Abstract
Successful expansion of hematopoietic stem cells would benefit the use of hematopoietic stem cell transplants in the clinic. Several angiopoietin-like proteins, including angiopoietin-like 7, can support the activity of hematopoietic stem cells. However, effects of ANGPTL7 on human hematopoietic stem cells and the downstream signaling cascade activated by ANGPTL7 are poorly understood. Here, we established a human hematopoietic stem and progenitor cell-supportive mouse fetal liver cell line that specifically expressed the Angptl7 protein. Furthermore, we found ANGPTL7 is capable of stimulating human hematopoietic stem and progenitor cell expansion and increasing the repopulation activities of human hematopoietic progenitors in xenografts. RNA-sequencing analysis showed that ANGPTL7 activated the expression of CXCR4, HOXB4 and Wnt downstream targets in human hematopoietic progenitors. In addition, chemical manipulation of Wnt signaling diminished the effects of ANGPTL7 on human hematopoietic stem and progenitor cells in culture. In summary, we identify the secreted growth factor ANGPTL7 as a regulator of both human hematopoietic stem and progenitor cell expansion and regeneration.
Introduction
Hematopoietic stem cells (HSCs), which are commonly used for HSC transplantation in patients with cancer or hematopoietic disorders, are capable of self-renewal and differentiation into all blood cell types.1,2 In mammals, both intracellular and extracellular signals contribute to the homeostasis of HSCs,3–6 but the mechanisms involved in the control of the fate of HSCs are still poorly understood.
Numerous attempts have been made to increase the long-term maintenance of HSCs in culture. Stromal cell lines derived from brain endothelial cells,7 aorta-gonads-mesonephros,8 and fetal liver hepatic progenitors9 have been established and evaluated to expand HSCs ex vivo, as stromal cells can not only secrete growth factors10 but mimic the HSC niche, which controls the balance between HSC self-renewal and differentiation.11,12
Several growth factors associated with HSC self-renewal and expansion have been identified. Treatment of mouse bone marrow HSCs with pleiotrophin (PTN)13 or Angptl family,14,15 especially Angptl 2 and Angptl 3, caused a marked increase in long-term repopulating HSC counts in culture and ex vivo. Growth factors like EGF-EGFR signaling regulate HSC regeneration after radiation injury.16 In addition, multiple reports have shown that the Wnt signaling pathway plays a key role in regulating HSC self-renewal14,17 and maintenance. In mice, activation of the canonical Wnt signaling through β-catenin overexpression18 or the treatment of cells with glycogen synthase kinase 3 (GSK-3) inhibitors,19 which indirectly activate β-catenin, preserve HSC stemness. The study of a signaling pathway that modulates HSC stemness is important for understanding the basic biology of HSC and will provide critical insight into their clinical applications.
ANGPTLs belong to a 7-member family of secreted glycoproteins that share 80% sequence homology with mouse Angptls. Each ANGPTL contains an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain. It is known that several members of the ANGPTL family have roles in regulating lipid metabolism and angiogenesis.20 ANGPTL proteins are also involved in the construction of extracellular matrix (ECM) formation, thus they are potential therapeutic targets for metabolic syndrome and cardiovascular disease.21,22 In particular, ANGPTL7 in the ECM of the trabecular mesh-work plays an important role in the deposition and organization of the matrix of the outflow tissue.21 Recently, several studies have suggested that Angptls play roles in the regulation of HSC activity. Angptl3, Angptl5 and Angptl7 have been shown to stimulate mouse HSC expansion ex vivo.14,23 Although Angptl5 substantially promotes the expansion of human HSCs in vitro and ex vivo,24 it is still not clear whether ANGPTL7 is also able to promote the expansion of human HSCs, and the downstream signaling cascade activated by ANGPTL7 in HSCs has not been well characterized.
In this study, we found that a stromal cell line established from mouse fetal liver expressed high levels of Angptl7 and promoted human HSPC expansion in cell culture conditions. We demonstrated that recombinant ANGPTL7 increased the engraftment capacity of human HSPCs in a xenograft mouse model and that ANGPTL7-stimulated human HSPC expansion depended on the Wnt signal pathway.
Methods
Mice
Animal experiments were performed in the Laboratory Animal Center of the Guangzhou Institutes of Biomedicine and Health (GIBH), and all animal procedures were approved by the Animal Welfare Committee of GIBH. NOD-scid-IL2Rg−/− (NSI) mice were derived at GIBH (W. Yei, unpublished data, 2014). All mice were maintained in Specific-pathogen-free cages and provided with autoclaved food and water. NSI mice were generated by injecting IL2Rg−/− TALENs mRNA pair into the cytoplasm of NOD pronuclear-stage embryos. Protocols were approved by the relevant institutional animal care and use committee (IACUC).
Cell culture
All primary samples were obtained after informed consent for research purposes, and the procedures were approved by the Research Ethics Board of GIBH. Human whole white blood cells (WBCs) were isolated with Lymphoprep (StemCell Technologies, Canada) according to the manufacturer’s instructions. Millicell Hanging Cell Culture Inserts (Millipore, Darmstadt, Germany) were used for PL08, PL08+M, PL01, and liquid hanging studies. A total of 2×105 PL01-PL20, PL08+M, and PL01 cells were pre-cultured in plastic tissue culture plates with StemSpan serum-free medium (StemSpan SFEM II, StemCell Technologies, Canada) as feeder cells; 2×106 WBCs were seeded in the inserts 24 h post-culture of the feeder cells unless otherwise stated. The WBCs were counted with a hemocytometer and analyzed by flow cytometry every three days. Human UCB CD34+ cells were enriched via magnetic cell sorting (Miltenyi, Bergisch Gladbach, Germany) and were seeded into the wells of a U-bottom 96-well plate. StemSpan serum-free medium was used as the basal medium. The basal medium supplemented with 100 ng/mL of human SCF (Peprotech, Rochy Hill, USA), 20 ng/mL of TPO (Peprotech, Rochy Hill, USA), 100 ng/mL of mouse PTN (Peprotech, Rochy Hill, USA), and 50 ng/mL human FLT3L (Peprotech, Rochy Hill, USA) was used as STPF medium. The STPF medium supplemented with 500 ng/mL ANGPTL7 was named ASTPF medium. Wnt3a (R&D, Minneapolis, MN, USA) protein was used at 100 μg/mL, IWP2 (Sigma-Aldrich, Munich, Germany) at 2 μM, CHIR99021 (Sigma-Aldrich, Munich, Germany) at 3 mM, and indomethacin at 1 μM and 50 ng/mL recombinant human DKK1 (Sigma-Aldrich, Munich, Germany). All reagents were changed daily in all the experiments reported here. Cells were cultured at 37°C in 5% CO2 and the normal level of O2. Cord blood samples were collected at the South China Medical University (SCMU) Department of Gynecology and Obstetrics after informed consent for research purposes only, and this process was monitored by the Institutional Review Boards of the SCMU.
NSI assay for ex vivo expanded cord blood HSCs
A total of 1×104 uncultured human umbilical cord blood (UCB) CD34+ cells or the progeny of 1×104 human UCB CD34+ cultured in STPF (SCF, TPO, PTN, and FLT3L) or ASTPF (ANGPTL7, SCF, TPO, PTN, and FLT3L) medium for seven days were pooled together and injected intravenously via the retro-orbital route into sub-lethally irradiated (1.5 Gy) 8–10-week-old NSI mice. After 8–16 weeks, mice were sacrificed, and peripheral blood mononuclear cells (PBMC), splenocytes, and bone marrow (BM) cells were analyzed by flow cytometry for the presence of human CD45+ cells. In our engraftment assay, the mice were considered to have engrafted successfully if we detected 0.1% or more human CD45+ cells in the BM at eight weeks after transplant. For limiting-dilution analysis, mice were considered positive for human HSC engraftment when at least 1% CD45+ human cells were detected among the mouse BM cells at eight weeks after transplant, unless otherwise indicated.
Homing assay
Sub-lethally irradiated NSI mice (1.5 Gy) were either transplanted with human CD34+ cells (1×106/mouse) without culture or cultured in STPF medium or ASTPF medium for seven days. Where indicated, part of the progeny of 1×106 CD34+ cells in ASTPF condition were incubated prior to injection with 10 ug/106 cells of anti-human CXCR4 mAb (clone 12G5; ebioscience, San Diego, CA, USA). 16 h post transplantation, BM and spleen were analyzed for the presence of human CD34+ cells.
Image acquisition
Images were acquired on a Leika DMI6000B fluorescence microscope using 2× objective lens. Images of PL08 cells in RPMI-1640 culture media were acquired at room temperature by DFC digital camera using Leika FW4000 software (version 1.2.1).
Colony-forming cell assays
Cell suspensions of 3×104 cells/mL for white blood cells (WBC) and 1.5×104 for CD34+ cells were used. Approximately 0.3 mL of cells was added to 3 mL of the MethoCult™ H4435 (StemCell Technologies, Canada) for duplicate cultures according to the manufacturer’s instructions. Granulocyte/macrophage CFUs and erythroid burst-forming units and CFU-E, BFU-E, CFU-GM, and CFU-GEMM were scored on day 14 of each culture. Cell suspensions of 2×104 cells/mL for mouse BM cells were used. Approximately 0.3 mL of cells was added to 3 mL of the MethoCult™ M3334 (StemCell Technologies, Canada) for duplicate cultures according to the manufacturer’s instructions. Granulocyte/macrophage CFUs and erythroid burst-forming units and CFU-E, BFU-E, CFU-GM, and CFU-GEMM were scored on day 10 of each culture.
Flow cytometry
Flow cytometric analysis was performed using Accuri C6 or FACSAria™ II (BD Biosciences, San Jose, CA, USA). PBMCs were labeled with Human Hematopoietic Lineage FITC Cocktail (eBioscience, San Diego, CA, USA), CD38-PE (eBioscience, San Diego, CA, USA), and CD34-APC (eBioscience, San Diego, CA, USA). BM cells from transplanted NSI mice were assessed using human CD45-PerCP Cy5.5 (eBioscience, San Diego, CA, USA), human CD19-PE (eBioscience, San Diego, CA, USA), human CD33-PE (eBioscience, San Diego, CA, USA), and human CD14-PE (eBioscience, San Diego, CA, USA).
Plasmid and protein purification
The plasmid PB-CAG human ANGPTL7 with a 6×His tag at the C terminus was transfected into 293T cells using Lipofectamine 2000 (Invitrogen, New York, NY, USA). After transfection, transfected cells were cultured overnight in Iscove Modified Dulbecco Medium (IMDM, Thermo Scientific, MA, USA) with 10% FBS and were then washed with IMDM before being cultured in serum-free StemSpan medium (StemSpan SFEM II, StemCell Technology) for another 24 h. We collected the conditioned medium cultured in IMDM with 10% FBS for 48 h or 72 h and incubated it with Ni-NTA according to the manufacturer’s instructions (Qiagen, Germany). We added 1 tablet/50 mL of the Complete Protease Inhibitor Cocktail (Roche, Basel, Switzerland) to the combined protein-bead mixture. The protein concentrations were determined with the Bio-Rad protein assay kit (Bio-Rad, California, USA) and BSA as the standard according to the manufacturer’s instructions. Western blots were performed to detect the ANGPTL7 protein using the rabbit ANGPTL7-specific antibody (ab118198, Abcam, Cambridge, UK) and the rabbit 6×His tag-specific antibody (ab1187, Abcam, Cambridge, UK). We sent the purified protein for mass spectrometry analysis for protein identification.
Gene expression analysis
The sequencing reads were mapped to the mouse RefSeq-RNA reference sequence (down-loaded from http://hgdownload.cse.ucsc.edu/downloads) using the FANSe 2 algorithm (available from: http://bioinformatics.jnu.edu.cn/software/fanse2/) with the parameters −L85 −E3 −U0 −S1015. Alternative splice variants were merged.25 Genes with at least 10 mapped reads were considered as reliably detected genes.26 These genes were further quantified using count values, which were raw counts of sequencing reads. The count values were imported into the DESeq software package to calculate the up-/downregulation of genes among PL08, PL01, and PL08−M cells. Mouse secreted protein gene sequences were downloaded from the Secreted Protein Database (SPD, http://spd.cbi.pku.edu.cn/sequence). We analyzed only those secreted protein genes that we could identify in the RefSeq-RNA sequence using SPD-RNA sequence by BLASTN (e value < 10–20). The up- or down-regulated secreted protein genes were identified by filtering the RNA-seq data with the following cut off: a 2-fold ratio in expression level and P<0.05. For human HSC gene expression RNA-seq analysis, all the count values of human gene with more than 2-fold change were imported into the DAVID database (available from: http://david.abcc.ncifcrf.gov/home.jsp). The up- or down-regulated Wnt signaling pathway genes were identified by filtering the RNA-seq data with the following cut off: a 2-fold ratio in expression level and P<0.05.
Statistical analysis
Data were analyzed using GraphPad Prism 4 with Student t-test. P<0.05 was considered statistically significant.
Results
Stromal cell clone PL08 supports human HSPC expansion in vitro
Fetal liver HSCs undergo dramatic expansion during embryonic development.27 Thus, we hypothesized that certain stromal cells in the fetal liver might secrete proteins to promote HSCs proliferation. In an attempt to establish immortalized fetal liver cell lines, we isolated primary stromal cells from livers of mouse embryos at 11.5 days of gestation (dpc) and immortalized them by transduction with SV40 large T antigen. Twenty clones (named PL01–PL20) were established after eight weeks. Out of these twenty clones, PL01 and PL08, both of which were adherent and grew robustly (Online Supplementary Figure S1), significantly supported the expansion of human umbilical cord blood nucleated cells (hUCBNCs)-derived HSPCs in StemSpan medium supplemented with hSCF, hTPO, PTN, and hFLT3L (STPF medium) (Online Supplementary Figure S1). Furthermore, hUCBNCs in PL08 hanging-culture conditions grew faster than the cells grown in PL01 hanging-culture condition (Figure 1A). Both the percentage and the total number of Lin−CD34+CD38− human HSPCs were significantly higher in PL08 hanging-culture conditions than in PL01 co-culture conditions or in liquid conditions after 12 days culture (Figure 1B and C). In addition, colony-forming cell (CFC) assay results showed that there were more GEMM (granulocyte, erythrocyte, monocyte, and megakaryocyte) colony-generating cells in PL08 co-cultures (Figure 1D). Interestingly, we noticed that PL08 cells lost the capacity to support HSPCs proliferation in vitro after treatment of mitomycin C (Mito), which is commonly used to prevent cell division and production of autocrine growth factors14 (Figure 1A–D). Taken together, these results demonstrate that mouse fetal liver-derived PL08 stromal cells support human HSPC expansion in vitro.
Figure 1.
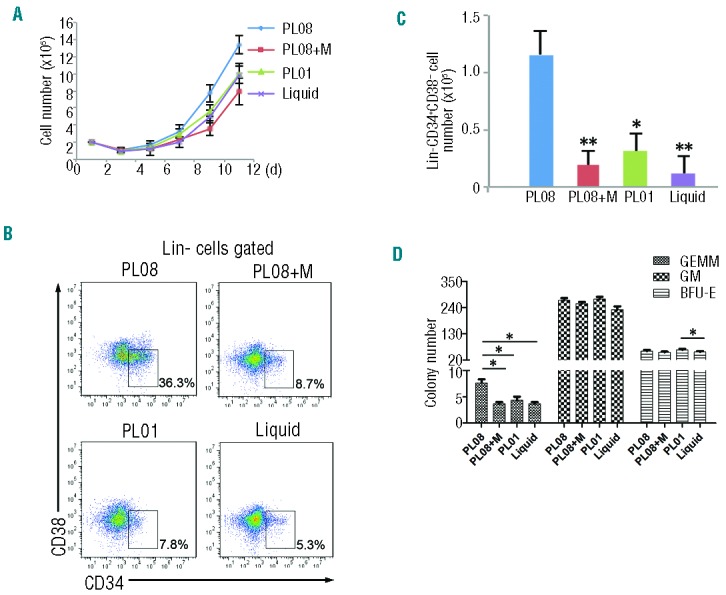
PL08 substantially stimulates the expansion of human hematopoietic stem cells (HSCs) in vitro. (A) A total of 2×105 human umbilical cord blood nucleated cells (hUCBNCs) were isolated and co-cultured with PL08 cells, PL08 cells that were previously treated with Mito (PL08+M), or PL01 cells in StemSpan medium supplemented with hSCF, hTPO, PTN, and hFLT3L (STPF). In culture, hUCBNCs were separated from stromal cells using Transwell membranes. In another control group (Liquid), 2×105 hUCBNCs were isolated and cultured without any stromal cells in StemSpan medium supplemented with STPF. Cultured hUCBNC were counted and analyzed every three days (n=4). (B) Representative FACS plots show the percentages of Lin-CD34+CD38− cells in PL08, PL08+M, PL01, and liquid culture conditions. (C) Summary of absolute numbers of Lin-CD34+CD38− cells cultured in PL08, PL08+M, PL01, or liquid conditions at day 12 from four independent experiments (n=4). Data represent mean+s.e.m. *P≤0.05 versus bar 3 for bar 1; **P≤0.01 versus bar 2 and bar 4 (for bar 1). (D) The colony numbers of CFU assays obtained from cultured cells as described in (A). Data represent mean+s.e.m. (n=4). *P≤0.05 versus bar 2, bar 3 and bar 4 (for bar 1). *P≤0.05 for bar 11 versus bar 12. BFU-E: erythroid colonies; GM: granulocyte-monocyte colonies; GEMM: granulocyte, erythrocyte, monocyte and megakaryocyte colonies.
Angptl7 is highly expressed in clonal PL08 cells
To identify the factors from PL08 cells that supported human HSPC proliferation, we performed RNA-seq to analyze the global gene expression profiles of PL08 cells, Mito-treated PL08 cells, and PL01 cells. Approximately 15,000 of the 20,861 Refseq genes were detectably expressed in each population (Online Supplementary Table S1). Overall, the three global gene expression profiles of PL08, Mito-treated PL08, and PL01 cells were different, with a Pearson correlation coefficient of 0.8 or under for each pair-wise comparison (Figure 2A and Online Supplementary Figure S2). We validated the RNA-seq results by measuring the mRNA levels of Pgk1,28 Sqstm1,29 S100a4,30 Arpc1b,31 Ptn,13 and Ldha,32 which were known to play important roles during hematopoiesis, angiogenesis, or actin polymerization. Consistent to RNA-seq analysis, quantitative reverse transcription PCR (qRT-PCR) showed that Sqstm1 and Ptn was highly expressed while Arpc1b, Ldha, and Pgk1 were expressed at low levels in PL08 cells (Online Supplementary Figure S3), indicating that the results of the RNA-seq analysis were reliable for further analysis.
Figure 2.
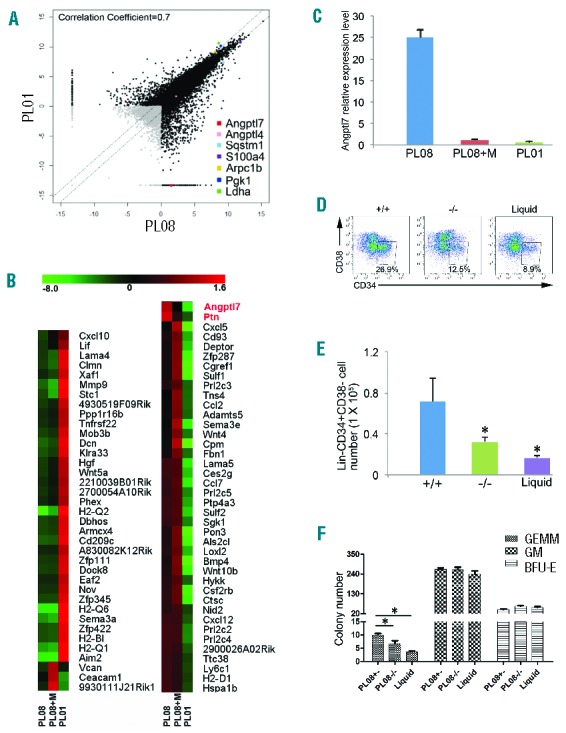
Angptl7 is specifically expressed in the PL08 cell line. (A) Pairwise comparison of poly(A)+ RNA-Seq analysis of the global expression profiles in PL08 versus PL01 cells. Pearson correlation coefficient is shown. (B) Unsupervised hierarchical cluster analysis of expression levels of 77 secreted protein genes in PL08, PL08+M, and PL01 cells (red: increased expression; green: decreased expression). (C) qRT-PCR analysis of Angptl7 mRNA levels in PL08, PL08+M, and PL01 cells. The results were normalized to the β-actin mRNA levels and represent mean+s.e.m. (n=3). (D) A total of 2×105 human umbilical cord blood nucleated cells (hUCBNCs) were isolated and co-cultured with PL08 cells, Angptl7-null PL08 cells in STPF conditions. In culture, hUCBNCs were separated from stromal cells using Transwell membranes. In another control group (Liquid), 2×105 hUCBNCs were isolated and cultured without any stromal cells in StemSpan medium supplemented with STPF. Representative FACS plots show the percentages of Lin-CD34+CD38− cells in PL08, Angptl7-null PL08, and liquid culture conditions. (E) Summary of absolute numbers of Lin-CD34+CD38− cells in liquid culture condition (Liquid), or in co-culture with WT PL08 (+/−), or Angptl7−/− PL08 (−/−) cells at day 12 from four independent experiments (n=4). Data represent mean+s.e.m. *P≤0.05 versus bar 2 and bar 3 (for bar 1). (F) The colony numbers of CFU assays obtained cultured hUCBNCs as described in (D). Data represent mean+s.e.m. (n=4). *P≤0.05 versus bar 2 and bar 3 (for bar 1).
Hierarchical clustering analysis showed that 77 genes encoding secreted proteins, including Angptl7, Ptn, Bmp4, and Wnt10b, were highly expressed in PL08 cells but not in PL01 cells (Figure 2B). Ptn, which is a growth factor supporting the expansion of HSCs,13 was highly expressed in both PL08 and Mito-treated PL08 cells (Online Supplementary Figure S2). These data suggest that there are additional cytokines specifically expressed in PL08 cells that contributed to the capacity of PL08 cells to support HSCs. Interestingly, Angptl7 can support mouse HSC expansion,14 and we found that it was specifically expressed in PL08 cells but not in PL01 cells or Mito-treated PL08 cells (Figure 2B and C). We then measured expression levels of angiopoietin-like protein families in PL08 cells and found both Angptl4 and Angptl7 were highly expressed in PL08 cells (Online Supplementary Figure S4). However, Angptl4 was also highly expressed in PL01 cells (Figure 2A), which did not support human HSPC growth in culture (Figure 1A–C). Thus, we speculated that Angptl7 but not Angptl4 was one of the key factors that contribute to the capacity of PL08 cells to support human HSPC expansion in vitro. To validate this hypothesis, we targeted genomic Angptl7 with highly active TALENs specific to exon 1 of Angptl7 plus a homologous donor vector, which used Angptl7 promoter to drive expression of Venus and disrupted Angptl7 expression (Online Supplementary Figure S5). We then generated homozygous of Angptl7 knockout PL08 cell lines (Online Supplementary Figures S6–S8). The percentages and the total number of Lin−CD34+CD38− human HSPCs were both significantly lower in Angptl7-null PL08 hanging-culture conditions than in normal PL08 co-culture conditions after 12 days culture (Figure 2D and E). In agreement with this, hUCBNCs co-cultured with Angptl7-null PL08 cells for seven days showed a significant reduction in total CFC content and a decrease in GEMM colony-generating cells compared to that cultured in normal PL08 cells (Figure 2F). Thus, the results suggested that the loss of Angptl7 led to PL08 failing to support human HSPC expansion in culture.
ANGPTL7 stimulates human HSPC expansion in vitro
To understand whether recombinant ANGPTL7 promotes human HSPC expansion, we constructed a plasmid containing the entire coding sequence of ANGPTL7 with a C-terminal 6xHis tag in a eukaryotic expression vector (Online Supplementary Figure S9). After transfection of HEK293T cells (293-ANGPTL7), the supernatant contained the secreted ANGPTL7-His fusion protein migrating at the expected size of 45kDa (Figure 3A). The identity of the purified recombinant ANGPTL7 protein was validated by mass spectrometry analysis (Online Supplementary Figure S10). hUCBNCs were cultured in both serum-free mock STPF (SCF, TPO, PTN, and FLT3L) medium and serum-free mock STPF medium supplemented ANGPTL7. The hUCBNCs grew faster in ASTPF medium than in STPF medium (Figure 3B). In addition, both the percentage and the absolute number of Lin-CD34+CD38− cells were significantly higher in ASTPF medium than that in STPF medium after seven days culture (Figure 3C and D). The colony-forming unit (CFU) assay showed that the total CFC content and GEMM colony-generating cells increased after ANGPTL7 treatment (Figure 3E). We next tested whether ANGPTL7 stimulated expansion of purified human HSPCs in vitro. Enriched CD34+ cells from hUCBNCs were cultured in ASTPF and STPF medium, respectively. Twelve days after culture, the percentages and the total number of Lin-CD34+CD38− cells were both higher in the culture condition supplemented with ANGPTL7 (Figure 3F and G), revealing that ANGPTL7 promoted the proliferation of CD34+ cells from hUCBNCs in vitro.
Figure 3.
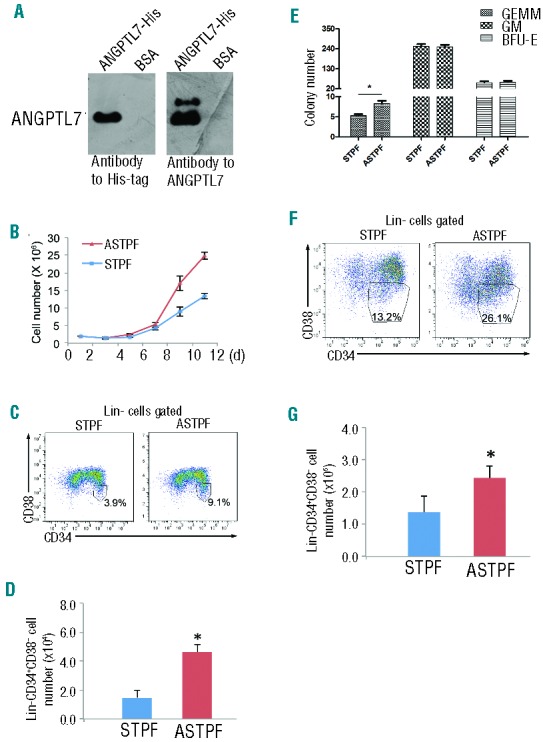
Effects of ANGPTL7 on human hematopoietic stem and progenitor cells (HSPCs). (A) Western blotting analysis of purified ANGPTL7 (left lanes) and control bovine serum albumin (BSA, right lanes) detected by antibodies as indicated. (B) Human umbilical cord blood nucleated cells (hUCBNC) cultures (2×106) in ASTPF (red triangles) and STPF (blue squares) conditions. Viable cells were counted at the indicated time points. Data represent mean+s.e.m. (n=3). (C and D) Representative FACS plots (C) and summary of the absolute numbers (D) of Lin-CD34+CD38− cells in the ASTPF and STPF conditions. Data represent mean+s.e.m. (n=3). *P≤0.05 for bar 1 versus bar 2. (E) The colony numbers of CFU assays obtained from 1×104 fresh hUCBNCs (day 0) or after culturing hUCBNCs in the ASTPF and STPF conditions. Data represent mean+s.e.m. (n=3). *P≤0.05 versus bar 1 for bar 2; **P≤0.01 for bar 2 versus bar 3. (F and G) Representative FACS profiles (F) and summary of absolute numbers (G) of purified CD34+ hUCBNCs cultured in the ASTPF and STPF conditions. Data represent mean+s.e.m. (n=3). *P≤0.05 for bar 1 versus bar 2.
ANGPTL7 stimulates the ex vivo expansion of human HSPCs
To examine whether ANGPTL7 treatment increases the engraftment capacity of human HSPCs after ex vivo expansion, ANGPTL7-stimulated and ANGPTL7-untreated CD34+ hUCBNCs cultured in STPF medium and freshly isolated CD34+ hUCBNCs were transferred into sub-lethally irradiated NOD-scid-IL2Rg−/− (NSI) recipients (Figure 4A), which were generated by TALEN-mediated gene targeting in the Il2rg locus (W. Ye, unpublished data, 2014) and did not have B, T, or NK cells (Online Supplementary Figure S11). Two to 4 months post transplantation, the percentages of human CD45+ cells in peripheral blood, BM, and spleen of NSI mice that had been injected with ANGPTL7-stimulated CD34+ hUCBNCs were significantly higher than those in the control group (Figure 4B). In addition, ANGPTL7-treated human HSPCs from the BM of the primary xenografts achieved higher engraftment efficiencies in the secondary transplantation compared to controls (Figure 4C). Limiting dilution analysis demonstrated that ANGPTL7 treatment increased the frequency of SCID repopulating cells in vivo (Figure 4D). HSPCs cultured in ASTPF medium reconstituted both lymphoid and myeloid lineages in the recipients (Figure 4E). Furthermore, the ratios of lymphoid and myeloid lineages in xenografts that had been injected with ANGPTL7-treated human HSPCs were not significantly different from those in recipients, in which ANGPTL7-untreated or freshly isolated human HSPCs were transferred (Online Supplementary Table S2). Taken together, these results suggested that ANGPTL7 enhanced the ex vivo expansion of human HSPCs but did not alter HSPC differentiation capacities.
Figure 4.
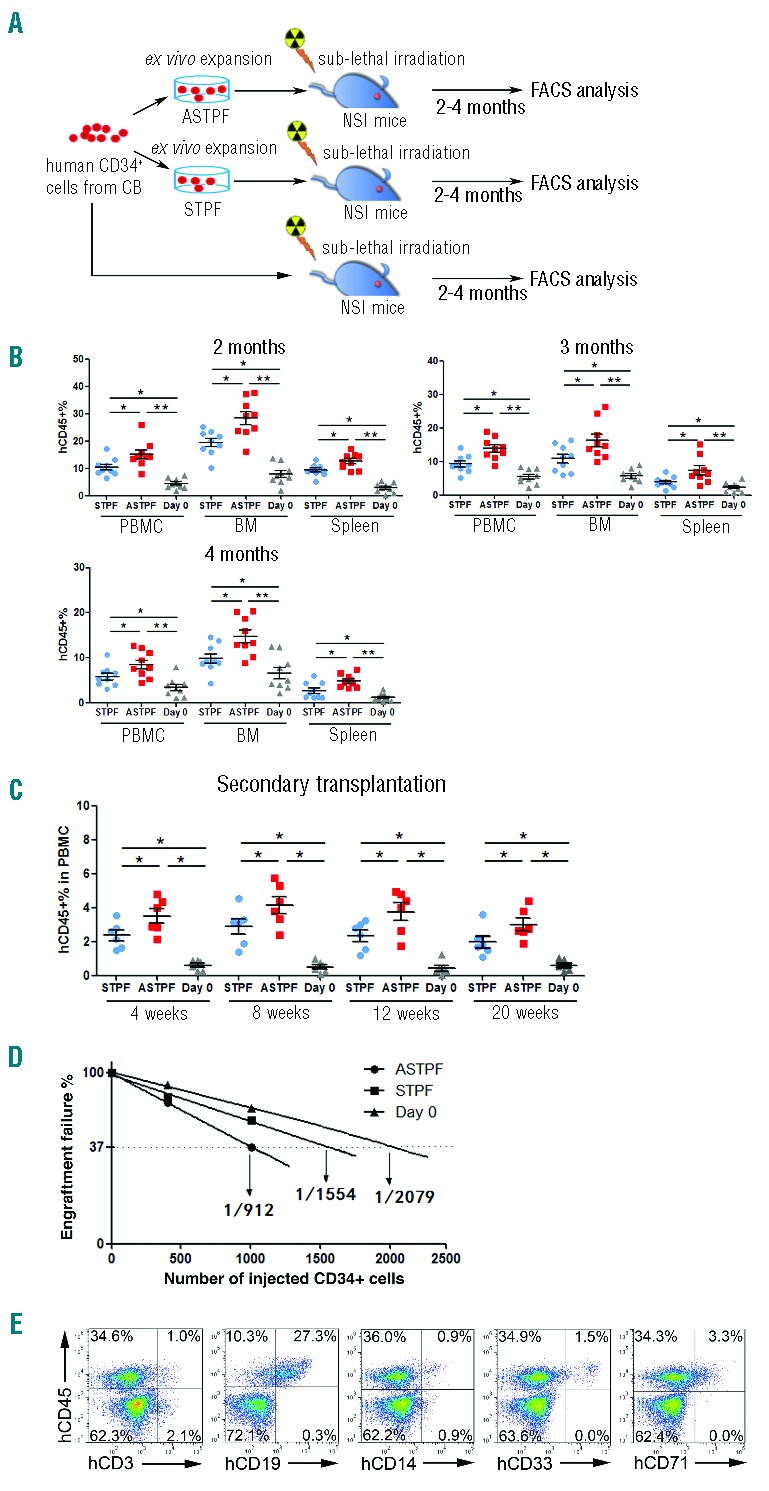
ANGPTL7 stimulates expansion of human hematopoietic stem and progenitor cells (HSPCs) ex vivo. (A) Experimental design for assessing the repopulation capacities of ex vivo expanded human HSPCs. Total of 1×104 fresh purified CD34+ human umbilical cord blood nucleated cells (hUCBNCs) and 1×104 purified CD34+ hUCBNCs cultured in ASTPF or STPF conditions for seven days were injected into three groups of sub-lethally irradiated NSI mice (9 mice per group). Two, three, and four months post transplantation, peripheral blood (PB), BM, and spleen (SP) samples from NSI mice from each group were subjected to FACS analysis. (B) Summary of percentages of human CD45+ cells in the PB, BM, and SP of NSI mice at two, three, or four months after injection with 1×104 purified CD34+ hUCBNCs or progeny of 1×104 purified CD34+ hUCBNCs that had been cultured in ASTPF or STPF conditions. Bars represent the mean percentages of human CD45+ cells in the PB, BM and SP of mice from each group (n=9). The experiments were repeated three times. *P≤0.05 for ASTPF group versus STPF group; **P≤0.01 for ASTPF group versus day 0 group; *P≤0.05 for STPF group versus day 0 group. (C) Bone marrow aspirate from one hind leg from a primary recipient was transplanted into 2 secondary recipients (n=6); *P≤0.05 for ASTPF group and STPF group versus day 0 group; ASTPF group versus STPF group. (D) Limiting-dilution analysis of the repopulating ability of cells (400 or 1000 cells) before culture (day 0) and after culture for seven days in STPF medium and ASTPF medium. Plots show percentage of recipient mice containing less than 1% human hematopoietic populations in recipient mouse bone marrow eight weeks after transplantation versus the number of input or input-equivalent cells injected (n=10 mice transplanted at each dose per condition; n=60 mice total). (E) Representative FACS analysis of percentages of multiple hematopoietic lineages in the BM of NSI mice from the ASTPF group two months post transplantation, as described in (B).
ANGPTL7-stimulated HSPC expansion is dependent on Wnt signaling
To dissect the mechanisms through which ANGPTL7 regulates HSPC expansion, we conducted RNA-seq analysis to profile the global gene expression of ANGPTL7-treated human HSPCs and ANGPTL7-untreated HSPCs (Figure 5A). We identified 330 genes with at least 2-fold expression changes upon ANGPTL7 stimulation (Online Supplementary Table S3). Ingenuity pathway analysis showed that the Wnt signaling that regulates hematopoiesis33–35 was activated in ANGPTL7-treated HSPCs (Figure 5B). We confirmed the results of the RNA-seq analysis by qRT-PCR (Figure 5C). The expression levels of AXIN1,36 AXIN2,37 TCF3,38 LEF1,39 CCND1,40 TCF7,41 and MYC42 that participated in Wnt pathway were increased upon ANGPTL7-stimulation. Interestingly, CXCR4, a critical gene for HSC homing,43 was up-regulated in HSPCs upon ANGPTL7 treatment (Figure 5C). We thus validated whether ANGPTL7 treatment promoted homing by injecting ANGPTL7-treated and ANGPTL7-untreated HSPCs into sub-lethally irradiated NSI mice. Sixteen hours after transplantation, we found that the percentages of human CD34+ cells in both BM and spleens of mice that were transferred with ANGPTL7-stimulated HSPCs were higher than that in other control groups (Figure 5D), suggesting that ANGPTL7 enhanced HSC homing, which was consistent with increased CXCR4 expression. In addition, the expression of HOXB4 that was important for HSC self-renewal44 was also augmented after ANGPTL7 treatment. However, ANGPTL7 treatment did not alter the expression of LAIR1 or LILRB2 that were both reported to be the receptors of ANGPTL7 in HSCs15 (Figure 5C).
Figure 5.
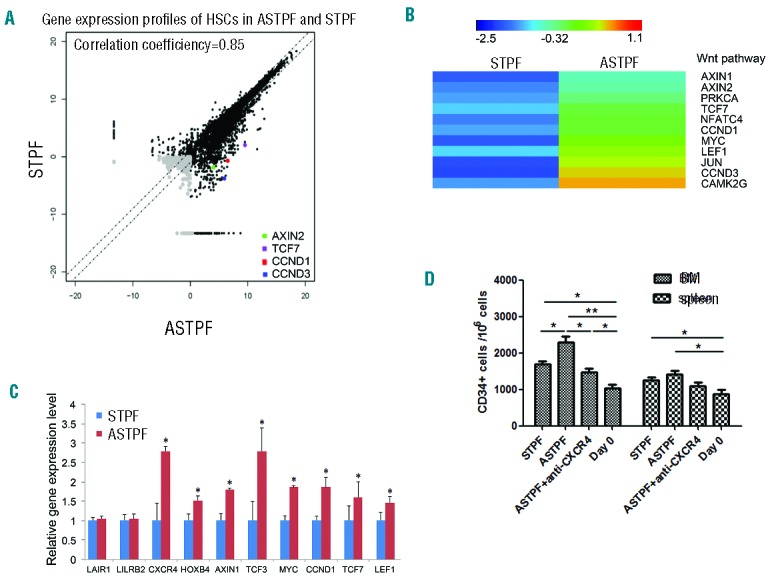
Genes activated in human hematopoietic stem and progenitor cells (HSPCs) by ANGPTL7. (A) CD34+ human umbilical cord blood nucleated cells (hUCBNCs) were cultured in STPF and ASTPF conditions for seven days. Then human HSPCs (Lin-CD34+CD38−) were purified from cultures and subjected for RNA-seq analysis. Pair-wise comparison of poly(A)+ RNA-Seq analysis of the global expression profiles of HSPCs (Lin-CD34+CD38−) in STPF and ASTPF conditions. (B) Hierarchical clustering of representative Wnt signaling genes that were up-regulated with more than 2-fold change in human HSPCs upon ANGPTL7 stimulation. (C) Relative expression levels of selected genes in purified CD34+ hUCBNCs cultured in ASTPF and STPF conditions. The results were normalized to β-ACTIN mRNA levels and represent mean+s.e.m. (n=3). *P<0.05 for ASPTF group versus STPF group. (D) Cord blood CD34+ cells without culture, or cultured in the STPF medium, ASTPF medium or ASTPF medium plus anti-CXCR4 antibody were injected intravenously into NSI mice. Bone marrow (BM) and spleens (SP) of recipient mice were analyzed for the presence of human CD34+ cells by flow cytometry 16 h after transplantation (n=6); *P≤0.05 for bar 1, and bar 3 versus bar 4, bar 1 versus bar 2, bar 2 versus bar 3, bar 5 and bar 6 versus bar8; **P≤0.01 for bar 2 versus bar 4.
To investigate whether ANGPTL7 activated canonical Wnt signaling in human HSPCs, we examined subcellular localization of β-catenin to the nuclei in human HSPCs (an indicator of canonical Wnt signaling activation45) upon ANGPTL7 stimulation. We found that β-catenin migrated to the nuclei after human HSPCs were treated with ANGPTL7 (Figure 6A). In addition, we assessed whether inactivation of Wnt signaling with Wnt inhibitors, IWP2,46 indomethacin (INDO)47,48 or DKK149 abolished ANGPTL7-mediated human HSPC expansion. ANGPTL7 had no effect on hUCBNCs when IWP2 or INDO was added into culture medium (Figure 6B). Conversely, activation of Wnt signaling by CHIR99021 (CHIR) or WNT3A increased the percentages of Lin-CD34+CD38− cells in ANGPTL7-treated hUCBNCs and ANGPTL7-untreated hUCBNCs to similar levels (Figure 6C). Taken together, these data indicate that Wnt signaling participated in ANGPTL7-mediated HSPC expansion.
Figure 6.
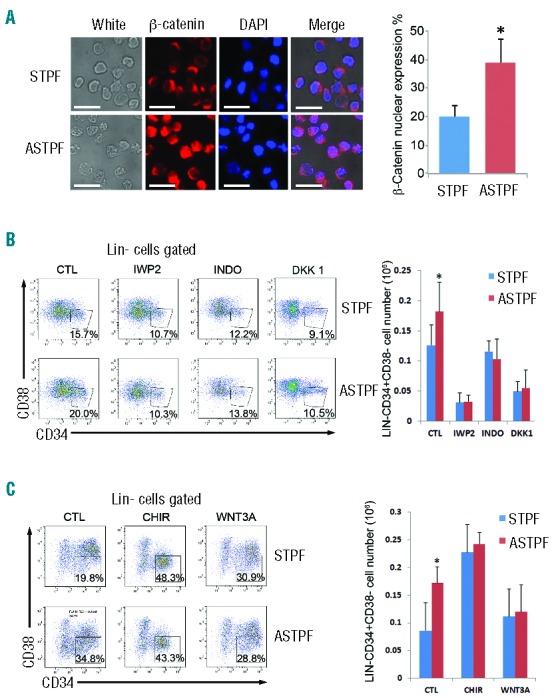
Wnt signaling participated in ANGPTL7-stimulation in hematopoietic stem and progenitor cells (HSPCs). (A) (Left) Immunofluorescence analysis of subcellular localization of β-catenin (red) in CD34+ human HSPCs treated with ANGPTL7 (ASTPF) or without ANGPTL7 (STPF) for 24 h. Scale bar: 100 μm. Nuclei were stained with DAPI (blue). In merged magnification images, the overlap of blue and red indicated the localization of β-catenin in nuclei. (Right) Chart depicting the percentage of β-catenin nuclear expression in CD34+ human HSPCs treated as indicated. Error bars show +/− s.e.m. of triplicates from three independent experiments; *P≤0.05 for bar 1 versus bar 2. (B) (Left) Representative FACS profiles of CD34+ purified human umbilical cord blood nucleated cells (hUCBNCs) cultured in ASTPF and STPF conditions with or without Wnt inhibitors (IWP2, INDO, or DKK1). (Right) Summary of absolute numbers of Lin-CD34+CD38− cells in purified CD34+ hUCBNCs cultured in ASTPF and STPF conditions with or without Wnt inhibitors (IWP2, INDO, or DKK1). Data represent mean+s.e.m. (n=3). *P≤0.05 for bar 1 versus bar 2. (C) (Left) Representative FACS profiles of CD34+ purified hUCBNCs cultured in ASTPF and STPF conditions with or without Wnt activators (CHIR or WNT3A). (Right) Summary of absolute numbers of Lin-CD34+CD38− cells in purified CD34+ hUCBNCs cultured in ASTPF and STPF conditions with or without Wnt activators (CHIR or WNT3A). Data represent mean+s.e.m. (n=3); *P≤0.05 versus bar 2 for bar 1.
Discussion
There are seven known members of the Angptls family sharing limited sequence homology with angiopoietins, which play crucial roles in control of HSC quiescence in the BM niche and angiogenesis.50 Angptls that are abundantly expressed by many types of cells, including those from fetal liver9 and adult BM niche,51 regulate the activities of HSCs and leukemia stem cells.15 Through binding to PirB, which is an inhibitory receptor suppressing activation of differentiated immune cells, Angptls support HSC repopulation and inhibit differentiation of AML cells in mice.15
Angptls are evolutionary conservative, as murine Angptls shows around 80% identity to the human ANGPTLs. Previous studies have shown that several Angptl proteins promote murine HSC expansion.14 However, the effects of ANGPTLs on human HSCs are not clear. Here, we demonstrate the important roles of ANGPTL7 in regulation of human HSPCs. In culture, the number and the colony-forming capacity of human HSPCs were increased upon stimulation of ANGPTL7. Furthermore, ANGPTL7-treated human HSPCs showed stronger repopulation capacity in immunodeficient mice. We speculate that the engraftment efficiency of human HSPCs can be further increased if the recipient mice transgenically over-express ANGPTL7.
In human HSPCs, ANGPTL7 activated several genes that are important for HSCs. We found that CXCR4, which is critical for HSC homing,52 was increased after ANGPTL7 stimulation. The upregulation of CXCR4 might help human HSPCs migrate to BM niche, contributing to the increase of repopulation in xenografts. Indeed, we found that ANGPTL7 promoted the homing of HSPCs into the BM, possibly via the increase of CXCR4 expression. It is also possible that ANGPTL7 promoted human HSPC proliferation by augmenting HOXB4, a strong positive regulator of hematopoietic stem cell (HSC) self-renewal,4 as HOXB4 expression increased in ANGPTL7-treated HSPCs. In agreement with this, Angpt1, a family member of Angptls, up-regulated expression of Hoxb4 in HSCs.53 Recently, Lair1 and PirB were reported to be the receptors of Angptls on HSC;15 nevertheless, we found that ANGPTL7 did not increase the expression of LAIR1 or LILRB2, the human ortholog of PirB, in human HPSCs.
Wnt signaling plays an important role in the regulation of HSC self-renewal.17,54,55 CCND1, CCND2, and CCND3 belong to the D-cyclin family that is important during late embryogenesis and hematopoiesis. Axin2 is a negative regulator of canonical Wnt/TCF signaling, which activates Axin2 expression in HSCs.56 TCF7, which is a downstream target of Wnt signaling, regulates self-renewal of HSPCs.57 We found that CCND1, CCND3, AXIN2, and TCF7 were all up-regulated in ANGPTL7-treated HSPCs. Translocation of β-CATENIN into nucleus in HSPCs following ANGPTL7 stimulation further validated that Wnt signaling was activated by ANGPTL7 treatment. Consistently, we detected upregulation of HOXB4, which was induced following activation of Wnt signaling,17 in ANGPTL7-treated HSPCs. In addition, ANGPTL7 failed to promote HSPC proliferation once Wnt signaling had been disturbed by chemicals. These results suggest that ANGPTL7 affected HSPCs through Wnt signaling. However, further investigations are required to understand whether ANGPTL7 receptors directly activated Wnt signaling and through which receptors ANGPTL7 activated Wnt signaling in HSPCs. We also need to know whether other signaling pathways were activated by ANGPTL7 in HSPCs besides Wnt signaling. These questions are important to judge whether ANGPTL7 can substitute chemical Wnt activators for expansion of human HSPCs in clinical practice58 because artificially elevated Wnt signaling may transform HSPCs into leukemia cells.59
Our identification of ANGPTL7 as growth factors for human HSCs suggests that ANGPTL7 may be useful in ex vivo expansion of human HSC for transplantation therapy or gene therapy protocol in the future.
Acknowledgments
We sincerely thank Dr. Xiaoping Chen and Dr. Jiekai Chen from Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences.
Footnotes
Funding
This study was supported in part by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA01020310), the National Natural Science Foundation of China (Grant No. 81272329, 81327801, 81272211, 81200381, and 81200255), the National Basic Research Program of China (973 Program) (2011CB504004 and 2010CB945500), and the Equipment Function Development & Technology Innovation Project of Chinese Academy of Sciences (Grant No. yg2010080, yg2011082, and yg2012049).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453(7193):306–313. [DOI] [PubMed] [Google Scholar]
- 2.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. [DOI] [PubMed] [Google Scholar]
- 4.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109(1):39–45. [DOI] [PubMed] [Google Scholar]
- 5.Will B, Vogler TO, Bartholdy B, et al. Satb1 regulates the self-renewal of hematopoietic stem cells by promoting quiescence and repressing differentiation commitment. Nat Immunol. 2013;14(5):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalaitzidis D, Sykes SM, Wang Z, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11(3):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chute JP, Saini AA, Chute DJ, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002; 100(13):4433–4439. [DOI] [PubMed] [Google Scholar]
- 8.Oostendorp RA, Harvey KN, Kusadasi N, et al. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99(4):1183–1189. [DOI] [PubMed] [Google Scholar]
- 9.Chou S, Lodish HF. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc Natl Acad Sci USA. 2010;107(17):7799–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu SH, Heiser D, Li L, et al. FLT3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm. Cell Stem Cell. 2012;11(3) 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011; 147(5):1146–1158. [DOI] [PubMed] [Google Scholar]
- 12.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himburg HA, Muramoto GG, Daher P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16(4):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12(2):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J, Umikawa M, Cui C, et al. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485(7400):656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doan PL, Himburg HA, Helms K, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19(3):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003; 423(6938):409–414. [DOI] [PubMed] [Google Scholar]
- 18.Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active beta-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J Immunol. 2006;177(4):2294–2303. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18(12):1778–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18(1):6–14. [DOI] [PubMed] [Google Scholar]
- 21.Comes N, Buie LK, Borras T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells. 2011;16(2):243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oike Y, Tabata M. Angiopoietin-like proteins–potential therapeutic targets for metabolic syndrome and cardiovascular disease. Circ J. 2009;73(12):2192–2197. [DOI] [PubMed] [Google Scholar]
- 23.Akhter S, Rahman MM, Lee HS, Kim HJ, Hong ST. Dynamic roles of angiopoietin-like proteins 1, 2, 3, 4, 6 and 7 in the survival and enhancement of ex vivo expansion of bone-marrow hematopoietic stem cells. Protein Cell. 2013;4(3):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111(7):3415–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Cui Y, Jin J, et al. Translating mRNAs strongly correlate to proteins in a multivariate manner and their translation ratios are phenotype specific. Nucleic Acids Res. 2013;41(9):4743–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong J, Cui Y, Guo J, et al. Resolving chromosome-centric human proteome with translating mRNA analysis: a strategic demonstration. J Proteome Res. 2014; 13(1):50–59. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Y, Yu B, Song S, et al. [Targeted exogenous EGFP gene editing in caprine fetus fibroblasts by zinc-finger nucleases]. Sheng Wu Gong Cheng Xue Bao. 2013; 29(11):1573–1580. [PubMed] [Google Scholar]
- 28.Rehn M, Olsson A, Reckzeh K, et al. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood. 2011;118(6):1534–1543. [DOI] [PubMed] [Google Scholar]
- 29.Huang S, Okamoto K, Yu C, Sinicrope FA. p62/sequestosome-1 up-regulation promotes ABT-263-induced caspase-8 aggregation/activation on the autophagosome. J Biol Chem. 2013;288(47):33654–333666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia W, Gao XJ, Zhang ZD, Yang ZX, Zhang G. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGF. Eur Rev Med Pharmacol Sci. 2013;17(11):1495–1508. [PubMed] [Google Scholar]
- 31.Zhu HL, Wei X, Qu SL, et al. Ischemic post-conditioning protects cardiomyocytes against ischemia/reperfusion injury by inducing MIP2. Exp Mol Med. 2011; 43(8):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011;469(11):3118–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trowbridge JJ, Moon RT, Bhatia M. Hematopoietic stem cell biology: too much of a Wnt thing. Nat Immunol. 2006;7(10): 1021–1023. [DOI] [PubMed] [Google Scholar]
- 35.Eaves CJ. Manipulating hematopoietic stem cell amplification with Wnt. Nat Immunol. 2003;4(6):511–512. [DOI] [PubMed] [Google Scholar]
- 36.Li VS, Ng SS, Boersema PJ, et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. [DOI] [PubMed] [Google Scholar]
- 37.Yu HM, Jerchow B, Sheu TJ, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005; 132(8):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merrill BJ, Pasolli HA, Polak L, et al. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004; 131(2):263–274. [DOI] [PubMed] [Google Scholar]
- 39.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a-/–like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13(6):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96(10):5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verbeek S, Izon D, Hofhuis F, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374(6517):70–74. [DOI] [PubMed] [Google Scholar]
- 42.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382): 1509–1512. [DOI] [PubMed] [Google Scholar]
- 43.Smith-Berdan S, Nguyen A, Hassanein D, et al. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8(1):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson M, Axton RA, Taylor AH, et al. HOXB4 can enhance the differentiation of embryonic stem cells by modulating the hematopoietic niche. Stem Cells. 2012; 30(2):150–160. [DOI] [PubMed] [Google Scholar]
- 45.Behrens J, Jerchow BA, Wurtele M, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–599. [DOI] [PubMed] [Google Scholar]
- 46.Blauwkamp TA, Nigam S, Ardehali R, Weissman IL, Nusse R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun. 2012; 3:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawcroft G, D’Amico M, Albanese C, Markham AF, Pestell RG, Hull MA. Indomethacin induces differential expression of beta-catenin, gamma-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis. 2002; 23(1):107–114. [DOI] [PubMed] [Google Scholar]
- 48.Kapitanovic S, Cacev T, Antica M, et al. Effect of indomethacin on E-cadherin and beta-catenin expression in HT-29 colon cancer cells. Exp Mol Pathol. 2006;80(1): 91–96. [DOI] [PubMed] [Google Scholar]
- 49.Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417(6889):664–667. [DOI] [PubMed] [Google Scholar]
- 50.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. [DOI] [PubMed] [Google Scholar]
- 51.Zheng J, Umikawa M, Zhang S, et al. Ex vivo expanded hematopoietic stem cells overcome the MHC barrier in allogeneic transplantation. Cell Stem Cell. 2011; 9(2):119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91(12):4523–4530. [PubMed] [Google Scholar]
- 53.Gomei Y, Nakamura Y, Yoshihara H, et al. Functional differences between two Tie2 ligands, angiopoietin-1 and -2, in regulation of adult bone marrow hematopoietic stem cells. Exp Hematol. 2010;38(2):82–89. [DOI] [PubMed] [Google Scholar]
- 54.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003; 423(6938):448–452. [DOI] [PubMed] [Google Scholar]
- 55.Tu X, Joeng KS, Nakayama KI, et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007; 12(1):113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugimura R, He XC, Venkatraman A, et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150(2):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huls G, van Es J, Clevers H, de Haan G, van Os R. Loss of Tcf7 diminishes hematopoietic stem/progenitor cell function. Leukemia. 2013;27(7):1613–1614. [DOI] [PubMed] [Google Scholar]
- 58.Rossi L, Lin KK, Boles NC, et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell. 2012;11(3):302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staal FJ, van Dongen JJ, Langerak AW. Novel insights into the development of T-cell acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2007;2(3):176–182. [DOI] [PubMed] [Google Scholar]


