Abstract
Background
Papillary thyroid carcinoma (PTC), the most common type of thyroid malignancy, usually possesses mutations, either RET/PTC rearrangement or BRAF mutation. Both mutations can activate the mitogen-activated protein kinase kinase/extracellular signal–related kinase signaling transduction pathway, which results in activation of transcription factors that regulate cellular proliferation, differentiation, and apoptosis.
Objective
To test the effects of CI-1040 (PD184352), a specific MEK1/2 inhibitor, on PTC cells carrying either an RET/PTC1 rearrangement or a BRAF mutation.
Design
The effects of CI-1040 on PTC cells were evaluated in vitro and in vivo.
Main Outcome Measures
The effects of CI-1040 on PTC cells were evaluated in vitro using a cell proliferation assay, cell cycle analysis, and immunoblotting. The antitumor effects of CI-1040 in vivo were evaluated in an orthotopic mouse model.
Results
The concentrations of CI-1040 needed to inhibit 50% cell growth were 0.052μM for PTC cells with a BRAF mutation and 1.1μM for PTC cells with the RET/PTC1 rearrangement. After 3 weeks of oral administration of CI-1040 (300 mg/kg/d) to mice with orthotopic tumor implants of PTC cells, the mean tumor volume of implants bearing the RET/PTC1 rearrangement (n=5) was reduced 47.5% compared with untreated mice (from 701.9 to 368.5 mm3), and the mean volume of implants with a BRAF mutation (n=8) was reduced 31.3% (from 297.3 to 204.2 mm3).
Conclusions
CI-1040 inhibits PTC cell growth in vitro and in vivo. Because RET/PTC rearrangements are unique to thyroid carcinomas and a high percentage of PTCs possess either mutation, these findings support the clinical evaluation of CI-1040 for patients with PTC.
Papillary thyroid carcinomas (PTCs) are the most common type of thyroid malignancy. BRAF mutations account for only 8% of mutations in all human cancers, but the percentage is much higher in PTC.1–4 The incidence of BRAF mutations ranges from 29% to 83% depending on the cohort being studied.1 The most common type of BRAF mutation in cancer is a T → A substitution at nucleotide 1799 that results in conversion of valine to glutamic acid at codon 600 (V600E) of the BRAF protein on exon 15.2–4 The negative charge introduced by glutamic acid at position 600 mimics the effect of phosphorylation at an adjacent site when BRAF is activated and results in constitutive activation of BRAF.3 The high rate of BRAF mutation in PTC makes PTC an ideal system for testing BRAF inhibitors. Other mutations commonly found in PTC are the RET/PTC rearrangements (11 different RET/PTC rearrangements have been reported; the RET/PTC1, RET/PTC2, and RET/PTC3 rearrangements have been studied most).5,6 The incidence of RET/PTC rearrangements in PTC ranges from 2.5% to 67.0% depending on the cohort being studied. The RET/PTC rearrangements result in constitutive activation of RET tyrosine kinase. Either mutation (RET/PTC rearrangement or BRAF mutation) can activate the mitogen-activated protein kinase kinase (MEK1/2 or MAPKK). The MEK1/2 then activates the MAPK (extracellular signal–related kinase 1/2 [ERK1/2]) signaling transduction pathway, resulting in activation of a variety of transcription factors that regulate cellular proliferation, differentiation, and apoptosis.2–4
Several specific MEK1/2 inhibitors have been developed in the past few years. The first commercially available MEK1/2 inhibitors were PD98059 and U0126. We previously tested both inhibitors in PTC cells and found that both could inhibit PTC cell proliferation and decrease the phosphorylation of ERK1/2 (p-ERK1/2).7 Despite the effects of these inhibitors on PTC cells, PD98059 and U0126 were used for in vitro study only owing to the poor solubility of PD98059 and the inactivity of U0126 in vivo.8
CI-1040 (PD184352) (Pfizer Global Research and Development, Ann Arbor, Michigan) is a small-molecule inhibitor with specific activity against MEK1/2. This orally active inhibitor has been tested in other cancers, including pancreatic, colon, breast, and non–small-cell lung cancers and was well tolerated by patients in phase 2 trials.9 In this study, we tested the effects of CI-1040 in PTC cell lines that possessed either a BRAF mutation or an RET/PTC1 rearrangement, both of which constitutively activate the BRAF-MEK1/2-ERK1/2 pathway. We found that CI-1040 inhibited PTC cell growth in vitro and in vivo at serologically achievable doses.
METHODS
CELL CULTURE
The PTC cell line carrying the RET/PTC1 rearrangement BHP2-7 (a subclone of TPC-1) was used in this study.10,11 The cells were maintained in RPMI1640 (Mediatech Inc, Herndon, Virginia) containing 10% fetal bovine serum (Hyclone, Logan, Utah), non-essential amino acid mixture (Cambrex BioScience, Walkers, Maryland), 1mM sodium pyruvate (Cambrex BioScience), and 2mM L-glutamine in a 37°C incubator supplied with 95% air and 5% carbon dioxide. A BRAF-mutated PTC cell line (K2) was maintained in Dulbecco Modified Eagle Medium: Nutrient Mixture F-12 (Mediatech Inc) containing 10% fetal bovine serum and 2mM L-glutamine.
REAGENTS
CI-1040 was dissolved in dimethyl sulfoxide (DMSO) as a 10mM stock solution and was stored at −20°C for in vitro study. For in vivo experiments, CI-1040 was dissolved in Cremophor EL–95% ethanol (50:50) (Sigma-Aldrich Corp, St Louis, Missouri) and was diluted with water before use.
CELL PROLIFERATION ASSAY
BHP2-7 and K2 cells (104) were plated in 24-well plates (Costar, Cambridge, Massachusetts) in triplicate for 4 days in a 37°C incubator. CI-1040 was added to the cells on day 0 or on days 0 and 2. For the MTT assay, 0.2 mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dissolved in 0.8% sodium chloride solution at 5 mg/mL was added to each well. After incubating the cells at 37°C for 3 hours, the liquid was aspirated from the wells and discarded. Stained cells were dissolved in 0.5 mL of DMSO, and their absorption at a wavelength of 570 nm (OD570) was ascertained using a multidetection microplate reader (Synergy HT; BioTek Instruments, Winooski, Vermont). For the concentration of CI-1040 needed to inhibit 50% cell growth (GI50), the MTT assay was performed on day 3. For cell growth curves, the MTT assay was performed every day on days 0 to 4. Cell growth was calculated as 100 × (T−T0)/(C−T0), where T is the OD570 reading after treating the cells with CI-1040 for 72 hours, T0 is the OD570 reading at day 0, and C is the OD570 reading of cells treated with DMSO only after 72 hours. The GI50 was determined using Prism 3.0 (GraphPad Software, San Diego, California) based on the cell growth at each CI-1040 concentration.
CELL CYCLE ANALYSIS
BHP2-7 and K2 cells (2×106) were plated the day before treatment in a 10-cm dish. On the day of treatment, CI-1040 was added to the cells for 24 hours at 37°C. Cells were treated with trypsin and collected, washed once in 0.8% sodium chloride solution, fixed in 5 mL of ethanol, and stored at 4°C overnight. Propidium iodide (50 μg/mL, 1 mL) containing DNase-free RNase (3.3 μg/mL) (Roche Applied Science, Indianapolis, Indiana) was added to the fixed cells, which were then incubated at 37°C for 30 minutes. Cell cycles were determined using a flow cytometer (FACSCalibur; BD Biosciences, San Jose, California).
WESTERN BLOT ANALYSIS
Protein extracts from PTC cells were prepared in lysis buffer containing 20mM Tris hydrochloride (pH 7.4), 1% Triton X-100, 300mM sodium chloride, 1mM phenylmethylsulfonyl fluoride, 50mM sodium fluoride, 1mM sodium vanadate, and 1×proteinase inhibitor cocktail III (Calbiochem, San Diego). Total protein concentrations were estimated using the Bradford assay (Bio-Rad, Hercules, California), with bovine serum albumin as a standard. For Western blot analysis, proteins were resolved on 10% or 4- to 20% gradient sodium dodecyl sulfate–polyacrylamide gels using an electrophoresis system (Mini-Protean II; Bio-Rad). The proteins were then transferred to nitrocellulose membranes (Hybond ECL; GE Healthcare Bioscience, Piscataway, New Jersey) using a mini-transblot electrophoretic transfer cell (Bio-Rad) at 80 V for 1 hour at room temperature. After transfer, the membranes were blocked and probed with antibodies at 4°C overnight as indicated by the manufacturer. Antibodies for p-ERK1/2, total ERK1/2, poly (ADP-ribose) polymerase (PARP), caspase-9, caspase-3, and survivin (Cell Signaling Technology, Danvers, Massachusetts) were used at a dilution of 1:1000; a monoclonal antibody against actin (Sigma-Aldrich Corp) was used at a dilution of 1:3000. The cyclin D1 antibody (Santa Cruz Biotechnology Inc, Santa Cruz, California) was used at a dilution of 1:400 and was incubated with membrane for 2 hours at room temperature.
TUNEL ASSAY
The PTC cells were treated with 2μM CI-1040 for 3 or 4 days and were trypsinized to single cells. Cisplatin, 1 mg/mL (Teva Parenteral Medicines Inc, Irvine, California), was used as a positive control for apoptosis. The TUNEL (terminal deoxynucleotide transferase–mediated dUTP nick-end labeling) assay was performed using a fluorometric TUNEL system (DeadEnd; Promega Corp, Madison, Wisconsin) according to the manufacturer’s protocol. Apoptotic cells were analyzed using a FACSCalibur flow cytometer.
TUMOR GROWTH IN ATHYMIC MICE USING AN ORTHOTOPIC MODEL
Athymic Ncr-nu/nu mice, aged 8 to 12 weeks, were obtained from the National Cancer Institute (Frederick, Maryland) and were housed for at least a week after arrival. All experimental procedures and care for mice were performed in accordance with the Institutional Animal Care and Use Committee and the Department of Veterinary Medicine at The University of Texas M. D. Anderson Cancer Center.
The procedures for an orthotopic thyroid carcinoma model in mice have been described elsewhere.12 The PTC cells (1×106 cells in 0.8% sodium chloride solution) were injected into the thyroid glands of 8 to 10 mice per group, and mice were monitored daily for tumor growth. Two to 3 weeks after inoculation, mice were given 150 mg/kg of CI-1040 dissolved in Cremophor EL–95% ethanol (50:50) and diluted with water twice daily (300 mg/kg/d) by means of oral gavage for 5 consecutive days each week for 3 weeks. Tumor sizes were measured using calipers at the end of the 3 weeks (ex vivo after harvesting the tissue), and tumor volume (V) was calculated using the following formula: (V=[length × width2]/2). Control mice were given diluted Cremophor EL–95% ethanol (50:50) only.
STATISTICAL ANALYSIS
Statistical analysis was performed using the unpaired t test. P≤.05 was considered to be statistically significant.
RESULTS
GI50 OF CI-1040 IN PTC CELLS
To monitor the effects of CI-1040 on inhibition of PTC cell growth, the GI50 was determined in PTC cells. Serial dilution (1:5) of CI-1040 was prepared, starting at 20μM to 3.2nM. After 3 days of incubation with varying concentrations of CI-1040, MTT assays were performed and cell growth was calculated. The GI50 for PTC cells with a BRAF mutation was 0.052μM; the GI50 for PTC cells with the RET/PTC1 rearrangement was 1.1μM (data not shown).
INHIBITION OF CELL GROWTH BY CI-1040
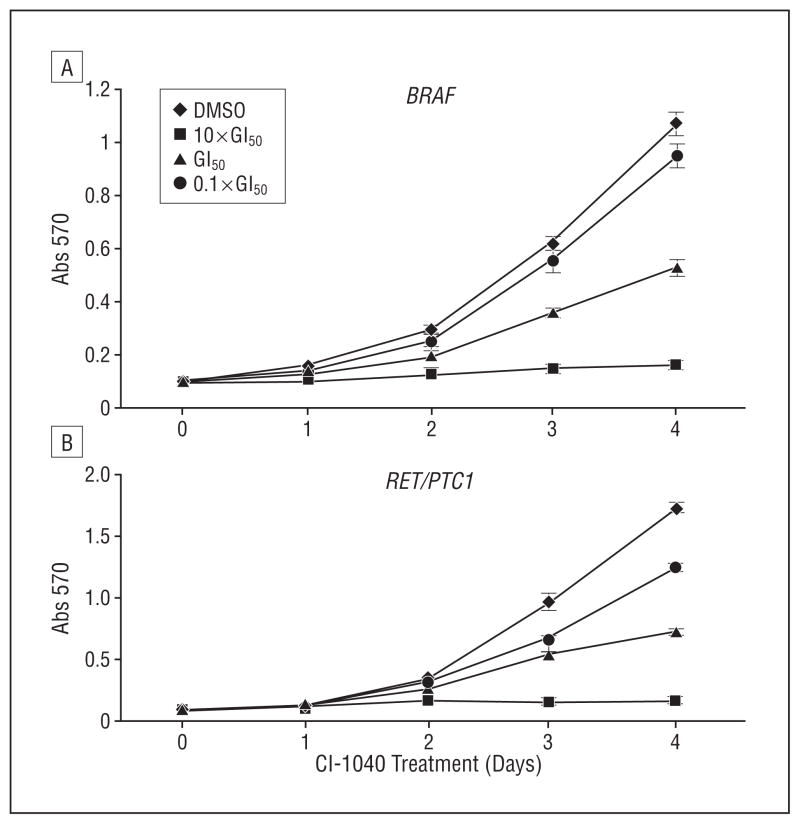
After the GI50 of CI-1040 in PTC cells was determined, PTC cells were divided into 3 groups, and each group was treated with a different concentration of CI-1040: approximately the GI50 (0.05μM for PTC cells with a BRAF mutation and 1μM for PTC cells with the RET/PTC1 rearrangement), 10-fold lower than the GI50 (0.005μM for PTC cells with a BRAF mutation and 0.1μM for PTC cells with the RET/PTC1 re-arrangement), or 10-fold higher than the GI50 (0.5μM for PTC cells with a BRAF mutation and 10μM for PTC cells with the RET/PTC1 rearrangement). Using MTT assays, after 4 days of treatment with CI-1040, PTC cells with a BRAF mutation showed 90% growth inhibition at a 0.5μM dose, 59% inhibition at 0.05μM, and 27% inhibition at 0.005μM compared with cells treated with DMSO only (P<.001 for all) (Figure 1). In PTC cells with the RET/PTC1 rearrangement, CI-1040 inhibited 86% of cell growth at 10μM (P=.001), 51% at 1μM (P<.001), and 11% at 0.1μM (P=.03) compared with cells treated with DMSO only (Figure 1). During 4-day treatment with CI-1040, growth was inhibited in the same percentage of PTC cells regardless of whether CI-1040 was added once (on day 0) or twice (on days 0 and 2) (data not shown). These results suggest that CI-1040 is a stable inhibitor that can inhibit the growth of PTC cells and that PTC cells bearing a BRAF mutation are significantly more sensitive to CI-1040 than their RET/PTC1 counterparts.
Figure 1.
CI-1040 potently inhibits papillary thyroid carcinoma (PTC) cell growth in vitro. The PTC cells were treated with CI-1040 for up to 4 days. CI-1040 was added to the cells on day 0. Each day, the cells were counted according to absorption at 570 nm (Abs 570) after staining with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MTT using a microplate reader. Cells treated with dimethyl sulfoxide (DMSO) only were used as positive controls. The CI-1040 concentrations used were 0.5μM (10×concentration needed to inhibit 50% cell growth [GI50]), 0.05μM (GI50), and 0.005μM (0.1×GI50) for PTC cells with a BRAF mutation (A) and 10μM (10×GI50), 1μM (GI50), and 0.1μM (0.1×GI50) for PTC cells with the RET/PTC1 rearrangement (B).
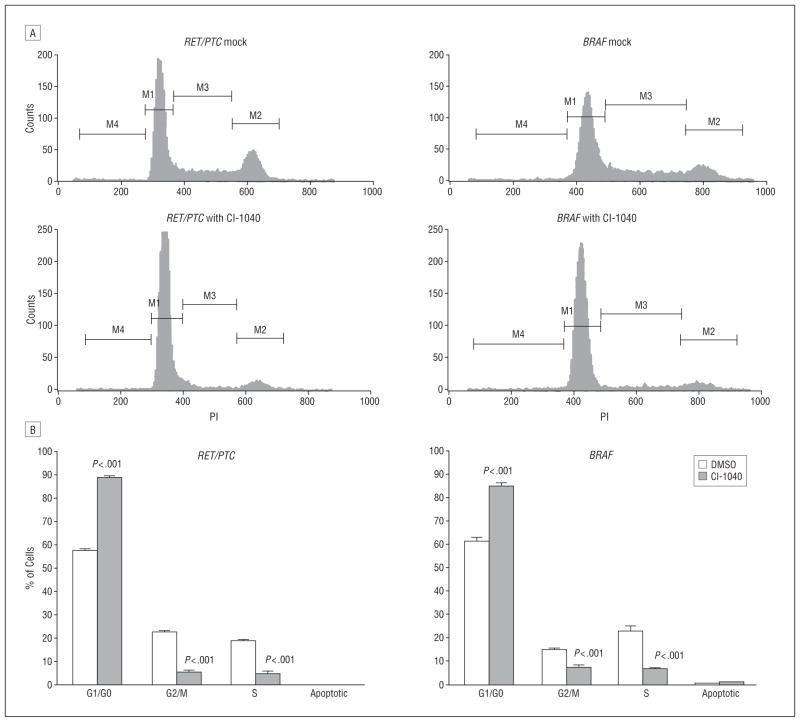
To determine the mechanism of cell growth inhibition in PTC cells after CI-1040 treatment, cell cycle analysis was performed after the PTC cells were treated with 1μM CI-1040 for 24 hours (Figure 2). More than 88% of PTC cells carrying the RET/PTC1 rearrangement were in G1 arrest, whereas 57% of untreated PTC cells were in the G1 phase. After CI-1040 treatment, only 5% of PTC cells were in the G2/M and S phases, whereas 23% of untreated cells were in the G2/M phase and 19% were in the S phase. Similar results were observed in PTC cells with a BRAF mutation. More than 85% of PTC cells with a BRAF mutation were in G1 arrest compared with untreated cells (61% in G1 phase). After CI-1040 treatment, only 7% of PTC cells with a BRAF mutation were in the G2/M phase and 6% were in the S phase, whereas 15% of untreated cells were in the G2/M phase and 23% were in the S phase. No significant change in the apoptotic phase was observed in either type of PTC cells after CI-1040 treatment for 24 hours (Figure 2).
Figure 2.
CI-1040 induces G1 arrest in RET/PTC1–rearranged and BRAF-mutated papillary thyroid carcinoma (PTC) cells. The PTC cells (RET/PTC1 rearranged or BRAF mutated) were treated with 1μM CI-1040 for 24 hours. Cells treated with dimethyl sulfoxide (DMSO) were used as a control. A, Flow cytometry data. M1 is the G1/G0 phase; M2, the G2/M phase; M3, the S phase; and M4, the apoptotic phase. B, The percentage of cells in the G1/G0, G2/M, S, and apoptotic phases after flow cytometry from 3 independent experiments. Error bars represent SD.
DEPHOSPHORYLATION OF ERK1/2 IN PTC CELLS BY CI-1040
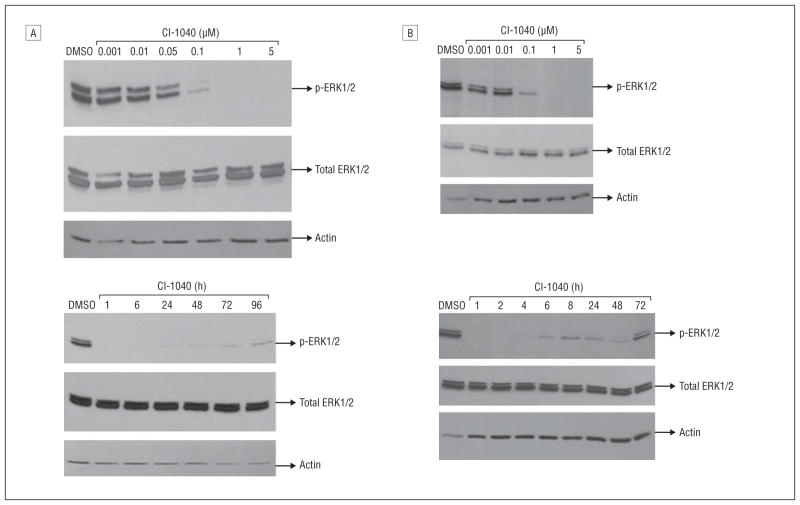
CI-1040 is a specific MEK1/2 inhibitor, and the downstream effectors of these kinases are ERK1/2. The ability of CI-1040 to decrease p-ERK1/2 was examined. The PTC cells were treated with different concentrations of CI-1040 for 1 hour, and the p-ERK1/2 was reduced at 0.1μM and was completely abolished at 1μM for PTC cells with a BRAF mutation (Figure 3A) or the RET/PTC1 rearrangement (Figure 3B). The duration of the dephosphorylation of ERK1/2 in PTC cells with a BRAF mutation lasted up to 96 hours (Figure 3A). For cells with the RET/PTC1 rearrangement, p-ERK1/2 became detectable as early as 6 hours after treatment with CI-1040, and p-ERK1/2 returned to its pretreatment level after 72 hours (Figure 3B). The ability of CI-1040 to decrease the expression of p-ERK1/2 was observed to be the same whether CI-1040 was added to PTC cells once (at day 0) or twice (at days 0 and 2) during the 96- and 72-hour treatments (data not shown). Total ERK1/2 expression remained the same during all treatments. These data suggest that the downstream effects of CI-1040 treatment could effectively result in the dephosphorylation of ERK1/2. The dephosphorylation effect lasted longer in PTC cells with a BRAF mutation than in those with the RET/PTC1 rearrangement.
Figure 3.
The extent and duration of dephosphorylation of extracellular signal–related kinase 1/2 (ERK1/2) after CI-1040 treatment depend on papillary thyroid carcinoma (PTC) genotype. The expression of phosphorylated ERK1/2 (p-ERK1/2) was detected using Western blot analysis. Total ERK1/2 and actin were used as loading controls, and cells treated with dimethyl sulfoxide (DMSO) only were used as positive controls for normal expression of p-ERK1/2. A, Cells with a BRAF mutation were treated with CI-1040 at 0.001μM, 0.01μM, 0.05μM, 0.1μM, 1μM, or 5μM for 1 hour (top) or with 1μM CI-1040 for 1, 6, 24, 48, 72, or 96 hours (bottom). B, Cells with the RET/PTC1 rearrangement were treated with 0.001μM, 0.01μM, 0.1μM, 1μM, or 5μM CI-1040 for 1 hour (top) or with 1μM CI-1040 for 1, 2, 4, 6, 8, 24, 48, or 72 hours (bottom).
CASPASES AND PARP CLEAVAGES DETECTED IN PTC CELLS WITH A BRAF MUTATION AFTER CI-1040 TREATMENT
Survivin and XIAP are antiapoptotic proteins. The expression of these proteins decreases in cells that undergo apoptosis.13,14 The PTC cells were treated with 1μM CI-1040, and the expression of both proteins was analyzed using Western blotting. In PTC cells with either a BRAF mutation or the RET/PTC1 rearrangement, the expression of survivin was lost after treating the cells with CI-1040 for 16 hours (data not shown) and did not recover during observations extending up to 72 hours. No change in the expression of XIAP was observed in PTC cells treated with 1μM CI-1040 for up to 4 days (data not shown).
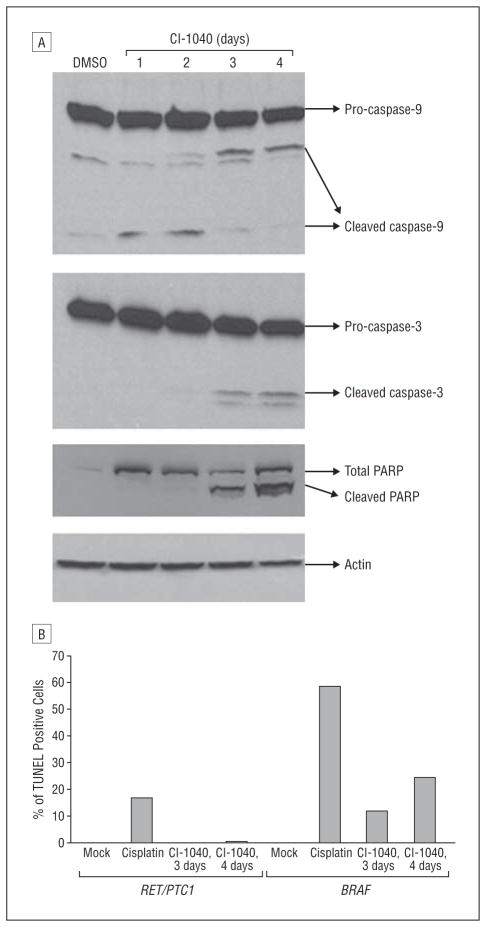
To further investigate the mechanism of cell growth inhibition in PTC cells with a BRAF mutation by CI-1040, we evaluated expression of the caspase-9, caspase-3, and caspase-3 substrate PARP as indicators of apoptosis using Western blot analysis. The PTC cells with a BRAF mutation showed cleaved caspase-9 after 1 day of treatment with CI-1040, cleaved caspase-3 after 2 days of treatment, and cleaved PARP after 2 days of treatment (Figure 4A). The expression of cleaved PARP remained detectable for up to 4 days. In cells with the RET/PTC1 rearrangement, cleaved caspase-3 and cleaved PARP were not detected even after up to 4 days of treatment (data not shown).
Figure 4.
Evidence of apoptosis in papillary thyroid carcinoma (PTC) cells with a BRAF mutation after CI-1040 treatment. A, Detection of cleaved caspases and cleaved poly (ADP-ribose) polymerase (PARP) in cells with a BRAF mutation after CI-1040 treatment. Cells with a BRAF mutation were treated with 1μM CI-1040 for 1 to 4 days. Expression of cleaved caspase-9, caspase-3, and cleaved PARP was detected by means of Western blot analysis. Cells treated with dimethyl sulfoxide (DMSO) only were used as controls. Actin was used as a loading control. B, The PTC cells with a BRAF mutation or the RET/PTC1 rearrangement were treated with 2μM CI-1040 for 3 or 4 days. Cells were harvested, and apoptotic cells were labeled using a fluorometric TUNEL (terminal deoxynucleotide transferase–mediated dUTP nick-end labeling) system. After analyzing cells using flow cytometry, the green cells that appeared in the R2 region (apoptotic cells) were counted, and the percentage of TUNEL-positive cells are shown. Cells treated with DMSO only (Mock) were used as a negative control, and cells treated with 40μM cisplatin for 48 hours were used as a positive control.
In addition to the observed PARP cleavage on Western blots, the effects of CI-1040 on PTC cells were further tested using the TUNEL assay. In BRAF-mutated PTC cells, 11% and 24% of cells were apoptotic after treating with CI-1040 for 3 and 4 days, respectively (Figure 4B). In RET/PTC1–rearranged PTC cells, less than 1% of cells were apoptotic after treating with CI-1040 for up to 4 days. These data confirm the results observed on Western blots that BRAF-mutated PTC cells undergo apoptosis after CI-1040 treatment, whereas PTC cells with the RET/PTC1 rearrangement do not.
INHIBITION OF TUMOR GROWTH BY CI-1040 IN AN ORTHOTOPIC MOUSE MODEL
The inhibitory effects of CI-1040 were tested in vivo using an orthotopic mouse model. The PTC cells were inoculated in situ into the mouse thyroid. After 2 to 3 weeks, CI-1040 was given to these mice (see the “Methods” section) for 3 weeks. At the end of the 3 weeks, tumor sizes were measured and tumor volume was calculated (Table). After CI-1040 treatment, the mean tumor volume was smaller in mice with tumor from PTC cells carrying a BRAF mutation than in untreated (vehicle) mice (204.2 mm3 compared with 297.3 mm3, 31.3% reduction, P<.001). The mean tumor volume was also smaller in mice with tumor from PTC cells carrying the RET/PTC1 rearrangement than in untreated (vehicle) mice (368.5 mm3 compared with 701.9 mm3, 47.5% reduction, P=.004). No toxic effects were observed in any mice when they were treated with CI-1040. These data suggest that CI-1040 inhibits tumor growth in vivo in PTC cells having either the RET/PTC1 rearrangement or a BRAF mutation.
Table.
Inhibitory Effects of CI-1040 on Tumor Growth in an Orthotopic Mouse Model
| Mutation | Treatment | Mice, No. | Tumor Volume, Mean (95% CI), mm3 | P Value |
|---|---|---|---|---|
| BRAF | Vehicle | 8 | 297.3 (237.1–357.5) | |
| CI-1040 | 8 | 204.2 (143.9–264.5) | <.001 | |
| RET/PTC1 | Vehicle | 4 | 701.9 (590.7–813.7) | |
| CI-1040 | 5 | 368.5 (256.7–480.3) | .004 |
Abbreviation: CI, confidence interval.
COMMENT
We examined the effects of the MEK1/2 inhibitor CI-1040 in PTC cells. We found that CI-1040 effectively inhibited PTC cell growth in vitro and in vivo. Previous studies15 have demonstrated that CI-1040 is a specific inhibitor of MEK1/2. After CI-1040 treatment in a colon cancer cell line, only the expression of p-ERK1/2 was affected, and no change in the expression of p-JNK, pP38, and pAKT was detected by means of Western blot analysis.15 In the present study, PTC cells with a BRAF mutation were sensitive to CI-1040 treatment in vitro, as the GI50 in these cells is only 0.052μM, which is in close agreement with the half-maximal inhibitory concentration of CI-1040 to the enzymatic activity of pure MEK1 at 0.017μM. In contrast, at 1.1μM, the GI50 of PTC cells with the RET/PTC1 rearrangement was much higher.
At the molecular level, the expression of p-ERK1/2 was suppressed in both types of PTC cells after treatment with 1μM CI-1040 for 1 hour. The rapid dephosphorylation of ERK1/2 has been reported in other types of cancer, including the breast cancer cell line MDA-MB-231,16 malignant schwannoma cell lines,17 and colon cancer cell lines.15 The duration of the dephosphorylation of ERK1/2 by CI-1040 was different in different types of PTC cells. Dephosphorylation of ERK1/2 by CI-1040 lasted longer in PTC cells with a BRAF mutation (>96 hours) than in PTC cells with the RET/PTC1 rearrangement (approximately 6 hours). The regulation of p-ERK1/2 in these cells could explain this difference. In PTC cells with a BRAF mutation, the only known substrates of BRAF are MEK1/2, which activate ERK1/2. However, in PTC cells with the RET/PTC1 rearrangement, the resulting constitutive activated RET kinase is regulated by other signal transduction pathways (JNK, PI3K, or PLCγ) in addition to the RAF-MEK1/2-ERK1/2 pathway.6 Studies have shown that the expression of p-MEK1/2 is higher in PTC cells with a BRAF mutation than in PTC cells with the RET/PTC1 rearrangement.18 Thus, the ability of CI-1040 to dephosphorylate ERK1/2 in the presence of the BRAF mutation is a strong and dramatic one.
We also observed that CI-1040 was a stable in vitro inhibitor of PTC cell proliferation and suppressed p-ERK1/2 expression in PTC cells. After the dephosphorylation of ERK1/2, both types of PTC cells showed suppression of survivin 16 hours after CI-1040 treatment and G1 arrest at 24 hours. The reduction in survivin expression and G1 arrest have been observed in other types of cancer cells, including colon cancer cells,15 ovarian cancer cells,19 and malignant schwannoma cells.17 Other researchers19 have reported that G1 arrest induced by CI-1040 is only observed in cells with a BRAF mutation or a KRAS mutation and is not seen in cells carrying the wild-type BRAF. In the present study, we observed G1 arrest in PTC cells with the RET/PTC1 rearrangement (with wild-type BRAF) and in those with a BRAF mutation. The events after G1 arrest seemed to be different in PTC cells with a BRAF mutation than in those with the RET/PTC1 rearrangement. As indicated by the detection of cleaved caspase-9, caspase-3, and cleaved PARP (3 or 4 days) and by TUNEL assay (3 or 4 days), CI-1040 induced apoptosis in PTC cells with a BRAF mutation after 24 hours of treatment. In PTC cells with the RET/PTC1 rearrangement, cytostasis was observed, activation of the caspase system was not detected, and no apoptotic cells were detected in TUNEL assay after up to 4 days of CI-1040 treatment. Because there are no available PTC cell lines carrying the RET/PTC2 and RET/PTC3 rearrangements, the effect of CI-1040 on these rearrangements was not tested. Although the mechanisms of the different responses to CI-1040 treatment by different types of PTC cells are unclear, we ruled out the involvement of p53 due to its mutation status in PTC cells. This p53-independent apoptosis induced by CI-1040 has been reported in malignant schwannoma cells by Mattingly et al,17 who showed that CI-1040 induced apoptosis in 3 different cell lines, 1 with a wild-type p53, another with a mutant p53, and the third with no p53 (p53 null).
CI-1040 was previously tested in a phase 2 trial in patients with advanced non–small-cell lung, breast, colon, or pancreatic cancer.9 No antitumor effect by CI-1040 was observed in these patients. A BRAF mutation was reported in 12% of patients with colon cancer1,20 and in less than 10% of patients with breast cancer21; no BRAF mutation was reported in patients with pancreatic cancer.22 These BRAF mutation rates are relatively lower than those reported in PTC (29%–83% depending on the cohort being studied).4 No report of stratifying patients with BRAF mutations was described in the phase 2 trial.9
We tested the effect of CI-1040 in vivo by measuring the tumor volume in an orthotopic mouse model to mimic the tumor microenvironment, as in the case of thyroid carcinomas occurring in patients. We observed moderate growth inhibition by CI-1040 in PTC tumors with either type of mutation (31.3% in BRAF and 47.5% in RET/PTC1). Moderate growth inhibition by CI-1040 was also observed by Liu et al23 using a xenograft mouse model, in which they observed 45% inhibition in KAT10 cells (a BRAF-mutated PTC cell line). At the dose of CI-1040 we were using, CI-1040 did not seem to be toxic to mice; as a result, no toxic effects were observed in mice. Although CI-1040 inhibited PTC cell growth in vitro, it was not as effective in vivo. Several factors may have contributed to this reduced effectiveness, including (1) the bioavailability of CI-1040 (CI-1040 dissolved completely in DMSO for in vitro study but dissolved only partially in Cremophor EL–95% ethanol for in vivo study), (2) the size of the tumor at the start of treatment, and (3) multiple mechanisms in vivo, such as angiogenesis and different growth factors and receptors. When PD184161, an analog of CI-1040, was tested in human liver cancer cell lines, it effectively inhibited tumor growth in mice when there was minimal tumor burden, but no inhibitory effect was observed when tumors were large at the time of treatment.24 Although the antitumor effect of CI-1040 in vivo was only moderate in mice in the present study, it was still statistically significant.
Based on these findings, we conclude that CI-1040 should be considered for testing in patients with PTC because both types of mutations in PTC respond equally to CI-1040 in vivo and because more than 70% of patients with PTC have either BRAF mutation or RET/PTC rearrangement,25 thus making this cancer an excellent system in which to test CI-1040. In addition, CI-1040 and other MEK1/2 inhibitors should be tested either alone or combined with other targeted compounds to further enhance the antitumor activity already observed.
Acknowledgments
Funding/Support: This work was partially supported by Independent Award R01 DE-13954 from the National Institutes of Health, grant 1P50-CA-97007 from the National Institutes of Health Specialized Program of Research Excellence in Head and Neck Cancer, support grant 5P30 CA 16672 from M. D. Anderson Cancer Center, the Michael A. O’Bannon Endowment for Cancer Research, the Betty Berry Cancer Research Fund, the State of Texas Tobacco Settlement Funds, and Cancer Center Support (CORE) grant CA 16672 from the National Cancer Institute for media production.
Footnotes
Financial Disclosure: None reported.
Previous Presentation: This study was presented at the Seventh International Conference on Head and Neck Cancer of the American Head and Neck Society; July 20, 2008; San Francisco, California.
Author Contributions: Dr Clayman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Henderson and Clayman. Acquisition of data: Henderson and Ahn. Analysis and interpretation of data: Henderson and Clayman. Drafting of the manuscript: Henderson, Ahn, and Clayman. Critical revision of the manuscript for important intellectual content: Henderson and Clayman. Obtained funding: Clayman. Administrative, technical, and material support: Henderson and Ahn. Study supervision: Clayman.
Additional Contributions: Judith Sebolt-Leopold, PhD, Pfizer Global Research and Development, provided CI-1040; Jerome Hershman, MD, VA Greater Los Angeles Healthcare System, provided the BHP2-7 PTC cell line; D. Wynford-Thomas, MB, FRCPath, DSc, FMEDSci, Cardiff University, provided the K2 PTC cell line; Wendy Schober-Ditmore conducted the flow cytometry; Diane Hackett and Joseph Munch provided text editing; Chandrani Chattopadhyay, PhD, Abhijit Mazumdar, PhD, Arumugam Jayakumar, PhD, Mitchell Fredrick, PhD, Mary Wang, MS, Katrina Briggs, Paula Holton, MS, and Kelli Cottingham provided technical support; and Sonia Perez provided manuscript preparation.
References
- 1.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5(11):875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 4.Ji H, Wang Z, Perera SA, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67(10):4933–4939. doi: 10.1158/0008-5472.CAN-06-4592. [DOI] [PubMed] [Google Scholar]
- 5.Jhiang SM, Mazzaferri EL. The RET/PTC oncogene in papillary thyroid carcinoma. J Lab Clin Med. 1994;123(3):331–337. [PubMed] [Google Scholar]
- 6.Jhiang SM. The RET proto-oncogene in human cancers. Oncogene. 2000;19(49):5590–5597. doi: 10.1038/sj.onc.1203857. [DOI] [PubMed] [Google Scholar]
- 7.Henderson YC, Fredrick MJ, Clayman GL. Differential responses of human papillary thyroid cancer cell lines carrying the RET/PTC1 rearrangement or a BRAF mutation to MEK1/2 inhibitors. Arch Otolaryngol Head Neck Surg. 2007;133 (8):810–815. doi: 10.1001/archotol.133.8.810. [DOI] [PubMed] [Google Scholar]
- 8.Sebolt-Leopold JS. MEK inhibitors: a therapeutic approach to targeting the Ras-MAP kinase pathway in tumors. Curr Pharm Des. 2004;10(16):1907–1914. doi: 10.2174/1381612043384439. [DOI] [PubMed] [Google Scholar]
- 9.Rinehart J, Adjei AA, LoRusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22(22):4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 10.Ohta K, Pang XP, Berg L, Hershman JM. Growth inhibition of new human thyroid carcinoma cell lines by activation of adenylate cyclase through the β-adrenergic receptor. J Clin Endocrinol Metab. 1997;82(8):2633–2638. doi: 10.1210/jcem.82.8.4136. [DOI] [PubMed] [Google Scholar]
- 11.Ohta K, Pang XP, Berg L, Hershman JM. Antitumor actions of cytokines on new human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1996;81(7):2607–2612. doi: 10.1210/jcem.81.7.8675585. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SH, Henderson Y, Kang Y, Chattopadhyay C, Briggs K, Clayman GL. An orthotopic model of papillary thyroid carcinoma in athymic nude mice. Arch Otolaryngol Head Neck Surg. 2008;134(2):190–197. doi: 10.1001/archoto.2007.36. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Yang J, Li F. Transcriptional and post-transcriptional controls of survivin in cancer cells: novel approaches for cancer treatment. J Exp Clin Cancer Res. 2006;25(3):391–402. [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Ling X. Survivin study: an update of “What is the next wave?”. J Cell Physiol. 2006;208(3):476–486. doi: 10.1002/jcp.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebolt-Leopold JS, Dudley DT, Herrera R, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5(7):810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 16.Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Semin Oncol. 2003;30(5 suppl 16):105–116. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Mattingly RR, Kraniak JM, Dilworth JT, et al. The mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor PD184352 (CI-1040) selectively induces apoptosis in malignant schwannoma cell lines. J Pharmacol Exp Ther. 2006;316(1):456–465. doi: 10.1124/jpet.105.091454. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura N, Carney JA, Jin L, et al. RASSF1A and NORE1A methylation and BRAF V600E mutations in thyroid tumors. Lab Invest. 2005;85(9):1065–1075. doi: 10.1038/labinvest.3700306. [DOI] [PubMed] [Google Scholar]
- 19.Pohl G, Ho CL, Kurman RJ, Bristow R, Wang TL, Shih IM. Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res. 2005;65 (5):1994–2000. doi: 10.1158/0008-5472.CAN-04-3625. [DOI] [PubMed] [Google Scholar]
- 20.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4(12):937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 21.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5(2):195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 22.Perren A, Schmid S, Locher T, et al. BRAF and endocrine tumors: mutations are frequent in papillary thyroid carcinomas, rare in endocrine tumors of the gastrointestinal tract and not detected in other endocrine tumors. Endocr Relat Cancer. 2004;11(4):855–860. doi: 10.1677/erc.1.00841. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Liu Z, Jiang D, Dackiw AP, Xing M. Inhibitory effects of the mitogen-activated protein kinase kinase inhibitor CI-1040 on the proliferation and tumor growth of thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol Metab. 2007;92(12):4686–4695. doi: 10.1210/jc.2007-0097. [DOI] [PubMed] [Google Scholar]
- 24.Klein PJ, Schmidt CM, Wiesenauer CA, et al. The effects of a novel MEK inhibitor PD184161 on MEK-ERK signaling and growth in human liver cancer. Neoplasia. 2006;8(1):1–8. doi: 10.1593/neo.05373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8(1):83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]