Abstract
Cytochromes c are heme proteins that require multiple maturation components, such as heme lyases, for cofactor incorporation. Saccharomyces cerevisiae has two heme lyases that are specific for apocytochromes c (CCHL) or c1 (CC1HL). CCHL can covalently attach heme b groups to apocytochrome c substrates of eukaryotic but not prokaryotic origin. Besides their conserved Cys-Xxx-Xxx-Cys-His heme-binding motifs, the amino-terminal regions of apocytochrome c substrates appear to be important for CCHL function. In this study, we show for the first time that only two amino acid changes in the amino-terminal region of the non-CCHL substrate apocytochrome c2 from Rhodobacter capsulatus are necessary and sufficient for efficient holocytochrome c formation by CCHL. This finding led us to propose a consensus sequence located at the amino-terminus of apocytochromes c and critical for substrate recognition and heme ligation by CCHL.
Keywords: Cytochrome c maturation, cytochrome c heme lyase, substrate specificity, apocytochrome c, heme attachment
1. Introduction
Cytochromes c are ubiquitious hemoproteins that act primarily as electron carriers between respiratory or photosynthetic energy transduction pathways, and are also involved in other cellular processes. They contain at least one heme b (protoporphyrin IX-Fe) cofactor bound covalently to the polypeptide chain via (usually) two thioether bonds between the vinyl groups of the porphyrin ring and the cysteine-sulfhydryl groups of a Cys-Xxx-Xxx-Cys-His heme-binding site [1–2]. Multiple distinct biogenesis machineries responsible for post-translational attachment of heme to apocytochromes c have been identified in various organisms and organelles [3–6]. One of these machineries (Ccm system III) is confined to mitochondria of fungi, metazoans and some protozoa. It includes one or two components with defined heme lyase activities towards apocytochrome c substrates, and a NAD(P)H-dependent heme reductase as an accessory factor [7–9]. Some eukaryotes like Saccharomyces (S.) cerevisiae contain two heme lyases with substrate specificities for either apocytochromes c (CCHL) or c1 (CC1HL) [10–11]. Other eukaryotes, including human and mouse, have only one heme lyase (also known as holocytochrome c synthetase or HCCS) with a broader substrate specificity, able to mature both cytochromes c and c1 [11–13]. CCHLs, CC1HLs and HCCSs show significant sequence similarity (~ 35% amino acid identity), and harbor one to four Cys-Pro-Val motifs [12,14], frequently found in proteins interacting with heme b groups [15], but shown to be not necessary for CCHL-dependent holocytochrome c formation [16].
Previous studies [17–32] have revealed that heme can be ligated to various apocytochromes c of eukaryotic origin in the cytoplasm of Escherichia (E.) coli hosts upon coproduction with S. cerevisiae CCHL, demonstrating that CCHL matures efficiently holocytochromes c in a redox-controlled heterologous environment (Fig. 1). However, holocyt c formation does not occur using apocyts c of prokaryotic origin, such as Paracoccus (P.) denitrificans cytochrome c550 [30]. Yet, a chimeric apocytochrome c formed of S. cerevisiae iso-2-cyt c (Cyc7p) and P. denitrificans cytochrome c550 can be ligated with heme by CCHL provided that at least the first 28 amino acids of Cyc7p, including its conserved heme binding site, are present [33]. Recently, data obtained with similar chimeric apocytochrome c constructs confirmed these findings [27,34], and established that the substrate recognition domain for CCHL is confined the amino-terminal region encompassing the heme binding motif of apocytochromes c. Thus, the determination of the amino acid composition of the amino-terminal portion of apocytochromes c is critical for the understanding of the mechanisms underlying substrate recognition and heme attachment by CCHL.
Figure 1.
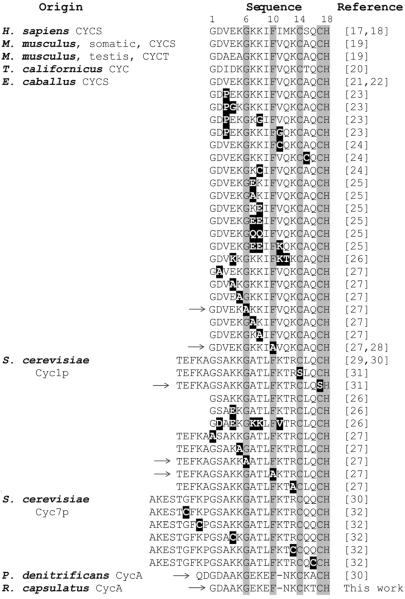
Sequence alignment of the amino-terminal portions of eukaryotic and prokaryotic cytochromes c. H. sapiens CYCS: Homo sapiens (or human) cytochrome c; M. musculus, somatic, CYCS: Mus musculus (or mouse) somatic cytochrome c; M. musculus, testis, CYCT: Mus musculus testis-specific cytochrome c; T. californicus CYC: Tigriopus (Tigerpod) californicus cytochrome c; E. caballus CYCS: Equus caballus (or horse) cytochrome c; S. cerevisiae Cyc1p and Cyc7p: Saccharomyces cerevisiae (or baker's yeast) iso-1-cytochrome c and iso-2-cytochrome c, respectively; P. denitrificans CycA: Paracoccus denitrificans cytochrome c550; R. capsulatus CycA: Rhodobacter capsulatus cytochrome c2. Absolutely conserved amino acid residues are highlighted in grey, and mutations in various cytochromes c tested experimentally for CCHL-dependent holocytochrome c formation are indicated in black. Cytochrome c derivatives that cannot be covalently ligated with heme by CCHL in the heterologous E. coli system are marked with an arrow in the left (→).
In this work, we first showed that, like P. denitrificans cytochrome c550, the prokaryotic Rhodobacter (R.) capsulatus cytochrome c2 (CycA) is produced in the cytoplasm of E. coli only as an apoform, both in the absence or presence of S. cerevisiae CCHL. Then, we carried out mutational analyses that stem from the comparison of the available amino-terminal sequences of mitochondrial cyts c, known to be matured by CCHL in the cytoplasm of E. coli (Fig. 1). We found that a Glu to Leu substitution at position 9, together with a Lys insertion at position 11 in R. capsulatus CycA are necessary and sufficient to render it an efficient CCHL substrate. These findings led us to propose a consensus sequence motif for apocytochrome c recognition and heme attachment by CCHL.
2. Materials and Methods
2.1 Cloning and Expression
Molecular biology techniques were performed according to Sambrook et al [35]. All constructs were confirmed by DNA sequencing. The bacterial strains and plasmids used in this work are described in Table 1. R. capsulatus cytochrome c2 (CycA) mutants were constructed by site-directed mutagenesis using the QuickChange site directed mutagenesis kit (Stratagene, La Jolla, CA) and the plasmid pCS1726 as a template. This plasmid contains an in-frame Strep-tag II epitope (WSHPQFEK) sequence fused at the 5'-end of cycA encoding CycA without its native signal peptide [36]. E. coli strain EC06, carrying a deletion of all cytochrome c maturation genes ccmABCDEFGH [37], was first transformed with pCS315 harboring the gene encoding S. cerevisiae CCHL fused with a 6xHis-tag sequence at its 5'-end [33]. Then, the EC06 strain harboring pCS315 was transformed with compatible plasmids containing appropriate apocytochrome c mutants. Overnight cultures (1 ml) of the EC06/pCS1726+pCS315 transformants (as well as the other apocytochromes variants) in Luria–Bertani (LB) broth medium, supplemented with 100 μg/ml ampicillin and 50 μg/ml chloramphenicol were used to inoculate 10 mL of the same medium in 50 mL tubes, and grown at 30 °C with gentle shaking until an OD600nm of 0.6. At this OD, cultures were induced by adding 1 mM IPTG for 4 h under the same growth conditions. Cells were then harvested by centrifugation and stored at −20 °C until further use.
Table 1.
Strains and Plasmids used in this work
| Strain/Plasmid | Relevant Characteristics | References |
|---|---|---|
|
| ||
| HB101 | E. coli strain used for cloning | Stratagene |
| EC06 | E. coli ccmABCDEFGH::kan strain used for gene expression | [37] |
| pCS3O8 | = pACCyc3p: pLysS (Novagen®) derivative with T7 lysozyme gene replaced by expression cassette containing T7 promoter/lac operator system and 6xHis-tag sequence fused CYC3 from S. cerevisiae, ClmR; CCHL | [30] |
| pCS315 | pCS308 derivative with T7 promoter region replaced by tac promoter region of pMal-c2 (New England BioLabs®,Inc), ClmR | This work |
| pCS905 | pET-3a (Novagen®) derivative with T7 promoter region replaced by tac promoter region of pMal-c2 (New England BioLabs®,Inc.), AmpR | [33] |
| pCS13O3 | pCS905 derivative containing a decahistidine sequence-fused GFP gene variant, AmpR | [45] |
| pCS703 | = pSCyc7p: pET-3a (Novagen®) derivative containing Strep-tag II (IBA®) sequence-fused CYC7 from S. cerevisiae, AmpR | [30] |
| pCS1000 | Strep-tag II (IBA) sequence-fused CYC7 from pCS703 cloned XbaI and BamHI into pCS1303, replacing 10xHis::GFP, AmpR; Cyc7p | This work |
| pCS1011 | pCS1000 derivative with CYC7 encoding the amino acid sequence C-terminal of its CXXCH heme binding site replaced by R. capsulatus cycA to generate a fusion of Cyc7p and CycA joined at Cyc7p His 18, AmpR ; Cyc7pH18_sl9CycA | This work |
| pCS1736 | pCS1000 derivative, like pCS1011 but Cyc7p and CycA are joined N-terminally of the CXXCH motif of CycA at Cys14, AmpR; Cyc7pR13_C14CycA | This work |
| pCS1769 | pCS1011 derivative, deletion of the codons encoding thirteen amino-terminal amino acid residues (Ala-9 to Lys4) of the Cyc7p portion AmpR; K5Cyc7pH18_Sl9CycA | This work |
| pCS1302 | pCS905 derivative, Strep-tag II (IBA) sequence-fused GFP gene variant expressed under control of tac promoter/lac operator system, AmpR | [36] |
| pCS1726 | pCS1302 derivative containing Strep-tag II (IBA) sequence-fused cycA (matured cyt c2 gene variant) from R. capsulatus CycA, AmpR; CycA | [36] |
| pCS1730 | pCS1726 derivative with a codon for a Lys inserted at position ll,AmpR; CycA-insK11 | This work |
| pCS1760 | pCS1726 derivative with the Glu9 codon exchanged to a Leu codon, AmpR; CycA-E9L | This work |
| pCS1748 | pCS1730 derivative with the Glu9 codon exchanged to a Leu codon, AmpR; CycA-insK11+E9L | This work |
| pCS1754 | pCS1730 derivative with the Asn12 codon exchanged to a Thr codon, AmpR; CycA-insK11+N12T | This work |
2.2 Protein Analysis
E. coli cells were resuspended in BugBuster® Protein Extraction Reagent (Novagen®) supplemented with 300 mM NaCl and 1 mM PMSF. After 45 min incubation with gentle stirring at room temperature, insoluble material was removed by centrifugation at 16 000 × g for 15 min at 4 °C. Protein concentration of the clear supernatants was determined using the Bicinchoninic Acid kit (Sigma, Inc.) with bovine serum albumin as a standard. SDS-PAGE was performed using 15% acrylamide-bisacrylamide gels according to [38]. Samples were resuspended in 2× loading buffer without addition of any reducing agent and incubated at 42 °C for 10 min. For immunodetection of Strep-tag fusions to cytochromes c, gel-resolved proteins were electroblotted onto Immobilon-PVDF membranes (Millipore, Inc.), and probed with Strep•Tag® II Monoclonal antibodies (Novagen®). Horseradish peroxidase conjugated anti-mouse IgGs (GE Healthcare, Inc.) were used as secondary antibodies, and detection was performed using the SuperSignal West Pico Chemiluminescent Substrate® (Thermo Scientific, Inc.). For detection of proteins containing covalently attached heme, SDS-PAGE resolved proteins were electroblotted onto nitrocelullose membranes (Schleicher and Schüll), which were washed with 50 mM Tris-HCl (pH 8.0) and 150 mM NaCl, and the presence of heme-dependent peroxidase activity was monitored using the SuperSignal West Pico Chemiluminescent Substrate® [39].
Visible spectra were recorded at room temperature in a Cary 60 spectrophotometer (Agilent Technologies, Inc) between 400 and 600 nm. Reduction of the total protein extracts was carried out by adding a few grains of sodium dithionite prior to recording the spectra.
3. Results and Discussion
3.1 Amino acid residues at the amino-terminal portion of apocytochromes c critical for CCHL activity
Mitochondrial cytochromes c have been reported to be covalently attached with heme by S. cerevisiae CCHL upon coproduction in the cytoplasm of E. coli whereas cytochromes c of prokaryotic origin are not [17–32]. Yet, fusion proteins containing the amino-terminal portion including the Cys-Xxx-Xxx-Cys-His heme binding motif of mitochondrial and the carboxyl-terminal portion of prokaryotic cytochromes c can be covalently ligated with heme by CCHL. Thus, only the amino-terminal portions of mitochondrial cytochromes c are needed for CCHL-dependent holocytochrome c formation, suggesting that the amino acid sequences and/or structural properties of the other apocytochrome regions are not essential for heme ligation [27,33–34].
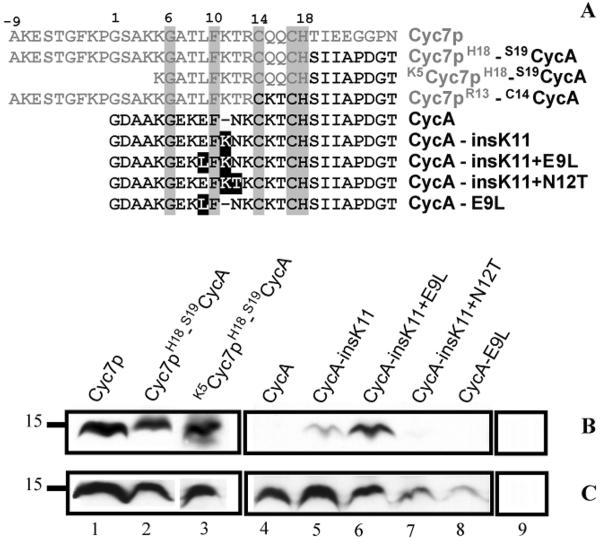
In this study, we used cytochrome c2 (CycA) from R. capsulatus as a prokaryotic, non-CCHL apoprotein substrate model and showed that it is produced in the cytoplasm of E. coli only as an apoform, independent of the presence or absence of S. cerevisiae CCHL (Fig. 2B and 2C, lanes 4). Then, we compared the amino-termini of mitochondrial cytochromes c that are matured by CCHL in the same heterologous host compartment with those of prokaryotic or mutant mitochondrial cytochromes c that are not CCHL substrates (Fig. 1). As a first mutational step, we fused the amino-terminus of S. cerevisiae Cyc7p, including its heme binding site Cys14-Gln15-Gln16-Cys17-His18, with the carboxyl-terminal portion of R. capsulatus CycA, creating Cyc7pH18-S19CycA (Fig. 2A). As expected, this fusion protein became competent for covalent heme ligation upon coproduction with CCHL (Fig. 2B and 2C, lanes 2). Next, we sought to define the amino acid residues in the amino-terminal portion of R. capsulatus CycA that are required for efficient heme ligation by CCHL.
Figure 2.
R. capsulatus apocytochrome c2 (CycA) fusion and mutant proteins tested for CCHL-dependent holocytochrome c formation in E. coli. Amino-termini sequences of different chimeric proteins formed of S. cerevisiae iso-2-cytochrome c (Cyc7p) and R. capsulatus CycA, and CycA mutants used are shown in panel A. Amino acid residues originating from Cyc7p and CycA are depicted in grey and black, respectively. Of the cytochromes c used only eight amino acids immediately following the Cys-Xxx-Xxx-Cys-His heme binding site are depicted to show the junction sequence of the chimera. In the fusion proteins amino acid residues flanking each portion are indicated. Absolutely conserved amino acids are boxed in grey, and mutations in the CycA derivatives are boxed in black. Panels B and C show the heme staining and Strep-tag detection data, respectively, for various cytochromes c coproduced with CCHL (lanes 1–8) or for CCHL produced alone (lane 9) in E. coli EC06 (ΔCcm) using 75 μg of total proteins in each case. Molecular markers (kDa) are shown on the left.
Amino acid residues Cys14, Cys17, and His18 (horse cytochrome c numbering) are conserved in virtually all cytochromes c, with the sole exception of some Euglenozoa mitochondrial cytochromes c [40]. However, a S. cerevisiae iso-1-cytochrome c (Cyc1p) variant with Cys14 to Ser substitution can be ligated with heme by CCHL, while a variant of the same cytochrome c with a Cys17 to Ser replacement cannot [31]. The fact that CCHL does not require Cys14 for proficient heme attachment to apocytochrome substrates, together with its mitochondrial location, suggest a plausible link between the CCHL (System III) and the proposed Euglenozoan Ccm system V, which ligates heme b groups to mitochondrial cytochromes c with a single Cys heme binding sequence (Xxx-Xxx-Xxx-Cys-His) [4]. However, this fact clearly distinguishes the substrate specificity of CCHL from that of Ccm system I (found in bacteria, archaea, and plant mitochondria), which requires a Cys-Xxx-Xxx-Cys-His heme binding motif for covalent and stereo-specific heme incorporation into cytochromes c [3,41]. Like Cys17, His18 is also essential for heme attachment (Fig. 1). It acts as a proximal axial ligand for the heme-iron and is invariantly present in all cytochromes c [1]. The heme binding motif of horse cytochrome c (Equus caballus CYCS) can be changed to Cys-Xxx-Xxx-Xxx-Cys-His (but not to Cys-Xxx-Xxx-Xxx-Xxx-Cys-His), and still remains a competent CCHL substrate, albeit at a lower efficiency [28]. A similar spacing flexibility between the Cys residues in the heme binding site sequence of apocytochrome c substrates has also been observed for the Ccm system I [42–43].
Besides the conserved Cys-Xxx-Xxx-Cys-His heme binding site, mitochondrial cytochromes c have in general conserved Gly1, Lys5, Gly6, Phe10 and Gln16, as well as a positively charged residue at position 13 (Lys or Arg) and an hydrophobic residue at position 9 (Iso or Leu) [44]. Mutation of Gly6 and Phe10 to Ala in the horse cytochrome c or the yeast Cyc1p eliminates CCHL-dependent holocytochrome c production [27–28], underlining their critical role for CCHL activity. In the contrary, mutation of Gln16 in Cyc7p [32] or Gln15 in horse cytochrome c [24] to Cys does not hinder the ability of CCHL to mature the corresponding cytochrome c variants. Similar flexibility for equivalent amino acid residues within the heme binding site has also been observed for Ccm system I and its apocytochromes c substrates [42]. To confirm this plasticity at positions 15 and 16, we constructed a fusion protein (Cyc7pR13-C14CycA) with the amino-terminal part of Cyc7p up to the residue Arg13, and the carboxyl-terminal portion of CycA, including its heme binding site Cys14-Lys15-Thr16-Cys17-His18 (Figure 2A). Indeed, this fusion protein was covalently ligated with heme upon coproduction with CCHL in E. coli (data not shown). The same results were also obtained with a fusion protein containing the amino-terminal portion of Cyc1p and the C-terminal portion of P. denitrificans cytochrome c550 including the latter heme binding sequence (Cys14-Lys15-Ala16-Cys17-His18) [27].
Change of Gly1, Lys5, or Arg13 to Ala in Cyc1p, Lys5 to Ala in horse cytochrome c, and Arg13 to Cys in Cyc7p do not affect production of the respective cytochrome c variants by CCHL [27,32]. When we constructed a fusion protein, K5Cyc7pH18-S19CycA, composed of a truncated amino terminus of Cyc7p from Lys5 to His18 (deletion of the amino acid residues from Ala-9 to Lys4) and the carboxyl terminal portion of CycA (Fig. 2A), we observed that it was ligated with heme upon coproduction with CCHL in E. coli (Fig. 2B and 2C, lanes 3). Therefore, our data confirm that fusion proteins containing the amino-terminal portion of mitochondrial cytochromes c (starting with Lys5 up to the heme binding site Cys14-Xxx-Xxx-Cys17-His18) and the carboxyl-terminal portion of prokaryotic cytochromes c can be efficient CCHL substrates. Overall, these observations suggest that the amino acid sequence and/or structural determinants required for heme ligation by CCHL reside between the positions 5 to 18 of the mitochondrial cytochromes c.
3.2 Mutational analysis of the positions necessary for holocytochrome c formation by CCHL
Besides the conserved amino acid residues described above, the sequence alignment shown in Fig. 1 highlights that all mitochondrial cytochromes c have a stretch of three amino acid residues between Phe10 and Cys14. In contrast, the non-CCHL substrates P. denitrificans cytochrome c550 and R. capsulatus cytochrome c2 (CycA) have at position 9 a negatively charged Glu instead of a hydrophobic residue, and only two amino acid residues between the conserved Phe10 and the first Cys at the heme binding site.
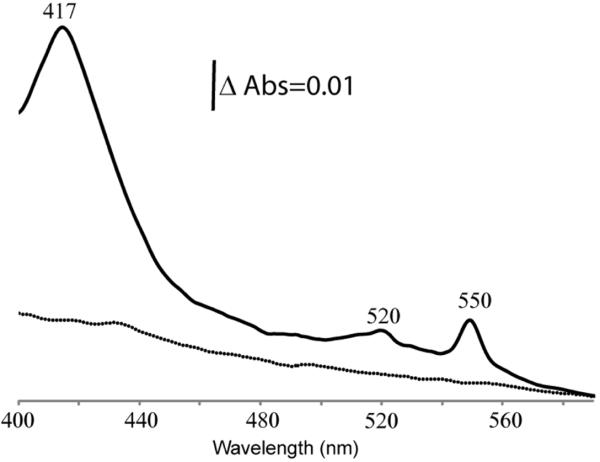
In order to assess the role(s) of these residues on heme ligation by CCHL, we constructed four mutants using the non-CCHL substrate CycA (Fig. 2A). We tested whether any of the mutant proteins is produced (immunodetection of fused Strep-tag epitope, Fig. 2C), and covalently attached with heme (chemiluminescence based detection of covalently attached heme in the presence of SDS, Fig. 2B) by a functional His-tagged S. cerevisiae CCHL in the cytoplasm of E. coli. First, the Glu residue at position 9 of CycA was replaced by the hydrophobic amino acid Leu, as in Cyc1p and Cyc7p (Fig. 1), originating the CycA-E9L variant. This variant was not matured upon coproduction with CCHL in E. coli, like the wild-type CycA (Fig. 2A, as well as Fig. 2B and 2C, lanes 4 and 8). Next, a Lys residue was inserted at position 11, producing the variant CycA-insK11 with a stretch of three amino acids between the conserved Phe10 and Cys14 as in mitochondrial cytochromes c, e.g., Cyc1p and Cyc7p. In the presence of CCHL, this variant yielded holocytochrome c although at a low efficiency (Fig. 2A, as well as Fig. 2B and 2C, lanes 5). Finally, two additional derivatives of CycA-insK11 were constructed to further probe the efficiency of CycA as a substrate for CCHL. In one construct, Asn12 was replaced by a Thr (as in Cyc1p and Cyc7p; other mitochondrial cytochromes c contain a Gln or Met at this position) to yield CycA-insK11+N12T (Fig. 2A). In the other construct, Glu9 was substituted with a Leu (like CycA-E9L) yielding CycA-insK11+E9L (Fig. 2A). In the presence of CCHL, the holocytochrome c form of CycA-insK11+N12T decreased to a quasi-undetectable level (Fig. 2B and Fig. 2C, lanes 7), but remarkably, with CycA-insK11+E9L the amount of holocytochrome c increased dramatically to that seen with Cyc7p, or with the fusion proteins (Fig. 2B and Fig. 2C, lanes 1, 2, 3, and 6). Covalent heme attachment by CCHL to CycA-insK11+E9L was further confirmed by visible spectroscopy using CycA as a control (Fig. 3). The spectrum of the dithionite-reduced total protein extract revealed a Soret band centered at 417 nm, as well as β- and α-bands at 520 and 550nm, respectively, typical for holocytochromes c. A α-band located at 550 nm is indicative of the successful formation of two thioether bonds between the vinyl groups of the heme tetrapyrrole ring and the two thiol groups of the Cys residues at the Cys-Lys-Thr-Cys-His heme binding site of R. capsulatus cytochrome c2.
Figure 3.
Visible spectra of the dithionite-reduced protein extracts (3.2 mg/mL) from E. coli coproducing S. cerevisiae CCHL with R. capsulatus CycA (dotted line) and CycA-insK11+E9L (solid line) variants.
Thus, by inserting a Lys at position 11 and by substituting Glu9 with Leu, we were able to convert for the first time a non-CCHL substrate (i. e., CycA) to a highly proficient CCHL substrate (i. e., CycA-insK11+E9L) in the used heterologous E. coli system. Finally, based on our overall findings, we propose that the first 14 amino acid residues including the conserved heme binding motif at the amino-terminal portions of cytochromes c (Fig. 1) define the consensus sequence “Lys/Ala5-Gly6-Xxx-Xxx-Leu/Ile9-Phe10-Xxx-Xxx-Xxx-Cys14-Xxx-Xxx-Cys17-His18” that is crucial for apoprotein substrate recognition and heme attachment by S. cerevisiae CCHL. Our protein engineering approach provides a new rationale for defining the substrate requirements of various mitochondrial heme lyases that are specific for apocytochromes c or c1, or both, and may help to elucidate the catalytic mechanism(s) of these poorly understood cytochrome c heme lyases.
Highlights.
We test the substrate specificity of Saccharomyces cerevisiae cytochrome c heme lyase (CCHL)
CCHL can only ligate heme b groups to apocytochromes c of eukaryotic origin
The amino-terminal region of eukaryotic apocytochromes c is critical for CCHL activity
We convert a prokaryotic apocytochrome c into an efficient CCHL substrate with two mutations
We define a consensus sequence for holocytochrome c formation by CCHL
Acknowledgements
This work was financed in part by J. S. and C. S. for the construction and the initial in vivo testing of the mutants used, and supported in part by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-FG02-91ER20052 to F.D. for biochemical characterization of the mutants and its recombinant proteins.
Abreviations
- Ccm
cytochrome c maturation
- CCHL
cytochrome c heme lyase
- CC1HL
cytochrome c1 heme lyase
- HCCS
holocytochrome c synthetase
- Cyc1p
Saccharomyces cerevisiae iso-1-cytochrome c
- Cyc7p
Saccharomyces cerevisiae iso-2-cytochrome c
- CycA
Rhodobacter capsulatus cytochrome c2
- IPTG
isopropylthiogalactoside
- PMSF
phenylmethylsulfonyl fluoride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pettigrew GW, Moore GR. Cytochrome c: Biological Aspects. Springer Verlag; Berlin: 1987. [Google Scholar]
- [2].Bowman SE, Bren KL. The chemistry and biochemistry of heme c: functional bases for covalent attachment. Nat Prod Rep. 2008;25:1118–1130. doi: 10.1039/b717196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sanders C, Turkarslan S, Lee DW, Daldal F. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 2010;18:266–274. doi: 10.1016/j.tim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allen JW. Cytochrome c biogenesis in mitochondria - Systems III and V. FEBS J. 2011;278:4198–4216. doi: 10.1111/j.1742-4658.2011.08231.x. [DOI] [PubMed] [Google Scholar]
- [5].Simon J, Hederstedt L. Composition and function of cytochrome c biogenesis System II. FEBS J. 2011;278:4179–4188. doi: 10.1111/j.1742-4658.2011.08374.x. [DOI] [PubMed] [Google Scholar]
- [6].de Vitry C. Cytochrome c maturation system on the negative side of bioenergetic membranes: CCB or System IV. FEBS J. 2011;278:4189–4197. doi: 10.1111/j.1742-4658.2011.08373.x. [DOI] [PubMed] [Google Scholar]
- [7].Bernard DG, Quevillon-Cheruel S, Merchant S, Guiard B, Hamel PP. Cyc2p, a membrane-bound flavoprotein involved in the maturation of mitochondrial c-type cytochromes. J Biol Chem. 2005;280:39852–39859. doi: 10.1074/jbc.M508574200. [DOI] [PubMed] [Google Scholar]
- [8].Giege P, Grienenberger JM, Bonnard G. Cytochrome c biogenesis in mitochondria. Mitochondrion. 2008;8:61–73. doi: 10.1016/j.mito.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [9].Corvest V, Murrey DA, Hirasawa M, Knaff DB, Guiard B, Hamel PP. The flavoprotein Cyc2p, a mitochondrial cytochrome c assembly factor, is a NAD(P)H-dependent haem reductase. Molecular Microbiology. 2012;83:968–980. doi: 10.1111/j.1365-2958.2012.07981.x. [DOI] [PubMed] [Google Scholar]
- [10].Dumont ME, Ernst JF, Hampsey DM, Sherman F. Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. Embo J. 1987;6:235–241. doi: 10.1002/j.1460-2075.1987.tb04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zollner A, Rodel G, Haid A. Molecular cloning and characterization of the Saccharomyces cerevisiae CYT2 gene encoding cytochrome c1-heme lyase. Eur J Biochem. 1992;207:1093–1100. doi: 10.1111/j.1432-1033.1992.tb17146.x. [DOI] [PubMed] [Google Scholar]
- [12].Bernard DG, Gabilly ST, Dujardin G, Merchant S, Hamel PP. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J Biol Chem. 2003;278:49732–49742. doi: 10.1074/jbc.M308881200. [DOI] [PubMed] [Google Scholar]
- [13].Schwarz QP, Cox TC. Complementation of a yeast CYC3 deficiency identifies an X-linked mammalian activator of apocytochrome c. Genomics. 2002;79:51–57. doi: 10.1006/geno.2001.6677. [DOI] [PubMed] [Google Scholar]
- [14].Steiner H, Kispal G, Zollner A, Haid A, Neupert W, Lill R. Heme binding to a conserved Cys-Pro-Val motif is crucial for the catalytic function of mitochondrial heme lyases. J Biol Chem. 1996;271:32605–32611. doi: 10.1074/jbc.271.51.32605. [DOI] [PubMed] [Google Scholar]
- [15].Zhang L, Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 1995;14:313–320. doi: 10.1002/j.1460-2075.1995.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moore RL, Stevens JM, Ferguson SJ. Mitochondrial cytochrome c synthase: CP motifs are not necessary for heme attachment to apocytochrome c. FEBS Lett. 2011;585:3415–3419. doi: 10.1016/j.febslet.2011.08.042. [DOI] [PubMed] [Google Scholar]
- [17].Jeng WY, Chen CY, Chang HC, Chuang WJ. Expression and characterization of recombinant human cytochrome c in E. coli. J Bioenerg Biomembr. 2002;34:423–431. doi: 10.1023/a:1022561924392. [DOI] [PubMed] [Google Scholar]
- [18].Olteanu A, Patel CN, Dedmon MM, Kennedy S, Linhoff MW, Minder CM, Potts PR, Deshmukh M, Pielak GJ. Stability and apoptotic activity of recombinant human cytochrome c. Biochem Biophys Res Commun. 2003;312:733–740. doi: 10.1016/j.bbrc.2003.10.182. [DOI] [PubMed] [Google Scholar]
- [19].Liu Z, Lin H, Ye S, Liu QY, Meng Z, Zhang CM, Xia Y, Margoliash E, Rao Z, Liu XJ. Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis. Proc Natl Acad Sci U S A. 2006;103:8965–8970. doi: 10.1073/pnas.0603327103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rawson PD, Burton RS. Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc Natl Acad Sci U S A. 2002;99:12955–12958. doi: 10.1073/pnas.202335899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dolgikh DA, Latypov RF, Abdullaev Z, Kolov V, Roder H, Kirpichnikov MP. Expression of mutant horse cytochrome c genes in Escherichia coli. Bioorg Khim. 1998;24:756–759. [PubMed] [Google Scholar]
- [22].Patel CN, Lind MC, Pielak GJ. Characterization of horse cytochrome c expressed in Escherichia coli. Protein Expr Purif. 2001;22:220–224. doi: 10.1006/prep.2001.1438. [DOI] [PubMed] [Google Scholar]
- [23].Rumbley JN, Hoang L, Englander SW. Recombinant equine cytochrome c in Escherichia coli: high-level expression, characterization, and folding and assembly mutants. Biochemistry. 2002;41:13894–13901. doi: 10.1021/bi026543y. [DOI] [PubMed] [Google Scholar]
- [24].Tenger K, Khoroshyy P, Kovacs KL, Zimanyi L, Rakhely G. Improved system for heterologous expression of cytochrome c mutants in Escherichia coli. Acta Biol Hung. 2007;58:23–35. doi: 10.1556/ABiol.58.2007.Suppl.3. [DOI] [PubMed] [Google Scholar]
- [25].Yu T, Wang X, Purring-Koch C, Wei Y, McLendon GL. A mutational epitope for cytochrome c binding to the apoptosis protease activation factor-1. J Biol Chem. 2001;276:13034–13038. doi: 10.1074/jbc.M009773200. [DOI] [PubMed] [Google Scholar]
- [26].Abdullaev Z, Bodrova ME, Chernyak BV, Dolgikh DA, Kluck RM, Pereverzev MO, Arseniev AS, Efremov RG, Kirpichnikov MP, Mokhova EN, Newmeyer DD, Roder H, Skulachev VP. A cytochrome c mutant with high electron transfer and antioxidant activities but devoid of apoptogenic effect. Biochem J. 2002;362:749–754. doi: 10.1042/0264-6021:3620749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stevens JM, Zhang Y, Muthuvel G, Sam KA, Allen JW, Ferguson SJ. The mitochondrial cytochrome c N-terminal region is critical for maturation by holocytochrome c synthase. FEBS Lett. 2011;585:1891–1896. doi: 10.1016/j.febslet.2011.04.058. [DOI] [PubMed] [Google Scholar]
- [28].Kleingardner JG, Bren KL. Comparing substrate specificity between cytochrome c maturation and cytochrome c heme lyase systems for cytochrome c biogenesis. Metallomics. 2011;3:396–403. doi: 10.1039/c0mt00086h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pollock WB, Rosell FI, Twitchett MB, Dumont ME, Mauk AG. Bacterial expression of a mitochondrial cytochrome c. Trimethylation of lys72 in yeast iso-1-cytochrome c and the alkaline conformational transition. Biochemistry. 1998;37:6124–6131. doi: 10.1021/bi972188d. [DOI] [PubMed] [Google Scholar]
- [30].Sanders C, Lill H. Expression of prokaryotic and eukaryotic cytochromes c in Escherichia coli. Biochim Biophys Acta. 2000;1459:131–138. doi: 10.1016/s0005-2728(00)00122-5. [DOI] [PubMed] [Google Scholar]
- [31].Rosell FI, Mauk AG. Spectroscopic properties of a mitochondrial cytochrome c with a single thioether bond to the heme prosthetic group. Biochemistry. 2002;41:7811–7818. doi: 10.1021/bi016060e. [DOI] [PubMed] [Google Scholar]
- [32].Pardo-Yissar V, Katz E, Willner I, Kotlyar AB, Sanders C, Lill H. Faraday Discuss. 2000. Biomaterial engineered electrodes for bioelectronics; pp. 119–134. [DOI] [PubMed] [Google Scholar]
- [33].Sanders C, Wethkamp N, Lill H. Transport of cytochrome c derivatives by the bacterial Tat protein translocation system. Mol Microbiol. 2001;41:241–246. doi: 10.1046/j.1365-2958.2001.02514.x. [DOI] [PubMed] [Google Scholar]
- [34].Richard-Fogal CL, San Francisco B, Frawley ER, Kranz RG. Thiol redox requirements and substrate specificities of recombinant cytochrome c assembly systems II and III. Biochim Biophys Acta. 2011;1817:911–919. doi: 10.1016/j.bbabio.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory manual. Cold Spring harbor Laboratory Press; NY: 1989. [Google Scholar]
- [36].Sanders C, Turkarslan S, Lee DW, Onder O, Kranz RG, Daldal F. The cytochrome c maturation components CcmF, CcmH, and CcmI form a membrane-integral multisubunit heme ligation complex. J Biol Chem. 2008;283:29715–29722. doi: 10.1074/jbc.M805413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thony-Meyer L, Fischer F, Kunzler P, Ritz D, Hennecke H. Escherichia coli genes required for cytochrome c maturation. J Bacteriol. 1995;177:4321–4326. doi: 10.1128/jb.177.15.4321-4326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [39].Feissner R, Xiang Y, Kranz RG. Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes. Anal Biochem. 2003;315:90–94. doi: 10.1016/s0003-2697(02)00658-9. [DOI] [PubMed] [Google Scholar]
- [40].Bertini I, Cavallaro G, Rosato A. Cytochrome c: occurrence and functions. Chem Rev. 2006;106:90–115. doi: 10.1021/cr050241v. [DOI] [PubMed] [Google Scholar]
- [41].Goddard AD, Stevens JM, Rondelet A, Nomerotskaia E, Allen JW, Ferguson SJ. Comparing the substrate specificities of cytochrome c biogenesis Systems I and II: bioenergetics. FEBS J. 2010;277:726–737. doi: 10.1111/j.1742-4658.2009.07517.x. [DOI] [PubMed] [Google Scholar]
- [42].Allen JW, Sawyer EB, Ginger ML, Barker PD, Ferguson SJ. Variant c-type cytochromes as probes of the substrate specificity of the E. coli cytochrome c maturation (Ccm) apparatus. Biochem J. 2009;419:177–184. doi: 10.1042/BJ20081999. [DOI] [PubMed] [Google Scholar]
- [43].Rios-Velazquez C, Cox RL, Donohue TJ. Characterization of Rhodobacter sphaeroides cytochrome c2 proteins with altered heme attachment sites. Arch Biochem Biophys. 2001;389:234–244. doi: 10.1006/abbi.2001.2330. [DOI] [PubMed] [Google Scholar]
- [44].Banci L, Bertini I, Rosato A, Varani G. Mitochondrial cytochromes c: a comparative analysis. J Biol Inorg Chem. 1999;4:824–837. doi: 10.1007/s007750050356. [DOI] [PubMed] [Google Scholar]
- [45].Verissimo AF, Yang H, Wu X, Sanders C, Daldal F. CcmI Subunit of CcmFHI Heme Ligation Complex Functions as an Apocytochrome c Chaperone during c-Type Cytochrome Maturation. J Biol Chem. 2011;286:40452–40463. doi: 10.1074/jbc.M111.277764. [DOI] [PMC free article] [PubMed] [Google Scholar]