancer risk the influence of sociodemographics and knowledge. Cancer Epidemiology Biomarkers & Prevention. 2002;11(1):89–95. [PubMed] [Google Scholar]
- Burak LJ, Meyer M. Using the Health Belief Model to examine and predict college women’s cervical cancer screening beliefs and behavior. Health Care for Women International. 1997;18(3):251–262. doi: 10.1080/07399339709516279. [DOI] [PubMed] [Google Scholar]
- Burn J, Gerdes A-M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S, Bertario L. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. The Lancet. 2011 doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaliki H, Loader S, Levenkron JC, Logan-Young W, Hall WJ, Rowley P. Women’s receptivity to testing for a genetic susceptibility to breast cancer. American Journal of Public Health. 1995;85(8_Pt_1):1133–1135. doi: 10.2105/ajph.85.8_pt_1.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhaoui Y, Gélinas H, Joseph L, Lance JM. Cost-minimization analysis of genetic testing versus clinical screening of at-risk relatives for familial adenomatous polyposis. International journal of technology assessment in health care. 2002;18(1):67–80. [PubMed] [Google Scholar]
- Finlay E, Stopfer JE, Burlingame E, Evans KG, Nathanson KL, Weber BL, Domchek SM. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genetic testing. 2008;12(1):81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves K, Peshkin B, Luta G, Tuong W, Schwartz M. Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics. 2011;14(3):178–189. doi: 10.1159/000324703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudgeon JM, Williams JL, Burt RW, Samowitz WS, Snow GL, Williams MS. Lynch syndrome screening implementation: business analysis by a healthcare system. Am J Manag Care. 2011;17(8):e288–300. [PubMed] [Google Scholar]
- Hudson KL, Murphy JA, Kaufman DJ, Javitt GH, Katsanis SH, Scott J. Oversight of US genetic testing laboratories. Nature Biotechnology. 2006;24(9):1083–1090. doi: 10.1038/nbt0906-1083. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Valdimarsdottier H, Brown KL, Offit K. Decision-making about genetic testing among women at familial risk for breast cancer. Psychosomatic Medicine. 1997;59(5):459–466. doi: 10.1097/00006842-199709000-00001. [DOI] [PubMed] [Google Scholar]
- Japsen B. 2014, Workers’ Share of Health Costs Nearly $5,000 At Large Companies. 2013 Oct 17; Retrieved November 11, 2013, 2013, from http://www.forbes.com/sites/brucejapsen/2013/10/17/in-2014-workers-share-of-health-costs-nearly-5000-at-large-companies/
- Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. Journal of clinical oncology. 2009;27(28):4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- Lerman C, Daly M, Masny A, Balshem A. Attitudes about genetic testing for breast-ovarian cancer susceptibility. Journal of clinical oncology. 1994;12(4):843–850. doi: 10.1200/JCO.1994.12.4.843. [DOI] [PubMed] [Google Scholar]
- Lerman C, Kerner J, Gomez-Caminero A, Hughes C, Reed MM, Biesecker B, Benkendorf JL. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. Journal of the National Cancer Institute. 1997;89(2):148–157. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- Lerman C, Schwartz MD, Miller SM, Daly M, Sands C, Rimer BK. A randomized trial of breast cancer risk counseling: Interacting effects of counseling, educational level, and coping style. Health psychology. 1996;15(2):75. doi: 10.1037//0278-6133.15.2.75. [DOI] [PubMed] [Google Scholar]
- Lerman C, Seay J, Balshem A, Audrain J. Interest in genetic testing among first-degree relatives of breast cancer patients. American journal of medical genetics. 1995;57(3):385–392. doi: 10.1002/ajmg.1320570304. [DOI] [PubMed] [Google Scholar]
- Leventhal H. Findings and theory in the study of fear communications. Advances in experimental social psychology. 1970;5:119–186. [Google Scholar]
- Leventhal H, Safer MA, Panagis DM. The impact of communications on the self-regulation of health beliefs, decisions, and behavior. Health Education & Behavior. 1983;10(1):3–29. doi: 10.1177/109019818301000101. [DOI] [PubMed] [Google Scholar]
- Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, Easton DF. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- Mecklin JP, Aarnio M, Läärä E, Kairaluoma MV, Pylvänäinen K, Peltomäki P, Järvinen HJ. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology. 2007;133(4):1093–1098. doi: 10.1053/j.gastro.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Miller AM, Champion VL. Attitudes about breast cancer and mammography: racial, income, and educational differences. Women & health. 1997;26(1):41–63. doi: 10.1300/J013v26n01_04. [DOI] [PubMed] [Google Scholar]
- Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. Journal of clinical oncology. 2006;24(4):700–706. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- Powell A, Chandrasekharan S, Cook-Deegan R. Spinocerebellar ataxia: patient and health professional perspectives on whether and how patents affect access to clinical genetic testing. Genetics in Medicine. 2010;12:S83–S110. doi: 10.1097/GIM.0b013e3181d67e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock I. The health belief model: explaining health behavior through expectancies. In: Glanz LF, Rimer KBK, editors. Health behavior and health education theory research and practice. San Francisco: Jossey-Bass; 1990. pp. 39–61. [Google Scholar]
- Schwartz MD, Lerman C, Miller SM, Daly M, Masny A. Coping disposition, perceived risk, and psychological distress among women at increased risk for ovarian cancer. Health psychology. 1995;14(3):232. doi: 10.1037//0278-6133.14.3.232. [DOI] [PubMed] [Google Scholar]
- Thomas R, Clarke V. Colorectal cancer: a survey of community beliefs and behaviours in Victoria. The Medical journal of Australia. 1998;169(1):37. doi: 10.5694/j.1326-5377.1998.tb141476.x. [DOI] [PubMed] [Google Scholar]
- Urban N, Anderson G, Peacock S. Mammography screening: how important is cost as a barrier to use? American Journal of Public Health. 1994;84(1):50–55. doi: 10.2105/ajph.84.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon SW. Participation in colorectal cancer screening: a review. Journal of the National Cancer Institute. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- Wang G, Kuppermann M, Kim B, Phillips KA, Ladabaum U. Influence of Patient Preferences on the Cost-Effectiveness of Screening for Lynch Syndrome. Journal of Oncology Practice. 2012;8(3S):e24s–e30s. doi: 10.1200/JOP.2011.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VW, Koh PK, Chow WL, Lim JFY. Predictive genetic testing of first degree relatives of mutation carriers is a cost-effective strategy in preventing hereditary non-polyposis colorectal cancer in Singapore. Familial cancer. 2012;11(2):279–289. doi: 10.1007/s10689-012-9513-y. [DOI] [PubMed] [Google Scholar]
- Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: a review. Familial cancer. 2010;9(4):691–703. doi: 10.1007/s10689-010-9380-3. [DOI] [PubMed] [Google Scholar]
- Wong YN, Hamilton O, Egleston B, Salador K, Murphy C, Meropol NJ. Understanding how out-of-pocket expenses, treatment value, and patient characteristics influence treatment choices. The oncologist. 2010;15(6):566–576. doi: 10.1634/theoncologist.2009-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KY, Caughey AB, Little SE, Cheung MK, Chen LM. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Familial cancer. 2011;10(3):535–543. doi: 10.1007/s10689-011-9444-z. [DOI] [PubMed] [Google Scholar]


