Abstract
Objective/Background
Prior work has not addressed sex differences in the incidence of severe postoperative pain episodes. The goal of this study was to examine sex differences in clinical postoperative pain scores across an array of surgical procedures using direct comparisons of numeric rating scale pain scores as well as using the incidence of severe pain events (SPEs).
Design/Setting
Retrospective cohort study of over 300,000 clinical pain score observations recorded from adult patients undergoing nonambulatory surgery at a tertiary care academic medical center over a 1-year period.
Methods/Patients
To test the hypothesis that the number of SPE on postoperative day (POD) 1 differed by sex after controlling for procedure, we calculated Cochran–Mantel–Haenszel statistics of sex by count of SPE, controlling for type of surgery.
Assessment Tools/Outcomes
Pain scores were collected from clinical nursing records where they were documented using the numeric rating scale.
Results
In female patients, 10,989 (25.09%) of 43,806 POD 1 pain scores were considered SPE compared with 10,786 (22.45%) of 48,055 POD 1 pain scores in male patients. This produced an overall odds ratio of 1.16 (99% confidence interval 1.11–1.20) for females vs males to report an SPE for a pain score on POD 1. Estimates of the odds that a given pain observation represents an SPE for female vs male patients after controlling for type of surgery yielded an odds ratio of 1.14 (99% confidence interval, 1.10–1.19).
Conclusion
Female patients experience greater mean pain scores, as well as a higher incidence of SPE, on POD 1 for a variety of surgical procedures.
Keywords: Sex, Gender, Pain, Surgery, Severe Pain Event, Numeric Rating Scale
Introduction
Each year, over 70 million patients undergo surgery in the United States alone. Surveys suggest that over 80% of these patients will experience postoperative pain and that for over 85% of these patients, the pain will be rated as moderate to severe [1]. Prior work in laboratory models of pain testing consistently demonstrate significant sex differences in response to a variety of nociceptive stimuli [2,3]. These findings have been replicated across numerous types of clinical chronic pain conditions, where data overwhelmingly point toward an excess prevalence of pain in females compared with males [4–7]. However, studies in the postoperative pain setting have yielded mixed results regarding sex differences in analgesic consumption and reported pain scores [8–15].
Examinations of averaged measures of pain scores, evaluated through standard regression techniques, may fail to fully evaluate a patient’s pain experience during recovery from surgery. For instance, mean pain scores may not reflect repeated patterns of severe episodic pain followed by temporary pain control via bolus doses of opioids. Prior work has not addressed potential sex differences in the incidence of severe postoperative pain episodes. However, the incidence of severe pain events (SPEs) after surgery is an important feature of postoperative pain characterization given new data associating severe postoperative pain scores with the development of chronic postsurgical pain [16–21]. Analysis of the incidence of SPE, as well as changes in pain during the early recovery period, may offer further insights into the patient postoperative pain experience.
If sex differences exist for severe pain episodes after surgery, acute pain services could use this information to create tailored approaches toward optimal postoperative pain management. The goal of this retrospective cohort study was to examine sex differences in postoperative pain control across an array of surgical procedures using direct comparisons of pain scores and the incidence of SPE. We hypothesized that the incidence of SPE, defined as pain scores greater than or equal to 7 of 10, on postoperative day (POD) 1 would be greater in female compared with male surgical patients.
Methods
The Institutional Review Board at the University of Florida approved this study, and study registration was not required given the retrospective nature of this project.
All data were obtained from the University of Florida’s Integrated Data Repository. Subjects were those adult patients aged 21 and over undergoing nonambulatory surgery at Shands at the University of Florida over a 1-year period beginning in May 2011. Exclusion criteria included obstetric surgery and those patients who received multiple separate surgeries within the study period to avoid contamination of pain scores from time domain interference with preceding or proceeding surgeries.
All pain scores were recorded using the numeric rating scale (NRS) on an 11-point system, ranging from 0 to 10. Pain scores were entered using the EPIC electronic medical record system; this particular implementation provides education on the administration of the NRS query at the point of data entry in order to improve the veracity of collected data. The NRS represents one of the most widely employed pain intensity measurement tools in hospitals in the United States for collection of pain scores in communicative adult patients [22]. To this end, the NRS has been widely used for clinical research involving acute pain outcomes, although there is data to suggest that the NRS may systematically underestimate patient pain states in some health care systems [23–25]. Furthermore, the NRS is a well-validated method for collecting pain intensity measurements within clinical and experimental settings, thus allowing for a common metric across experimental and clinical pain research. Therefore, we elected to employ the NRS so that our results can be clinically translated to other U.S. civilian hospital populations.
Pain scores generally were recorded every 4 hours, per nursing protocol, with a repeat query within 1 hour after administration of analgesic medications for breakthrough pain, and increased numbers of observations for patients in higher acuity patient care settings. When the patient was listed as “asleep” during the charting of pain scores, the pain score was converted to a missing value rather than zero to account for the fact that some patients had received additional sedatives that may have facilitated sleep despite a strong nociceptive load. Missing values were considered as missing at random. All pain scores were recorded with a corresponding data/time stamp, which was converted to a “time in minutes following surgery.” Pain scores were filtered to include only those obtained after the listed end-surgery time through the end of POD 5. General descriptions of postoperative pain here focused on POD 1 because this was the time period with the most frequent pain observations and minimal censuring of data due to hospital discharge. Severe postoperative pain was defined as a numeric rating score between 7 and 10 on a scale ranging from 0 to 10.
Types of surgery were identified using current procedural terminology (CPT) codes. Given the large number of CPT codes, surgeries were grouped into 244 different categories using the Clinical Categorization Software (CCS) for Services and Procedures provided by the Agency for Healthcare Research and Quality (http://www.hcup-us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp). Only those CCS groups with at least 41 subjects per group were included due to prior evidence suggesting that this minimum group size was necessary to detect differences in pain score by sex [3].
Statistical Analyses
To evaluate the general influence of sex on postoperative pain and to place our results within the context of prior work, female vs male pain scores were compared using t tests on a per-procedural basis using the Satterthwaite approximation for degrees of freedom to account for unequal variance between groups. Prior work suggests that parametric methods may be used for analyzing numeric pain scores, given that parametric methods reflect similar power and false positive rates when compared with nonparametric methods for large samples [26,27]. Mean differences between groups were also reported using Satterthwaite confidence intervals (CIs). Data are presented as the mean with 99% CI.
To test the hypothesis that the number of SPE on POD 1 differed by sex after controlling for procedure, we calculated Cochran–Mantel–Haenszel statistics of sex by a count of SPE, controlling for CCS groups. Overall sex differences in the frequency of SPE reported between the end of surgery and the conclusion of POD 5 were calculated for comparison. Additionally, the difference in proportions of POD 1 pain scores considered SPE between females and males was calculated globally and on a per-procedural level via chi-squared testing.
Given the large number of observations, an overall significance level of 0.01 was chosen. To correct for the many procedure-wise comparisons, corrections for multiple comparisons was performed using the method of Holm [28]. The Holm method is similar to that of Bonferroni but uses a step-down process that is less conservative while still maintaining the family-wise error rates of the Bonferroni method [29]. Given the retrospective nature of this study and the prespecified number of included observations, no power analysis was conducted. All analyses were conducted using sas version 9.3 (SAS Institute, Cary, NC, USA).
Results
A total of 349,797 pain observations from 8,332 subjects undergoing 147 different CCS categories of surgery were reviewed. The median number of observations was 38 (interquartile range of 20–60, total range of 1–181). A total of 69 CCS categories, representing 601 patients, and 16,351 pain observations were removed because female and/or male sex groups had less than 41 subjects for a given CCS category. The analyzed dataset included 333,446 pain observations from 7,731 subjects undergoing 78 different CCS categories of surgery.
Patient Demographics
An overview of patient demographics is given in Table 1. The mean age for females was 56.4 years (99% CI 55.7–57.1), and for males, 56.6 years (99% CI 55.9–57.3), a difference that was not statistically significant (P = 0.7). The mean body mass index for females was 29.5 kg (99% CI 29.2–29.9), and for males, 28.5 kg (99% CI 28.2–28.9), with a statistically significant mean difference of 0.99 kg (99% CI 0.5–1.5, P < 0.0001). The mean number of separate CPT codes per surgery was 1.74 (99% CI, 1.69–1.78) for females vs 1.65 (99% CI 1.61–1.70) for males, with a mean difference of 0.08 (99% CI 0.02–0.15, P = 0.0001). The mean Charlson Comorbidity Index for females was 1.04 (99% CI, 0.99–1.09) and for males, 1.18 (99% CI, 1.12–1.23), with a mean difference of 0.14 (99% CI, 0.07–0.21, P < 0.0001), indicating that males had more comorbid conditions than females.
Table 1.
Characteristics of male and female patients
| Characteristic | Female (Number) | Male (Number) | P value |
|---|---|---|---|
| Total patient count | 3,739 | 3,992 | 0.4947 |
| Age group | 0.0829 | ||
| 21–39 | 639 | 677 | |
| 40–64 | 1,794 | 1,891 | |
| 65–84 | 1,197 | 1,340 | |
| 85 or greater | 109 | 84 | |
| BMI category | <0.0001 | ||
| Morbidly obese (BMI ≥ 40) | 366 | 184 | |
| Normal (BMI 19–24) | 820 | 769 | |
| Obese (BMI 30–40) | 937 | 946 | |
| Overweight (BMI 25–29) | 686 | 1,080 | |
| Underweight (BMI < 19) | 532 | 526 | |
| Unknown BMI status | 398 | 487 | |
| Number of CPT codes per surgery | 0.0015 | ||
| 3–5 | 650 | 591 | |
| Greater than 5 | 61 | 47 | |
| Less than 3 | 3,028 | 3,354 | |
| Charlson comorbidity index | 0.0127 | ||
| <3 | 3,305 | 3,430 | |
| 3–6 | 418 | 541 | |
| 7–10 | 6 | 6 | |
| Unknown | 10 | 15 |
BMI = body mass index; CPT = current procedural terminology.
Pain Scores
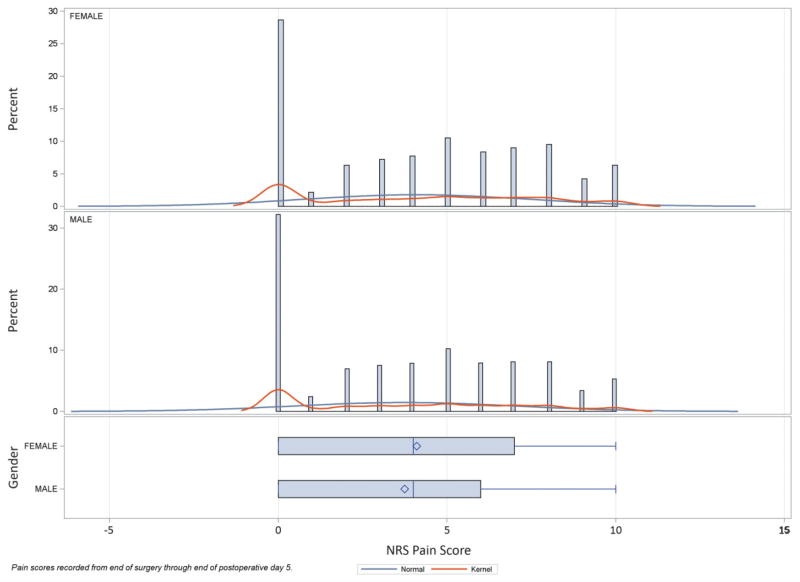
Pain scores recorded between the end of surgery and the end of POD 5 were statistically different between female and male patients (mean difference 0.36, 99% CI 0.33–0.40, P < 0.0001), with a mean score of 4.11 (99% CI 4.08–4.13) for females and 3.74 (99% CI, 3.72–3.76) for males (Figure 1). Given the change in pain scores over time for many patients, this comparison was repeated for pain scores obtained on POD 1. For POD 1 pain scores, there was a small but statistically significant difference according to sex (mean difference 0.22, 99% CI 0.16–0.28, P < 0.0001), with female mean pain scores of 4.20 (99% CI, 4.15–4.24) and male pain scores of 3.98 (99% CI, 3.94–4.02).
Figure 1.
Distribution of pain scores by sex. Pain scores for female and male patients recorded between the end of surgery and the end of postoperative day (POD) 5 are shown. The sample comprised 333,446 pain scores, documented using the numeric rating scale (NRS), from 7,731 subjects undergoing 78 separate Clinical Categorization Software (CCS) categories of surgery. There was a statistically significant difference between female and male patients (mean difference 0.36, 99% confidence interval [CI] 0.33–0.4, P < 0.0001), with a mean score of 4.1 (99% CI 4.1–4.1) for females and 3.74 (99% CI 3.7–3.8) for males.
Table 2 compares the mean pain scores for female and male patients on POD 1 for the CCS categories of surgery. The mean difference between female and male patients, in addition to the 99% CI, are included to demonstrate the small magnitude of difference between female and male patients for a given surgery. These values range in absolute value from 0 to 2.03. There were 15 CCS categories for which the Holm’s correction changed the significance from <0.01 to greater than the cutoff.
Table 2.
Comparison of pain scores by sex on POD 1
| CCS Group | Female
|
Male
|
Mean Difference (99% CI) Female–Male | t Test P Value | Holm’s Corrected P Value | ||
|---|---|---|---|---|---|---|---|
| Number of Observations | Mean Pain Score ± SD | Number of Observations | Mean Pain Score ± SD | ||||
| Amputation of lower extremity | 1,683 | 5.5 ± 3.6 | 1,561 | 5.5 ± 3.2 | 0.03 (−0.64, 0.71) | 0.8954 | 1 |
| Aortic resection, replacement, or anastomosis | 538 | 3.0 ± 3.1 | 378 | 2.6 ± 3.0 | 0.35 (0.01, 0.70) | 0.0085 | 0.3650569 |
| Appendectomy | 125 | 4.8 ± 2.8 | 251 | 5.6 ± 2.8 | −0.82 (−1.52, −0.11) | 0.0028 | 0.1433189 |
| Arthroplasty knee | 286 | 4.1 ± 3.2 | 578 | 4.2 ± 3.0 | −0.09 (−0.34, 0.15) | 0.3303 | 1 |
| Arthroplasty other than hip or knee | 490 | 4.2 ± 3.3 | 1,274 | 3.5 ± 3.1 | 0.68 (0.23, 1.13) | 0.0001 | 0.0057522 |
| Cholecystectomy and common duct exploration | 392 | 4.9 ± 3.4 | 307 | 4.9 ± 3.0 | 0.00 (−0.56, 0.55) | 0.9871 | 1 |
| Colonoscopy and biopsy | 165 | 2.1 ± 3.0 | 233 | 2.1 ± 3.5 | −0.07 (−1.30, 1.15) | 0.8797 | 1 |
| Colorectal resection | 326 | 4.0 ± 3.3 | 339 | 4.2 ± 3.4 | −0.17 (−0.67, 0.34) | 0.4017 | 1 |
| Colostomy, temporary and permanent | 1,334 | 4.4 ± 3.5 | 1,006 | 4.4 ± 3.5 | 0.08 (−1.67, 1.83) | 0.9016 | 1 |
| CABG | 310 | 4.2 ± 3.6 | 387 | 2.9 ± 2.7 | 1.27 (0.74, 1.81) | 0 | 1.04E-07 |
| Creation, revision, and removal of arteriovenous fistula or vessel-to-vessel cannula for dialysis | 282 | 4.8 ± 3.6 | 262 | 3.1 ± 3.4 | 1.70 (0.95, 2.46) | 0 | 7.67E-07 |
| Debridement of wound, infection, or burn | 1,712 | 5.6 ± 3.2 | 2,214 | 5.2 ± 2.9 | 0.43 (0.05, 0.82) | 0.0033 | 0.1599523 |
| Embolectomy and endarterectomy of lower limbs | 569 | 3.6 ± 3.2 | 672 | 4.7 ± 3.4 | −1.09 (−2.14, −0.03) | 0.0079 | 0.3480715 |
| Endarterectomy, vessel of head and neck | 453 | 1.6 ± 2.5 | 421 | 2.1 ± 2.7 | −0.47 (−1.10, 0.16) | 0.0559 | 1 |
| ERCP | 790 | 4.5 ± 3.3 | 852 | 3.1 ± 3.2 | 1.35 (0.67, 2.04) | 0 | 3.14E-05 |
| Endoscopy and endoscopic biopsy of the urinary tract | 367 | 4.3 ± 3.7 | 565 | 3.4 ± 2.9 | 0.88 (−0.52, 2.28) | 0.104 | 1 |
| Excision of skin lesion | 823 | 3.8 ± 2.7 | 606 | 4.4 ± 3.4 | −0.58 (−1.78, 0.62) | 0.2082 | 1 |
| Exploratory laparotomy | 396 | 4.2 ± 3.3 | 611 | 5.1 ± 3.4 | −0.84 (−1.26, −0.43) | 0 | 1.40E-05 |
| Extracorporeal lithotripsy, urinary | 1,246 | 4.8 ± 3.8 | 1,805 | 3.1 ± 3.4 | 1.68 (0.34, 3.02) | 0.0013 | 0.0703855 |
| Gastric bypass and volume reduction | 752 | 3.8 ± 3.0 | 727 | 4.6 ± 3.0 | −0.71 (−1.56, 0.14) | 0.0308 | 1 |
| Gastrostomy, temporary and permanent | 147 | 4.6 ± 3.5 | 78 | 2.6 ± 3.2 | 2.03 (0.16, 3.90) | 0.0054 | 0.2421155 |
| Heart valve procedures | 954 | 3.2 ± 3.0 | 1,078 | 2.7 ± 2.8 | 0.51 (0.24, 0.79) | 0 | 7.06E-05 |
| Hip replacement, total and partial | 301 | 3.9 ± 3.3 | 435 | 4.1 ± 3.0 | −0.24 (−0.49, 0.01) | 0.0142 | 0.5684356 |
| Ileostomy and other enterostomy | 155 | 3.9 ± 3.1 | 95 | 3.7 ± 3.1 | 0.19 (−0.39, 0.78) | 0.3957 | 1 |
| Incision and drainage, skin and subcutaneous tissue | 489 | 5.2 ± 3.3 | 894 | 4.9 ± 3.2 | 0.26 (−0.23, 0.75) | 0.1761 | 1 |
| Incision and excision of CNS | 1,635 | 3.3 ± 3.2 | 1,384 | 3.4 ± 3.2 | −0.11 (−0.37, 0.16) | 0.2953 | 1 |
| Insertion, replacement, or removal of extracranial ventricular shunt | 698 | 4.0 ± 3.4 | 1,481 | 3.2 ± 3.2 | 0.75 (0.27, 1.23) | 0.0001 | 0.0041286 |
| Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator | 192 | 3.6 ± 3.0 | 325 | 1.6 ± 2.8 | 2.00 (1.11, 2.90) | 0 | 1.20E-06 |
| Kidney transplant | 41 | 3.9 ± 3.1 | 50 | 3.9 ± 3.3 | −0.03 (−0.68, 0.62) | 0.9023 | 1 |
| Laminectomy, excision intervertebral disc | 846 | 5.6 ± 3.1 | 1,196 | 4.8 ± 3.2 | 0.78 (0.48, 1.07) | 0 | 2.21E-09 |
| Laparoscopy | 1,105 | 3.5 ± 3.4 | 1,345 | 3.7 ± 2.5 | −0.13 (−0.91, 0.65) | 0.663 | 1 |
| Lobectomy or pneumonectomy | 528 | 3.8 ± 3.2 | 1,040 | 2.8 ± 2.9 | 1.02 (0.39, 1.65) | 0 | 0.0020145 |
| Nephrectomy, partial or complete | 462 | 4.3 ± 3.2 | 686 | 3.5 ± 3.1 | 0.76 (0.32, 1.20) | 0 | 0.0005741 |
| Nephrotomy and nephrostomy | 61 | 4.4 ± 3.3 | 53 | 4.9 ± 2.9 | −0.53 (−1.20, 0.15) | 0.0433 | 1 |
| No procedure | 147 | 3.9 ± 3.1 | 180 | 3.5 ± 3.0 | 0.39 (0.05, 0.73) | 0.0034 | 0.1642764 |
| Other diagnostic nervous system procedures | 1,019 | 2.8 ± 3.1 | 2,461 | 1.6 ± 2.7 | 1.21 (0.30, 2.11) | 0.0006 | 0.0357079 |
| Other diagnostic procedures on lung and bronchus | 174 | 2.9 ± 2.8 | 300 | 3.4 ± 3.1 | −0.50 (−0.95, −0.05) | 0.0042 | 0.193488 |
| Other diagnostic procedures on musculoskeletal system | 345 | 4.2 ± 3.0 | 449 | 3.8 ± 3.0 | 0.38 (−0.32, 1.08) | 0.1568 | 1 |
| Other diagnostic radiology and related technique | 2,353 | 3.0 ± 3.6 | 2,116 | 4.1 ± 3.5 | −1.14 (−3.10, 0.83) | 0.1302 | 1 |
| Other fracture and dislocation procedure | 320 | 4.6 ± 3.2 | 542 | 5.2 ± 3.1 | −0.61 (−1.10, −0.12) | 0.0014 | 0.0752244 |
| Other hernia repair | 2,908 | 5.4 ± 3.0 | 1,686 | 5.2 ± 3.1 | 0.20 (−0.33, 0.73) | 0.3254 | 1 |
| Other non-OR therapeutic cardiovascular procedures | 592 | 2.8 ± 3.3 | 444 | 4.3 ± 3.3 | −1.42 (−2.68, −0.16) | 0.0039 | 0.1812885 |
| Other OR gastrointestinal therapeutic procedures | 108 | 3.9 ± 3.4 | 106 | 3.1 ± 3.3 | 0.80 (0.34, 1.25) | 0 | 0.0004353 |
| Other OR heart procedures | 743 | 2.9 ± 3.1 | 646 | 3.4 ± 2.9 | −0.52 (−1.07, 0.02) | 0.0136 | 0.5588774 |
| Other OR lower GI therapeutic procedures | 80 | 4.1 ± 3.1 | 55 | 4.2 ± 3.1 | −0.03 (−0.69, 0.62) | 0.8926 | 1 |
| Other OR procedures on vessels of head and neck | 239 | 3.6 ± 3.1 | 343 | 3.7 ± 3.2 | −0.18 (−0.69, 0.33) | 0.3635 | 1 |
| Other OR procedures on vessels other than head and neck | 86 | 4.1 ± 3.5 | 77 | 3.4 ± 3.3 | 0.74 (0.18, 1.31) | 0.0007 | 0.037895 |
| Other OR therapeutic nervous system procedures | 82 | 4.5 ± 3.1 | 130 | 4.5 ± 3.1 | −0.02 (−0.35, 0.32) | 0.8978 | 1 |
| Other OR therapeutic procedures of urinary tract | 712 | 4.5 ± 3.4 | 109 | 3.5 ± 3.1 | 1.01 (0.51, 1.51) | 0 | 1.38E-05 |
| Other OR therapeutic procedures on bone | 492 | 5.9 ± 2.9 | 500 | 4.5 ± 3.1 | 1.4 | 0 | 2.61E-08 |
| Other OR therapeutic procedures on joints | 613 | 4.8 ± 3.2 | 740 | 4.3 ± 2.8 | 0.45 (−0.14, 1.05) | 0.0478 | 1 |
| Other OR therapeutic procedures on musculoskeletal system | 2,299 | 4.6 ± 2.5 | 2,042 | 4.8 ± 3.4 | −0.14 (−1.41, 1.13) | 0.7684 | 1 |
| Other OR therapeutic procedures on nose, mouth, and pharynx | 335 | 3.3 ± 3.2 | 378 | 3.7 ± 3.4 | −0.35 (−0.99, 0.29) | 0.16 | 1 |
| Other OR therapeutic procedures on respiratory system | 329 | 2.1 ± 2.8 | 192 | 3.4 ± 3.4 | −1.29 (−1.97, −0.61) | 0 | 7.83E-05 |
| Other OR therapeutic procedures on skin and breast | 386 | 4.5 ± 2.7 | 272 | 5.1 ± 2.7 | −0.65 (−1.50, 0.19) | 0.0463 | 1 |
| Other OR upper GI therapeutic procedures | 351 | 3.8 ± 3.0 | 365 | 2.8 ± 3.0 | 0.99 (0.33, 1.66) | 0.0001 | 0.0078213 |
| Other organ transplantation | 1,050 | 3.3 ± 2.7 | 423 | 3.3 ± 3.1 | −0.03 (−0.67, 0.62) | 0.915 | 1 |
| Other therapeutic ear procedures | 1,359 | 3.9 ± 2.7 | 1,248 | 2.1 ± 2.2 | 1.82 (0.25, 3.39) | 0.0031 | 0.1545763 |
| Other therapeutic endocrine procedures | 41 | 2.4 ± 3.0 | 190 | 2.2 ± 2.9 | 0.24 (−0.30, 0.78) | 0.2567 | 1 |
| Other therapeutic procedures on muscles and tendons | 286 | 4.0 ± 3.0 | 669 | 4.9 ± 3.2 | −0.83 (−1.37, −0.30) | 0.0001 | 0.0041286 |
| Other therapeutic procedures, hemic and lymphatic system | 286 | 4.0 ± 3.6 | 121 | 4.4 ± 3.5 | −0.47 (−1.71, 0.77) | 0.325 | 1 |
| Other vascular bypass and shunt, not heart | 694 | 4.7 ± 2.7 | 818 | 4.3 ± 3.2 | 0.38 (−0.47, 1.22) | 0.2465 | 1 |
| Other vascular catheterization, not heart | 442 | 4.3 ± 3.3 | 200 | 2.8 ± 3.2 | 1.52 (0.74, 2.31) | 0 | 5.20E-05 |
| Partial excision bone | 41 | 4.7 ± 3.4 | 67 | 3.2 ± 3.3 | 1.53 (0.91, 2.15) | 0 | 2.31E-08 |
| Peripheral vascular bypass | 457 | 4.1 ± 3.3 | 529 | 4.4 ± 3.6 | −0.36 (−1.08, 0.36) | 0.1973 | 1 |
| Skin graft | 242 | 5.7 ± 3.1 | 487 | 5.3 ± 3.1 | 0.46 (0.07, 0.86) | 0.0027 | 0.1394749 |
| Small bowel resection | 540 | 4.0 ± 3.1 | 376 | 4.7 ± 3.0 | −0.76 (−1.95, 0.42) | 0.0955 | 1 |
| Spinal fusion | 77 | 5.3 ± 3.1 | 299 | 4.9 ± 3.2 | 0.39 (0.07, 0.70) | 0.0017 | 0.0875771 |
| Thyroidectomy, partial or complete | 148 | 4.3 ± 2.9 | 300 | 3.9 ± 3.1 | 0.40 (−0.37, 1.18) | 0.1756 | 1 |
| Tracheoscopy and laryngoscopy with biopsy | 308 | 2.7 ± 3.2 | 589 | 3.8 ± 3.2 | −1.12 (−2.74, 0.50) | 0.072 | 1 |
| Tracheostomy, temporary and permanent | 108 | 1.2 ± 2.3 | 120 | 1.3 ± 2.3 | −0.03 (−0.74, 0.68) | 0.9097 | 1 |
| Transurethral excision, drainage, or removal urinary obstruction | 323 | 4.0 ± 3.6 | 195 | 4.5 ± 3.2 | −0.52 (−1.86, 0.83) | 0.3157 | 1 |
| Treatment, facial fracture or dislocation | 75 | 4.9 ± 3.4 | 79 | 4.7 ± 3.2 | 0.25 (−1.09, 1.59) | 0.6275 | 1 |
| Treatment, fracture or dislocation of hip and femur | 137 | 4.7 ± 3.3 | 205 | 4.9 ± 3.1 | −0.21 (−0.55, 0.13) | 0.1142 | 1 |
| Treatment, fracture or dislocation of lower extremity (other than hip or femur) | 72 | 5.2 ± 3.2 | 205 | 5.7 ± 3.0 | −0.47 (−0.78, −0.16) | 0.0001 | 0.0047918 |
| Treatment, fracture or dislocation of radius and ulna | 70 | 6.0 ± 3.3 | 168 | 5.2 ± 3.1 | 0.83 (−0.03, 1.69) | 0.0124 | 0.5220135 |
| Upper gastrointestinal endoscopy, biopsy | 1,426 | 4.1 ± 4.0 | 1,254 | 2.3 ± 3.2 | 1.82 (1.08, 2.57) | 0 | 5.34E-08 |
| Ureteral catheterization | 258 | 4.5 ± 3.2 | 191 | 4.7 ± 3.5 | −0.23 (−1.15, 0.69) | 0.522 | 1 |
CABG = coronary artery bypass graft; CI = confidence interval; CCS = Clinical Categorization Software; ERCP = endoscopic retrograde cannulation of pancreas; GI = gastrointestinal tract; OR = operating room; POD = postoperative day; SD = standard deviation.
SPE by Sex from End of Surgery to End of POD 5
Of the 7,731 subjects, 6,797 (87.92%) reported at least one SPE between the end of surgery and the conclusion of POD 5. Of 3,739 female subjects, 3,166 (84.68%) reported at least one SPE between the end of surgery and POD 5 compared with 3,058 of 3,992 (76.60%) male subjects (chi-squared 80.92, P < 0.0001), giving an odds ratio of 1.69 (99% CI 1–1.96), which indicates female patients are at greater risk than male patients for experiencing at least one SPE.
Between the end of surgery and the conclusion of POD 5, 77,419 of 256,027 (23.22%) pain scores were considered SPE. Of the 160,709 pain scores recorded in female patients, 40,470 (25.18%) were considered SPE compared with 36,949 (21.39%) SPE of 172,737 pain scores for male patients. This suggested an overall odds ratio of 1.24 (99% CI 1.21–1.26) for female vs male patients for any given pain score to be an SPE from the end of surgery through the conclusion of POD 5, indicating that females are 24% more likely than males to have a SPE when they have pain.
SPE by Sex on POD 1
On POD 1, 7,485 subjects had recorded pain scores; this difference from the 7,731 subjects with scores documented between the end of surgery and POD 5 reflects those subjects who were intubated and/or had undocumented pain scores on POD 1 but subsequent recordings on POD 2 through POD 5. Of the 7,485 patients with pain scores recorded on POD 1, 4,559 (60.91%) reported at least one SPE. For females, 1,292 (64.44%) of 3,633 patients reported at least one SPE on POD 1 compared with 1,634 (57.58%) of 3,852 male patients (chi-squared 36.98, P < 0.0001). This suggested an overall odds ratio of 1.34 (99% CI 1.18–1.51) for the risk of female vs male patients experiencing at least one SPE on POD 1.
On POD 1, 21,775 (23.7%) of 91,861 pain observations were rated as an SPE. In female patients, 10,989 (25.09%) of 43,806 POD 1 pain scores were considered an SPE compared with 10,786 (22.45%) of 48,055 POD 1 pain scores as an SPE for male patients. This suggested an overall odds ratio of 1.16 (99% CI 1.11–1.20) for female vs male patients, indicating that female patients were 16% more likely than male patients to report an SPE for a pain score on POD 1.
SPE on POD 1 by Sex and Type of Surgery
To further characterize differences in postoperative pain experience while accounting for differing lengths of stay and for different types of surgical procedures, we examined the overall frequencies of POD 1 SPE in female and male patients for each CCS category of surgical procedures (Table 3). Cochran–Mantel–Haenszel statistics of sex by overall number of SPE on POD 1, controlling for CCS categories, supported the hypotheses for nonzero correlation, difference in mean scores, and general association of SPE frequency with sex, all at the P < 0.0001 level of significance. Estimates of the odds that a given pain observation represents an SPE for female vs male patients after controlling for CCS group yielded an odds ratio of 1.14 (99% CI 1.10–1.19).
Table 3.
Severe pain episodes on postoperative day 1 by procedure and sex
| CCS Group | Female
|
Male
|
Chi-Squared
|
|||||
|---|---|---|---|---|---|---|---|---|
| Severe Pain Episode?
|
Percent of Pain Scores as SPE | Severe Pain Episode?
|
Percent of Pain Scores as SPE | |||||
| No | Yes | No | Yes | P Value | Adjusted P Value (Holm) | |||
| CABG | 379 | 111 | 22.7 | 1,165 | 109 | 8.6 | 1.00E-15 | 7.81E-14 |
| Upper gastrointestinal endoscopy, biopsy | 192 | 94 | 32.9 | 498 | 80 | 13.8 | 5.30E-11 | 4.08E-09 |
| Other OR therapeutic procedures on bone | 254 | 199 | 43.9 | 313 | 108 | 25.7 | 1.55E-08 | 1.18E-06 |
| Laminectomy, excision intervertebral disc | 998 | 685 | 40.7 | 1,074 | 487 | 31.2 | 1.80E-08 | 1.35E-06 |
| Heart valve procedures | 1,474 | 238 | 13.9 | 2,030 | 184 | 8.3 | 2.04E-08 | 1.51E-06 |
| Arthroplasty other than hip or knee | 519 | 233 | 31.0 | 589 | 138 | 19.0 | 1.02E-07 | 7.45E-06 |
| Other OR therapeutic procedures of urinary tract | 383 | 145 | 27.5 | 870 | 170 | 16.3 | 2.08E-07 | 1.50E-05 |
| Creation, revision, and removal of arteriovenous fistula or vessel-to-vessel cannula for dialysis | 261 | 131 | 33.4 | 256 | 51 | 16.6 | 5.03E-07 | 3.57E-05 |
| ERCP | 213 | 97 | 31.3 | 325 | 62 | 16.0 | 1.81E-06 | 0.000126355 |
| Partial excision bone | 373 | 165 | 30.7 | 313 | 65 | 17.2 | 3.67E-06 | 0.000252904 |
| Lobectomy or pneumonectomy | 296 | 71 | 19.3 | 510 | 55 | 9.7 | 2.76E-05 | 0.001873536 |
| Debridement of wound, infection or burn | 490 | 356 | 42.1 | 800 | 396 | 33.1 | 3.48E-05 | 0.002330097 |
| Nephrectomy, partial or complete | 612 | 178 | 22.5 | 721 | 131 | 15.4 | 0.000209892 | 0.01385288 |
| Other OR therapeutic procedures on respiratory system | 275 | 26 | 8.6 | 356 | 79 | 18.2 | 0.000281347 | 0.018287569 |
| Exploratory laparotomy | 1,023 | 311 | 23.3 | 705 | 301 | 29.9 | 0.000317732 | 0.02033485 |
| Other therapeutic procedures on muscles and tendons | 309 | 87 | 22.0 | 417 | 194 | 31.8 | 0.0007238 | 0.045599419 |
| Treatment, fracture or dislocation of lower extremity (other than hip or femur) | 804 | 442 | 35.5 | 1,056 | 749 | 41.5 | 0.000803041 | 0.049788549 |
| Other OR therapeutic procedures on joints | 226 | 119 | 34.5 | 342 | 107 | 23.8 | 0.0009658 | 0.058913772 |
| Treatment, fracture or dislocation of radius and ulna | 91 | 83 | 47.7 | 202 | 98 | 32.7 | 0.001164523 | 0.069871351 |
| Other vascular catheterization, not heart | 205 | 77 | 27.3 | 220 | 42 | 16.0 | 0.001481174 | 0.087389257 |
| Endoscopy and endoscopic biopsy of the urinary tract | 57 | 29 | 33.7 | 67 | 10 | 13.0 | 0.001950886 | 0.111807554 |
| Insertion, replacement, or removal of extracranial ventricular shunt | 623 | 200 | 24.3 | 500 | 106 | 17.5 | 0.001927716 | 0.111807554 |
| Other OR gastrointestinal therapeutic procedures | 783 | 171 | 17.9 | 934 | 144 | 13.4 | 0.004533025 | 0.253849427 |
| Gastrostomy, temporary and permanent | 40 | 21 | 34.4 | 46 | 7 | 13.2 | 0.008662414 | 0.476432791 |
| Spinal fusion | 1,055 | 580 | 35.5 | 955 | 429 | 31.0 | 0.009368234 | 0.505884662 |
| Gastric bypass and volume reduction | 566 | 146 | 20.5 | 75 | 34 | 31.2 | 0.012025097 | 0.637330162 |
| Embolectomy and endarterectomy of lower limbs | 117 | 30 | 20.4 | 122 | 58 | 32.2 | 0.016564906 | 0.861375126 |
| Amputation of lower extremity | 175 | 145 | 45.3 | 316 | 226 | 41.7 | 0.300358967 | 1 |
| Aortic resection, replacement or anastomosis | 887 | 132 | 13.0 | 2,209 | 252 | 10.2 | 0.020050457 | 1 |
| Appendectomy | 137 | 55 | 28.6 | 204 | 121 | 37.2 | 0.046546518 | 1 |
| Arthroplasty knee | 2,195 | 713 | 24.5 | 1,272 | 414 | 24.6 | 0.977836555 | 1 |
| Cholecystectomy and common duct exploration | 404 | 188 | 31.8 | 315 | 129 | 29.1 | 0.35020456 | 1 |
| Colonoscopy and biopsy | 94 | 14 | 13.0 | 87 | 19 | 17.9 | 0.314976975 | 1 |
| Colorectal resection | 579 | 164 | 22.1 | 492 | 154 | 23.8 | 0.434512083 | 1 |
| Colostomy, temporary and permanent | 62 | 18 | 22.5 | 39 | 16 | 29.1 | 0.386030278 | 1 |
| Endarterectomy, vessel of head and neck | 230 | 9 | 3.8 | 316 | 27 | 7.9 | 0.043079805 | 1 |
| Excision of skin lesion | 66 | 16 | 19.5 | 94 | 36 | 27.7 | 0.177604219 | 1 |
| Extracorporeal lithotripsy, urinary | 105 | 50 | 32.3 | 73 | 22 | 23.2 | 0.122998947 | 1 |
| Hip replacement, total and partial | 1,768 | 585 | 24.9 | 1,605 | 511 | 24.1 | 0.58039881 | 1 |
| Ileostomy and other enterostomy | 404 | 88 | 17.9 | 422 | 78 | 15.6 | 0.334809448 | 1 |
| Incision and drainage, skin and subcutaneous tissue | 400 | 213 | 34.7 | 503 | 237 | 32.0 | 0.290447557 | 1 |
| Incision and excision of CNS | 1,886 | 413 | 18.0 | 1,659 | 383 | 18.8 | 0.50102412 | 1 |
| Insertion, revision, replacement, removal of cardiac pacemaker or cardioverter/defibrillator | 140 | 25 | 15.2 | 214 | 19 | 8.2 | 0.02830473 | 1 |
| Kidney transplant | 260 | 75 | 22.4 | 287 | 91 | 24.1 | 0.594967736 | 1 |
| Laparoscopy | 268 | 61 | 18.5 | 165 | 27 | 14.1 | 0.188110915 | 1 |
| Nephrotomy and nephrostomy | 277 | 109 | 28.2 | 196 | 76 | 27.9 | 0.933456037 | 1 |
| No procedure | 885 | 220 | 19.9 | 1,109 | 236 | 17.5 | 0.134816463 | 1 |
| Other OR heart procedures | 404 | 58 | 12.6 | 605 | 81 | 11.8 | 0.703772563 | 1 |
| Other OR lower GI therapeutic procedures | 270 | 81 | 23.1 | 274 | 91 | 24.9 | 0.561453966 | 1 |
| Other OR procedures on vessels of head and neck | 864 | 186 | 17.7 | 340 | 83 | 19.6 | 0.391278803 | 1 |
| Other OR procedures on vessels other than head and neck | 379 | 110 | 22.5 | 737 | 157 | 17.6 | 0.026267453 | 1 |
| Other OR therapeutic nervous system procedures | 1,013 | 346 | 25.5 | 899 | 349 | 28.0 | 0.148491084 | 1 |
| Other OR therapeutic procedures on musculoskeletal system | 34 | 7 | 17.1 | 125 | 65 | 34.2 | 0.03166685 | 1 |
| Other OR therapeutic procedures on nose, mouth, and pharynx | 232 | 54 | 18.9 | 538 | 131 | 19.6 | 0.801945679 | 1 |
| Other OR therapeutic procedures on skin and breast | 216 | 70 | 24.5 | 90 | 31 | 25.6 | 0.807010758 | 1 |
| Other OR upper GI therapeutic procedures | 269 | 57 | 17.5 | 298 | 41 | 12.1 | 0.04995933 | 1 |
| Other diagnostic nervous system procedures | 107 | 18 | 14.4 | 227 | 24 | 9.6 | 0.160599232 | 1 |
| Other diagnostic procedures on lung and bronchus | 619 | 75 | 10.8 | 720 | 98 | 12.0 | 0.475011038 | 1 |
| Other diagnostic procedures on musculoskeletal system | 335 | 107 | 24.2 | 159 | 41 | 20.5 | 0.301541924 | 1 |
| Other diagnostic radiology and related technique | 32 | 9 | 22.0 | 47 | 20 | 29.9 | 0.368685688 | 1 |
| Other fracture and dislocation procedure | 413 | 156 | 27.4 | 449 | 223 | 33.2 | 0.027930624 | 1 |
| Other hernia repair | 288 | 169 | 37.0 | 332 | 197 | 37.2 | 0.932903016 | 1 |
| Other non-OR therapeutic cardiovascular procedure | 120 | 27 | 18.4 | 55 | 23 | 29.5 | 0.056213611 | 1 |
| Other organ transplantation | 215 | 27 | 11.2 | 417 | 70 | 14.4 | 0.228509762 | 1 |
| Other therapeutic ear procedures | 37 | 4 | 9.8 | 50 | 0 | 0.0 | 0.02389377 | 1 |
| Other therapeutic endocrine procedures | 482 | 58 | 10.7 | 332 | 44 | 11.7 | 0.649099393 | 1 |
| Other therapeutic procedures, hemic and lymphatic system | 55 | 22 | 28.6 | 216 | 83 | 27.8 | 0.887341347 | 1 |
| Other vascular bypass and shunt, not heart | 121 | 27 | 18.2 | 229 | 71 | 23.7 | 0.191538478 | 1 |
| Peripheral vascular bypass | 247 | 61 | 19.8 | 437 | 152 | 25.8 | 0.044894373 | 1 |
| Skin graft | 428 | 270 | 38.7 | 910 | 571 | 38.6 | 0.954716721 | 1 |
| Small bowel resection | 86 | 22 | 20.4 | 96 | 24 | 20.0 | 0.944527047 | 1 |
| Thyroidectomy, partial or complete | 256 | 67 | 20.7 | 153 | 42 | 21.5 | 0.829624189 | 1 |
| Tracheoscopy and laryngoscopy with biopsy | 68 | 7 | 9.3 | 67 | 12 | 15.2 | 0.269336331 | 1 |
| Tracheostomy, temporary and permanent | 132 | 5 | 3.6 | 196 | 9 | 4.4 | 0.734822204 | 1 |
| Transurethral excision, drainage, or removal urinary obstruction | 52 | 20 | 27.8 | 137 | 68 | 33.2 | 0.397805739 | 1 |
| Treatment, facial fracture or dislocation | 48 | 22 | 31.4 | 114 | 54 | 32.1 | 0.914236798 | 1 |
| Treatment, fracture or dislocation of hip and femur | 984 | 442 | 31.0 | 842 | 412 | 32.9 | 0.302725453 | 1 |
| Ureteral catheterization | 171 | 87 | 33.7 | 140 | 51 | 26.7 | 0.110986071 | 1 |
CABG = coronary artery bypass graft; CCS = Clinical Categorization Software; ERCP = endoscopic retrograde cannulation of pancreas; GI = gastrointestinal tract; OR = operating room; POD = postoperative day.
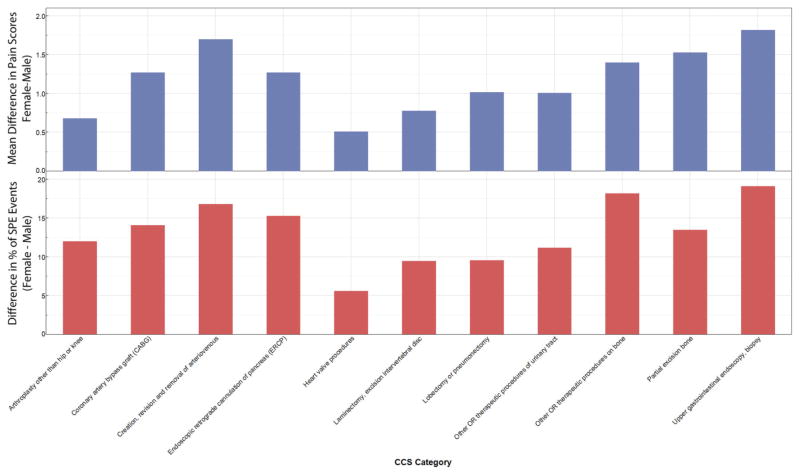
For those procedures where the mean differences in pain score and the proportions of pain scores that were SPE were statistically different for females vs males, there was general agreement in the direction of difference (e.g., female greater than male incidence for SPE comparison along with female greater than male pain score for mean difference comparison) for those 11 procedures where the mean difference in pain scores and the incidence of SPE were statistically different according to sex (Figure 2).
Figure 2.
Comparison of mean difference in pain scores with the difference in percentage of severe pain episode events between female and male patients. There were 11 procedures with statistically significant sex differences in mean difference in pain scores and incidence of severe pain event (SPE) for pain scores recorded on postoperative day (POD) 1.
Discussion
Our results support earlier clinical findings that suggest an overall sex difference in pain after surgery when pain scores are measured using the NRS in a clinical setting. Furthermore, these results demonstrate that for a wide variety of surgical procedures, there are differences in the incidence of SPE between female and male patients. These results were not limited to a single type of surgery but instead encompassed cardiothoracic, orthopedic, visceral, vascular, and soft tissue surgeries. In addition, these differences were observed despite a very conservative approach to avoiding type I errors. Despite the relatively small magnitude of the differences, the scale of our data allowed us to demonstrate the observed differences with a very high degree of certainty. Our aggregate results suggest that female patients may be at slightly higher risk for the severe pain scores that have been associated with the development of chronic postsurgical pain, although the nature of our retrospective study design obviously cannot identify the mechanisms driving these effects.
When evaluating differences in pain intensity within the postoperative setting, it is important to consider the multiple variables that influence the pain experience, including preoperative pain, psychological status, pain modulatory function, the degree of tissue injury posed by the surgery, and the patient’s response to analgesic interventions [7,30–34]. All of these factors (and more) interact within an individual patient to generate a rating of pain intensity, which can generate skepticism regarding the value of simple self-report measures of pain, such as the NRS. However, it is important to note that the NRS has been well validated in clinical and experimental settings [35–37].
Moreover, single-item ratings of experimental pain intensity correspond to activation in pain-related brain regions in response to the same pain stimulus [38]. More recently, grey matter density in pain-related brain regions was found to predict intensity ratings of experimental heat pain [39]. Thus, single-item pain ratings remain a highly efficient and valid window into an individual’s pain experience.
Our data build upon prior work suggesting higher pain scores for female patients after surgery, all of which also used the NRS within clinical settings [4]. The results shown in Table 2, where the differences in pain scores between females and males are compared using mean differences in pain scores on POD 1, concur with these prior findings. By grouping types of surgeries into broader categories of associated procedures, we were able to identify small differences in pain scores between sexes in a manner similar to that of Ruau et al. [13]. These findings are highly consistent with abundant data demonstrating greater experimental pain sensitivity and higher risk for clinical pain among women compared with men [4]. Although our findings reflect small mean differences between sexes on a per-procedural basis, even for procedures with high statistical significance (see Table 2), the results still have important implications. Specifically, the observed sex differences were highly reliable and suggest the need for additional research to identify the contributing factors. Moreover, at a public health level, interventions designed to improve postoperative pain management at either the hospital or systems level should take into account the higher risk for severe postoperative pain among women.
Eleven of the procedures with sex differences in mean pain scores also had statistically significant differences between the sexes in the incidence of SPE. Unlike the direct global comparison of pain scores in Table 2, the comparison of SPE in Table 3 demonstrates a number of procedures for which the per-procedure difference in percentages of SPE between the sexes was quite large. The concordance between SPE and mean differences in pain scores for lobectomy or pneumonectomy is especially notable, given that the requisite thoracotomy used for such procedures often leads to the development of chronic post-thoracotomy pain and that poorly controlled acute postoperative pain is associated with the development of chronic postsurgical pain as well [20,21,40]. Our results suggest that despite the widespread use of thoracic epidural analgesia at our institution for female and male patients undergoing thoracotomies, females are at greater risk for SPE compared with male patients.
Our findings should be interpreted in light of the study’s limitations, one of which is the lack of data regarding analgesic administration because sex differences in the effectiveness or administration of analgesics could influence the results. Previous evidence regarding sex differences in opioid analgesia are mixed, but on balance, the data suggest that females experience greater analgesia in response to mu-opioid and mixed-action opioids when administered for postoperative pain [41]. Any sex differences in reported pain scores should ideally also account for the possibility of differences in analgesic responsiveness related to sex; clinically, partitioning the effects of nociceptive loading and analgesic efficacy on reported pain intensity scores remains challenging. This informed our decision to focus on POD 1 for the testing of SPE using the logic that the patient’s care team likely optimized a pain management regimen throughout the hours after surgery on POD 0 (zero). Thus, continued SPE suggested effects from atypical analgesic requirements not addressed by the customary processes and the effects attributable to underlying surgical nociception along with the biopsychosocial characteristics of patients.
Our study shared additional limitations inherent to large-outcomes studies. First, the documentation of pain scores certainly deviated from the ascribed clinical protocol of a recording at least every 4 hours, with more frequent assessments conducted after analgesic interventions or in patient care settings with more intensive monitoring. Patients suffering from pain may have had their pain scores documented more frequently, whereas those comfortably sleeping may have been undersampled. Furthermore, the process used for collecting NRS pain scores used no standardization or specific training on pain assessment beyond that of the routine clinical education of nurses; this was an unavoidable salutary effect of such a large-scale collection of NRS data and mimics the limitation inherent to pain score assessments employed throughout the United States. Importantly, such limitations may be minimized through the use of multi-item pain assessment scales such as the Defense and Veterans Pain Rating Scale (DVPRS) [42]. Although the assignment of surgical procedures to CCS groups degrades the granularity of modeling particular procedures, CCS group assignment has been well validated in prior studies [43–45]. Interpretation of CCS group differences was most complicated for catch-all “other” categories of procedures, which is an inescapable effect of examining outcomes from a variety of types of surgery in a quaternary care facility. The inclusion of these “other” categories also minimized the attribution of component procedures to less appropriate categories, thus preserving low-variance categories and allowing for improved interpretation. Further work is necessary using multidimensional pain assessment tools (e.g., DVPRS), as well as with even larger data platforms and methods that enable testing of interactions and conditioning of factors upon pain outcomes.
In conclusion, our results suggest that females, on average, report higher numeric ratings of pain intensity in a clinical environment, and they experience a higher incidence of SPE on POD 1 for a variety of surgical procedures. Furthermore, the difference in clinically reported NRS pain scores between female and male patients increases through POD 5, reflecting a more rapid decrease in male compared with female patients. These results may inform future work in delineating which patient characteristics and treatment regimens are likely to influence the risk of severe acute postoperative pain. Further work is necessary to better characterize the use of SPE incidence in selecting patient cohorts to help health care providers better anticipate not just average pain needs but also comprehensive pain experience over the duration of the early postoperative recovery period.
Acknowledgments
Funded by a grant from the National Institutes of Health (no. K23GM102697 to Patrick J. Tighe, MD MS).
Footnotes
None of the authors report a conflict of interest.
References
- 1.Apfelbaum JL, Chen C, Mehta SS, Gan ATJ. Postoperative pain experience: Results from a National Survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 2.Racine ML, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception: Part 1: Are there really differences between women and men? Pain. 2012;153(3):602–18. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Riley J, Robinson M, Wise E, Myers C, Fillingim R. Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain. 1998;74(2–3):181–7. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 4.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurley RW, Adams MCB. Sex, gender, and pain: An overview of a complex field. Anesth Analg. 2008;107(1):309–17. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogil JS. Perspectives. Nat Rev Neurosci. 2012;13(12):859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 7.Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–8. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia Y-Y, Chow L-H, Hung C-C, et al. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled IV analgesia: A prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002;49(3):249–55. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 9.Gagliese L, Gauthier LR, Macpherson AK, Jovellanos M, Chan VW. Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Med. 2008;9(3):299–314. doi: 10.1111/j.1526-4637.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 10.Lau H, Patil NG. Acute pain after endoscopic totally extraperitoneal (TEP) inguinal hernioplasty: Multivariate analysis of predictive factors. Surg Endosc. 2004;18(1):92–6. doi: 10.1007/s00464-003-9068-y. [DOI] [PubMed] [Google Scholar]
- 11.Ritter MA, Wing JT, Berend ME, Davis KE, Meding JB. The clinical effect of gender on outcome of total knee arthroplasty. J Arthroplasty. 2008;23(3):331–6. doi: 10.1016/j.arth.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: Women report more pain than men after arthroscopic surgery. Pain. 2004;112(3):248–53. doi: 10.1016/j.pain.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13(3):228–34. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taenzer AH, Clark C, Curry CS. Gender affects report of pain and function after arthroscopic anterior cruciate ligament reconstruction. Anesthesiology. 2000;93(3):670–5. doi: 10.1097/00000542-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama K, Kawai M, Tani M, et al. Gender differences in postoperative pain after laparoscopic cholecystectomy. Surg Endosc. 2006;20(3):448–51. doi: 10.1007/s00464-005-0406-0. [DOI] [PubMed] [Google Scholar]
- 16.Callesen T, Bech K, Kehlet H. Prospective study of chronic pain after groin hernia repair. Br J Surg. 1999;86(12):1528–31. doi: 10.1046/j.1365-2168.1999.01320.x. [DOI] [PubMed] [Google Scholar]
- 17.Hickey OT, Burke SM, Hafeez P, et al. Severity of acute pain after breast surgery is associated with the likelihood of subsequently developing persistent pain. Clin J Pain. 2010;26(7):556–60. doi: 10.1097/AJP.0b013e3181dee988. [DOI] [PubMed] [Google Scholar]
- 18.Iohom G, Abdalla H, O’Brien J, et al. The associations between severity of early postoperative pain, chronic postsurgical pain and plasma concentration of stable nitric oxide products after breast surgery. Anesth Analg. 2006;103(4):995–1000. doi: 10.1213/01.ANE.0000240415.49180.4A. [DOI] [PubMed] [Google Scholar]
- 19.Katz J, Jackson M, Kavanagh B, Sandler A. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12(1):50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: Risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 21.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 22.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: A ratio measure? Pain Pract. 2003;3(4):310–6. doi: 10.1111/j.1530-7085.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 23.Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16(1):22–8. doi: 10.1097/00002508-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 25.Goulet JL, Brandt C, Crystal S, et al. Agreement between electronic medical record-based and self-administered pain numeric rating scale: Clinical and research implications. Med Care. 2013;51(3):245–50. doi: 10.1097/MLR.0b013e318277f1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philip BK. Parametric statistics for evaluation of the visual analog scale. Anesth Analg. 1990;71(6):710. doi: 10.1213/00000539-199012000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology. 1995;82(4):896–902. doi: 10.1097/00000542-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 29.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726–8. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson KO, Reyes-Gibby CC. Biopsychosocial approach to persistent post-mastectomy pain: What can we conclude? Pain. 2013;154(5):623–4. doi: 10.1016/j.pain.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Pincus T, Kent P, Bronfort G, et al. Twenty-five years with the biopsychosocial model of low back pain: Is it time to celebrate? A report from the Twelfth International Forum for Primary Care Research on Low Back Pain. Spine. 2013;38(24):2118–23. doi: 10.1097/BRS.0b013e3182a8c5d6. [DOI] [PubMed] [Google Scholar]
- 32.Carey ET, Martin CE, Siedhoff MT, Bair ED, As-Sanie S. Biopsychosocial correlates of persistent postsurgical pain in women with endometriosis. Int J Gynaecol Obstet. 2014;124(2):169–73. doi: 10.1016/j.ijgo.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Slade GD, Fillingim RB, Sanders AE, et al. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: Implications and future directions. J Pain. 2013;14(12 suppl):T116–24. doi: 10.1016/j.jpain.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lötsch J, Ultsch A. A machine-learned knowledge discovery method for associating complex phenotypes with complex genotypes. Application to pain. J Biomed Inform. 2013;46(5):921–8. doi: 10.1016/j.jbi.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83(2):157–62. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 37.Jensen MP, Engel JM, McKearnan KA, Hoffman AJ. Validity of pain intensity assessment in persons with cerebral palsy: A comparison of six scales. J Pain. 2003;4(2):56–63. doi: 10.1054/jpai.2003.9. [DOI] [PubMed] [Google Scholar]
- 38.Coghill RC, McHaffie JG, Yen Y-F. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100(14):8538–42. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emerson NM, Zeidan F, Lobanov OV, et al. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain. 2013;155(3):566–73. doi: 10.1016/j.pain.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: A critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 2009;36(1):170–80. doi: 10.1016/j.ejcts.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Niesters M, Dahan A, Kest B, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151(1):61–8. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Buckenmaier CC, Galloway KT, Polomano RC, et al. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med. 2013;14(1):110–23. doi: 10.1111/j.1526-4637.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen K, Battles JB, Marks ES, et al. Serious Injury Surveillance System that Includes Adverse Event Hospitalizations. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Developing a Veterans Health Administration (VHA) [PubMed] [Google Scholar]
- 44.Robinson JW. Regression tree boosting to adjust health care cost predictions for diagnostic mix. Health Serv Res. 2008;43(2):755–72. doi: 10.1111/j.1475-6773.2007.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabak YP, Sun X, Nunez CM, Johannes RS. Using electronic health record data to develop inpatient mortality predictive model: Acute Laboratory Risk of Mortality Score (ALaRMS) J Am Med Inform Assoc. 2014;21(3):455–63. doi: 10.1136/amiajnl-2013-001790. [DOI] [PMC free article] [PubMed] [Google Scholar]