Abstract
In a prospective multicenter phase II study, we evaluated the effect of three courses of vincristine, doxorubicin and dexamethasone followed by high-dose melphalan and autologous stem cell transplantation on an intention-to-treat basis. Sixty-nine newly diagnosed patients with amyloid light chain amyloidosis were included between November 2000 and January 2006: 37 men and 32 women with a median age of 56 years, including 46% of patients with cardiac and 22% of patients with involvement of 3 or 4 organs. Initial results presented in 2008 showed a 4-year overall survival rate of 62% among all the patients, while the 4-year survival rate after transplantation was 78%. Here we report the long-term follow-up data after a median follow up of 115 months of the patients still alive. Median survival of all patients was 96 months from registration and for the transplanted patients ten years from the date of transplantation. Twelve (12%) patients died during induction therapy with vincristine, doxorubicin and dexamethasone, including 8 patients (12%) due to treatment-related mortality. Two patients died within one month following high-dose melphalan. We conclude that vincristine, doxorubicin and dexamethasone should not be applied as induction therapy for intensification in amyloid light chain amyloidosis. However, a 2-step approach consisting of a non-intensive less toxic induction therapy followed by high-dose melphalan and autologous stem cell transplantation may result in extended survival in newly diagnosed patients with amyloid light chain amyloidosis (clinicaltrials.gov identifier: 01207094).
Introduction
Amyloid light chain (AL) amyloidosis is caused by clonal lambda or kappa restricted bone marrow plasma cell proliferation, producing amyloidogenic light chains. Deposition of amyloid fibrils causes progressive organ failure, which eventually leads to death. The primary goal of therapy in systemic amyloidosis is to eliminate the supply of precursor protein and thereby stop further amyloid deposition.1 Many treatment options are currently available, including proteasome inhibitors, immunomodulatory drugs (IMIDS) and high-dose chemotherapy.2 Recently, Palladini et al. reported the results in 259 patients of risk-adapted melphalan-dexamethasone which induced a high rate of hematologic response (76%) and complete remissions in 31% of cases.3 The median overall survival was 7.4 years in the patients that could tolerate high-dose dexamethasone. The role of high-dose melphalan (HDM) and autologous stem cell transplantation (ASCT) is a subject of debate. Although extended survival can be obtained with ASCT,2 data on the efficacy are scarce and almost all data are retrospective.4–6 No survival benefit was found for ASCT in a recent meta-analysis of 12 studies.7 Moreover, in the randomized trial of the French Myeloma Collaborative Group, auto-SCT was not found to improve survival as compared to melphalan-dexamethasone.8 A major limitation of the French study was the high incidence of non-relapse mortality (NRM; 24%) of HDM (the majority of patients received 200 mg/m2) and ASCT given as front-line therapy. We hypothesized that a 2-step approach consisting of induction therapy followed by HDM would improve the overall response rate and subsequent survival. An important aspect of this approach is that effective induction therapy may increase the number of patients eligible for intensification and will reduce NRM. Here, we present the extended follow up of our prospective study after a median follow up of 115 months.
Methods
Study design and end points
In this prospective multicenter phase II study, the effect of three courses of vincristine, doxorubicin, dexamethasone (VAD) administered as rapid infusion9,10 followed by HDM with ASCT was evaluated in AL amyloidosis. Patients who did not meet the eligibility criteria for HDM after VAD went off protocol treatment and were followed for survival. Study end points were efficacy with regard to response rate, overall survival, feasibility and the value of risk factors at diagnosis. The trial was carried out in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Boards of all participating hospitals, and all patients provided written informed consent before inclusion in the study.
Patients and evaluation of organ involvement
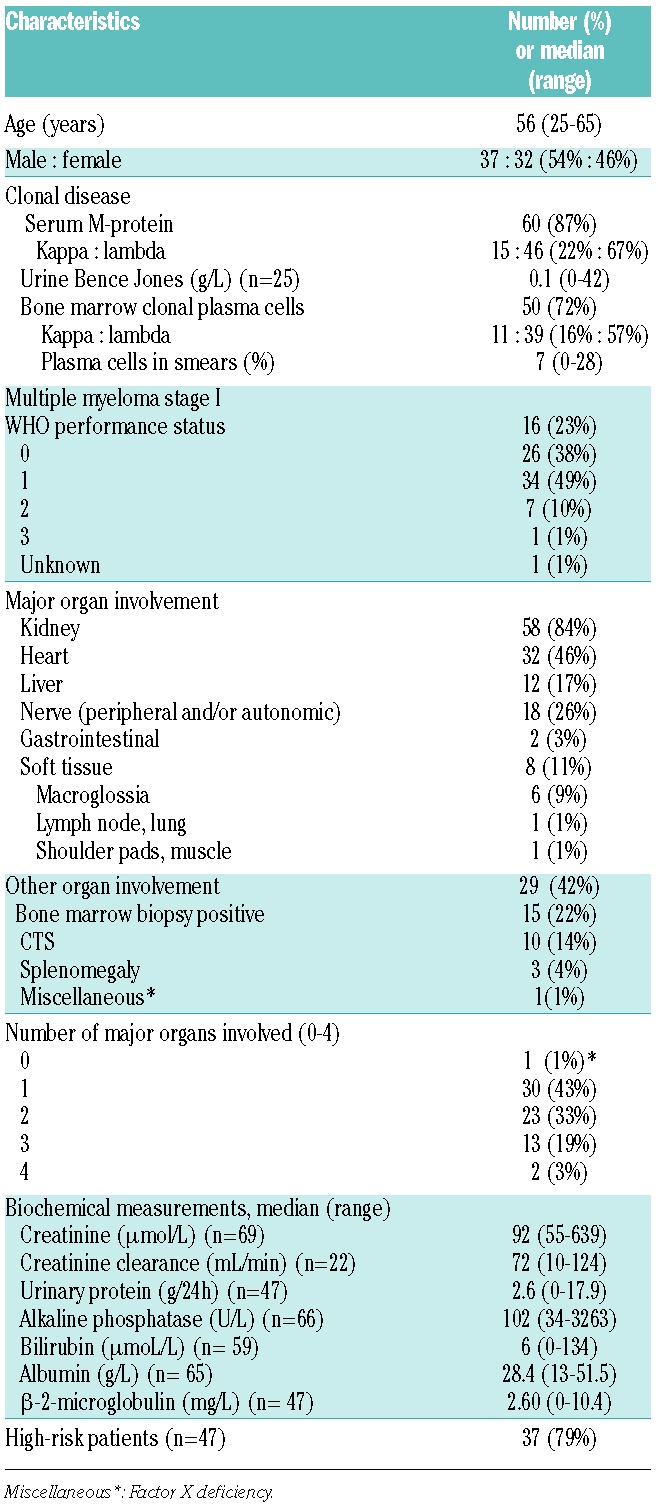
Patients under 66 years of age with biopsy-proven AL amyloidosis and monoclonal gammopathy or Durie & Salmon multiple myeloma stage I were included (n=16, 23%). Patients were either treatment naïve or had previously been treated with a maximum three courses of melphalan and prednisone. Patients with prior malignancy diagnosed less than five years ago [except non-melanoma skin tumors or stage 0 (in situ) cervical carcinoma], other types of amyloidosis, and severe other pulmonary, neurological, psychiatric, cardiac, liver or metabolic diseases not related to AL amyloidosis were excluded. Patients’ characteristics are summarized in Table 1
Table 1.
Characteristics at entry of 69 patients with AL amyloidosis.
Amyloid was diagnosed by apple green birefringence in polarized light of a Congo red-stained biopsy of heart, kidney, liver, gut, nerve, or abdominal fat tissue. All patients with AL amyloidosis should have signs of a clonal plasma cell dyscrasia by immunoelectrophoresis, free light chain in serum or urine, or immunophenotyping of plasma cells in bone marrow. In patients with isolated nephropathy or gastroenteropathy, or an underlying inflammatory condition, AA amyloid had to be excluded by immunohistochemistry. In patients with isolated cardiomyopathy or neuropathy, or a positive family history ATTR amyloid, had to be excluded by immunohistochemistry and by DNA analysis of the TTR gene.
Definition of major organ involvement
Renal involvement: defined as proteinuria more than 0.5 g/24 h or serum creatinine more than 106 μmol/L (or an endogenous creatinine clearance below 90 mL/min) in the absence of other causes of renal disease.
Cardiac involvement: defined as the presence of a mean left ventricular wall thickness on cardiac ultrasound more than 11 mm in the absence of a history of hypertension or valvular heart disease or as the presence of unexplained low voltage (<0.5 mV) on the electrocardiogram or by congestive heart failure as per NYHA heart failure class. Patients who were NYHA class 1 with evidence of cardiac amyloid by echocardiogram or electrocardiogram were categorized as having asymptomatic cardiac involvement. Patients who were NYHA class 2 or higher with evidence of cardiac involvement were categorized as having predominant cardiac involvement (amyloid cardiomyopathy).
Hepatic involvement: defined as hepatomegaly (liver span >15 cm) or alkaline phosphatase more than 200 U/L in the absence of other causes of liver disease. Peripheral polyneuropathy was defined as the typical complaints of sensory or motor neuropathy and confirmed by a neurologist.
Autonomic neuropathy: defined as the presence of orthostatic hypotension (a drop in systolic blood pressure of 20 mm Hg or more and a drop in diastolic blood pressure of 10 mm Hg or more and a rise in heart rate of 10 or less beats per minute during the first three minutes after a change from the supine to the upright posture) or abnormal autonomic testing by the method of Ewing and Clark (score >5 points) not caused by medication.
Gastrointestinal tract: defined by positive biopsy combined with symptoms.
Soft tissue: defined by tongue enlargement, arthropathy, skin, myopathy by biopsy or pseudohypertrophy, lymph node (may be localized), carpal tunnel syndrome all combined with an AL amyloid positive biopsy.
In the final analysis, high-risk patients were defined retrospectively as having one or more of the following major risk factors: cardiac septum thickness 15 mm or more, cardiac ejection fraction 55% or less, serum creatinine more than 177 μmol/L, or serum bilirubin more than 34 μmol/L, while patients were considered low risk if all four risk factors were absent.11
Treatment
The VAD regimen could be modified in case of risk of enhanced toxicity. Vincristine was not used in patients with more than grade 2 neuropathy (neurosensory, neuromotory) and in patients with signs of autonomic neuropathy. Dexamethasone only, according to the above schedule, was given in patients with WHO performance status 4, severe cardiac disease (NYHA grade 4 heart failure or cardiac ejection fraction <45% or mean left ventricular wall thickness on echocardiogram >15 mm or syncope caused by dysrythmia or severe conduction defect), or inadequate liver function probably related to AL amyloidosis, i.e. bilirubin 2.5 times the upper normal value. Supportive care during VAD consisted of pneumococcal and pneumocystis prophylaxis with cotrimoxazol 2×480 mg daily, prophylaxis of infections in case of severe neutropenia (ANC< 0.5×109/L), fluconazole 50 mg daily, and antacids (or otherwise according to local protocols).
Patients were re-evaluated after the third course and judged eligible for stem cell collection and ASCT if WHO performance status was grade 0–2, NYHA class was grade 1–3, cardiac ejection fraction was more than 45%, absence of active infections, absence of severe pulmonary, neurological and psychiatric disease, the serum bilirubin and transaminase concentrations not exceeding 2.5 times the upper reference limit, and normal blood counts (WBC ≥2×109/L and platelets >100×109/L).
Autologous PBSC were collected at day 4 or 5 after granulocyte-colony stimulating factor (G-CSF) at a dose of 10 μg/kg. High-dose melphalan and ASCT started between 2–6 weeks after stem cell collection. Melphalan 100 mg/m2/day was given as rapid infusions on days -3 and -2. In patients with a creatinine clearance of 40 mL/min or less, melphalan 100 mg/m2 was administered on day -2 only.
Treatment response and outcome evaluation
The main end points were hematologic and clinical response, the percentage of patients that ultimately received HDM and ASCT, and overall survival.
Hematologic response was defined as complete response (disappearance of monoclonal protein in blood and urine, and no excess of clonal of plasma cells in bone marrow), partial response (>50% reduction in serum and urine monoclonal proteins), persistence, relapse (recurrence of monoclonal protein after complete response), and progression (doubling of monoclonal protein in serum or urine).
Organ response was retrospectively defined according to the published guidelines as per the Tenth International Symposium on Amyloidosis.11 Grading of toxicity and adverse events was performed using the NCI Common Terminology Criteria for Adverse Events (CTCAE, version 3.0, published December 12, 2003).
Statistical analysis
It was intended that 60 patients would be included in this trial, with interim analyses after the first 20 and 40 patients, with the possibility of discontinuing the trial early when the true percentage of transplanted patients would be 20% or less. The percentage of patients ultimately transplanted was considered to be the most relevant end point for early discontinuation of the study. It was expected that approximately one-third of the patients would receive an autologous transplant. Should this be less than 20%, the study should be considered for early discontinuation. A multiple-stage procedure12 was used with two interim analyses after 20 and 40 evaluable patients with the possibility of early discontinuation. The stopping rules were determined in such a way that the probability of early stopping was small if the true percentage of transplanted patients was 35% or more, while there would be a considerable probability that the trial would be stopped early if the true percentage of transplanted patients was 20% or less.
If the true percentage of transplanted patients were 20%, there would be a probability of 41% to discontinue the trial after reaching 20 evaluable patients, while the probability of (undeserved) early discontinuation would be only 4% if the true percentage of transplanted patients were 35%. So the null hypothesis was that the proportion of transplanted patients would be 20% or less, while the alternative hypothesis is that this proportion would be 35% or more. The actuarial Kaplan-Meier method was used to calculate overall survival (OS) of all patients from registration until death from any cause, and for the transplanted patients OS from the date of transplantation until death. Patients still alive or lost to follow up were censored at the last day they were known to be alive. As exploratory analyses, univariate logistic regression was used to evaluate the association of base-line characteristics (sex, age, number of renal/cardiac/hepatic/neurologic organs involved (0–2 vs. 3–4), and risk score (low vs. high) with the probability of receiving an autologous transplant, while univariate Cox regression analysis was used to evaluate the association of these baseline characteristics with OS from registration and OS from transplantation. P values have not been corrected for multiple testing, and a two-sided significance level α=0.05 was used.
Results
Patients’ characteristics
Seventy patients with AL amyloidosis were included in the study between November 2000 and January 2006. One patient was ineligible (no MGUS, nor MM stage 1) and was excluded from all further analyses. The reason for extending the inclusion to 69 patients (60 intended) was the intention to start a follow-up trial. However, that took longer than expected and it was decided to close the trial on February 1, 2006.
Characteristics of the 69 eligible patients are shown in Table 1. Forty-four patients were in NYHA class 1 (64%), 12 were in class 2 (17%), 10 were in class 3 (14%), 2 were in class 4 (3%), and classification was missing for one. Left ventricular wall thickness was median 13 mm (range 7–30 mm), cardiac ejection fraction was median 61% (range 20%–89%), and low voltage ECG was present in 11 patients (16%). Lung diffusion abnormalities were found in 8 patients (12%). Fifty-eight (84%) had renal involvement; however, none of these patients were dialysis dependent at diagnosis or at transplant. Of 47 patients with available data, 37 (79%) could retrospectively be classified as high-risk patients.11
VAD treatment and stem cell collection preceding HDM and ASCT
Seven patients received pre-treatment before study inclusion. Previous treatment consisted of 1–4 days of dexamethasone in 4 patients, 2–3 cycles of melphalan and prednisolone in 2 patients, and 4 days prednisolone and cyclophosphamide in another patient.
Ten patients received only one cycle of VAD, 5 patients received two cycles of VAD, and 54 patients received three cycles of VAD. The dose of vincristine was reduced in 2 patients and was not given in 15 patients. The dose of doxorubicin was reduced in 3 patients and was not given in 5 patients. The dose of dexamethasone was reduced in 5 patients and was not given in one patient.
Stem cells were mobilized and collected in 46 patients (67%) who could proceed to HDM after VAD induction. Mobilization started median 104 days after registration (range 83–193 days). G-CSF was used in 45 patients and cyclophosphamide in 4 patients. The number of collected stem cells was median 5.1×106 CD34/kg (range 2.1–17.3×CD34/106/kg).
High-dose melphalan and autologous stem cell transplantation
High-dose melphalan was administered median 141 days after registration (range 87–224 days). Thirty-four patients (74%) received full dose (200 mg/m2) melphalan, one received 140 mg/m2, 9 received 100 mg/m2, and 2 patients received 70 mg/m2. The treating physicians’ reasons for the divergent dosages administered were cardiac insufficiency, WHO performance status 2, and renal insufficiency, respectively. Twenty-three patients were not transplanted: 11 patients died during/after VAD, 7 patients were not eligible for mobilization, 3 patients refused, and 2 patients had progressive disease. The stem cell transplants were performed in 10 transplantation centers in the Netherlands and in one Belgian center. Median infused number of stem cells was 3.6×106 CD34/kg (range 0.4–28.0×106 CD34/kg). Median time of hospital care was 21 days (range 0–86 days). Median number of platelet transfusions was 2 (range 0–38) and median number of red blood cell units was 3 (range 0–24). Median hematologic recovery time of ANC more than 1.0×109/L and platelets more than 50×109/L was 17 and 21 days, respectively.
Treatment-related mortality and toxicity
VAD chemotherapy: 12 patients (18%) died during induction therapy with VAD. Causes of death were heart and neurological toxicity in 2 patients, cardiac in 2, infectious in 2, unknown in 2, and amyloidosis in 4 patients. Seven of the 12 patients had cardiac involvement; of these, 5 patients had involvement of 3 major organs. Side-effects CTC grade 2 or over were recorded in 46%, 49%, and 48% of patients during each of the respective three cycles of VAD induction. Side-effects were diverse, but most frequent were gastrointestinal, cardiovascular function, and neurological problems. Infections CTC grade 2 or over were recorded in 13%.
Stem cell mobilizing chemotherapy and leukapheresis: no patient died during or after stem cell mobilization and leukapheresis. Side-effects CTC grade 2 or over were recorded in 15% of patients; infections CTC grade 2 or over were recorded in 9%.
High-dose melphalan and autologous stem cell transplantation: 2 of the 46 patients (4%) died from treatment-related mortality (TRM) within 100 days after administration of HDM; at day 1 from a cardiac cause and one after one month from combined renal and cardiac failure, respectively. Side-effects CTC grade 2 or over were recorded in 87% of patients after HDM and infections CTC grade 2 or over were recorded in 65%. Of the 32 women, 28 (88%) received ASCT, in contrast to only 18 of 37 (49%) male patients (P<0.01). Forty-three of 54 (80%) patients with 0–2 major risk factors were transplanted versus only 3 of 15 (20%) with 3–4 major risk factors (P<0.001). Furthermore, while all 10 patients with low risk were transplanted, only 20 of 37 (54%) with high-risk disease received ASCT (P<0.01; Fisher exact test).11
Treatment response
Survival: median OS of all patients from registration was 90 months; the 1-year, 5-year and 10-year OS were 70%, 54% and 34%, respectively (Figure 1A). All 23 patients who did not receive HDM have died (median OS was 3 months; max. 112). Median follow up after HDM and ASCT in 25 patients still alive is 115 months from inclusion and 111 months from ASCT. The 1-year OS for patients from transplantation was 93%, while the 5-year OS was 74%, and the 10-year OS was 51%. Median survival in this group is 122 months (Figure 1B). Male sex and 3–4 major organs involved were also associated with worse overall survival from registration. Also the survival of the 9 patients with previous treatment (usually dexamethasone or MP) was significantly worse than the 60 patients without previous treatment (median OS 14 vs. 101 months; P=0.004). The outcome of the 16 patients (of whom 9 were transplanted) with AL amyloidosis and stage I MM was not statistically different although their survival tended to be shortened [hazard ratio (HR) 1.87, 95%CI: 0.97–3.60; P=0.07]. Among the transplanted patients, sex, age, 3–4 major organs, high risk were not significantly associated with OS from transplantation; however, the median OS was 93 months in the high-risk patients versus 122 months in low-risk (P=0.12).
Figure 1.
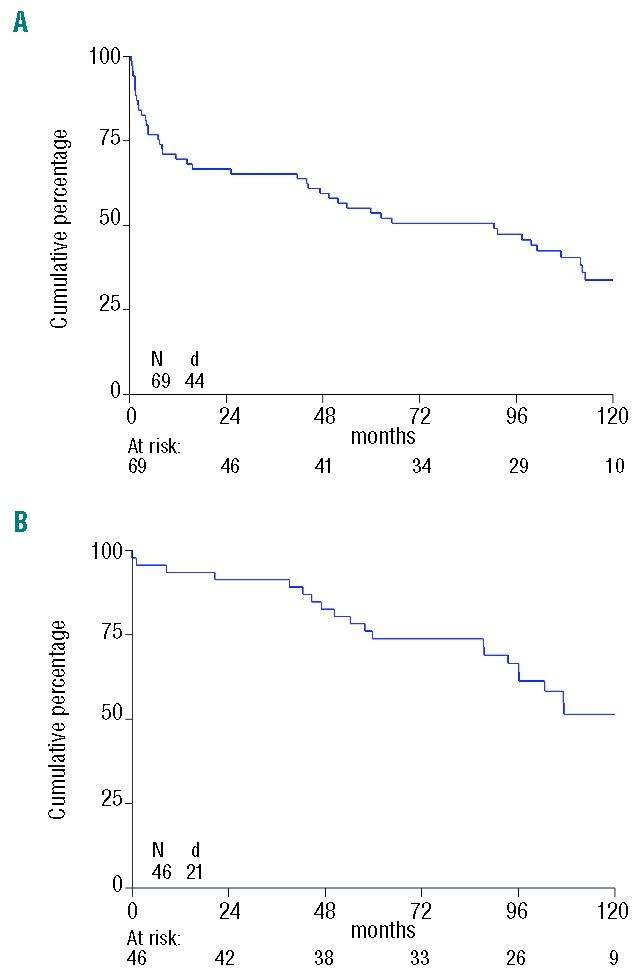
Kaplan-Meier survival curve showing overall survival in months of study group. (A) From registration in the study. (B) From autologous stem cell transplant (ASCT). N: number of patients; d: number of deaths.
Hematologic response of plasma cell disease: overall hematologic response after the third cycle of VAD was achieved in 16 patients (23%) of whom 4 patients (6%) had a complete response. Twenty-one patients (46%) obtained an overall hematologic response after ASCT, including 6 patients (13%) with a CR. Stable persistent disease was seen in 8 patients (17%) and response status was not assessed in 17 patients (37%).
Clinical response: overall clinical improvement after the third cycle of VAD was present in 11 (16%) patients (6 renal, 1 cardiac, 0 hepatic, 4 neuropathic, 1 albumin, and 4 other improvement). Overall clinical improvement after stem cell transplantation was present in 19 (41%) patients (8 renal, 1 cardiac, 2 hepatic, 4 neuropathic, 6 albumin, and 7 other improvement), stabilization was seen in 14 (30%) patients, progression in 10 patients, and unknown in 3 patients.
Discussion
Long-term follow up of our prospective study shows that a 2-step approach of induction therapy followed by ASCT results in prolonged survival in AL amyloidosis; however, patients who qualified for the intensification phase benefitted most. Median survival on an intention-to-treat basis was 90 months and 122 months for the ASCT patients. VAD treatment did not affect stem cell mobilization and 67% of the patients could proceed to HDM and ASCT, which was associated with low TRM (4%). However, initial mortality during VAD was high (18%), indicating toxicity of this regimen in AL amyloidosis. It should be noted, however, that high-risk patients with multi-organ involvement were not excluded from our study. Increased toxicity of VAD as induction to auto-SCT was also found by Perz et al. with 25% of patients experiencing grade 3–4 toxicity and a 7% mortality.13 We must conclude that VAD should not be given to patients with AL amyloidosis.
A limitation of this evaluation is that the study was performed between 2000–2006. We did not have the availability of cardiac biomarkers and serum free light chains, which are now considered the most important prognostic factors for survival, and also since then new consensus response criteria have been introduced.14–17 Other limitations are the high numbers of patients without an assessed hematologic (37%) and clinical response (also 37%), partly explained by the strict requirements of our response criteria, e.g. repeated bone marrow investigation.
The debate about the role of ASCT for AL amyloidosis will continue. Being eligible for ASCT is probably one of the most important favorable prognostic factors.14,18,19 In our prospective trial, median overall survival was 10.1 years in the patients who underwent auto-SCT versus only a median three months for the patients who were not able to undergo the transplant, mainly due to early death.
A major challenge remains the treatment of high-risk disease. Only a minority of our patients with 3–4 risk factors could proceed to ASCT. More effective and less toxic regimens than VAD are nowadays available for the treatment of AL amyloidosis that can also be used as induction before auto-SCT.20–25 In our study, hematologic response was 23% after VAD and 48% after HDM and ASCT. Novel anti-MM regimens, including bortezomib, induce impressive response rates (>90%) of excellent quality which lead to a high percentage of complete remissions (up to 70%) following intensification in younger patients with MM.26,27 It is likely that with such induction regimens high-risk patients are stabilized more rapidly and have a greater chance of improvement of their organ damage and performance status. This will qualify a higher percentage of patients eligible for subsequent intensification. As complete eradication of the amyloid producing clone may be the best prerequisite for improvement of AL amyloid-related symptoms and survival in standard and high-risk patients, we are in favor of continuing a 2-step approach for treatment of younger AL amyloidosis patients. Preliminary data from a study in patients with renal amyloidosis comparing bortezomib/dexamethasone followed by auto-SCT with auto-SCT alone seem to confirm the rationale of such a 2-step approach.28 This design with bortezomib/dexamethasone induction followed by HDM 200 and ASCT is also prospectively explored in a collaborative study of HOVON and the German Amyloidosis Center in Heidelberg, Germany (registered at www.trialregister.nl; NTR3220).
Footnotes
Funding
Supported by a grant from the Dutch Cancer Society: CKVO 2003–03.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Merlini G, Belotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349:583–596. [DOI] [PubMed] [Google Scholar]
- 2.Chaulagain C, Comenzo R. New Insights and Modern Treatment of AL Amyloidosis. Curr Hematol Malig Rep. 2013;8:291–298. [DOI] [PubMed] [Google Scholar]
- 3.Palladini G, Milani P, Foli A, et al. Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: long-term results of a risk-adapted approach. Haematologica. 2014; 99:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parmar S, Kongtim P, Champlin R, et al. Auto-SCT improves survival in systemic light chain amyloidosis: a retrospective analysis with 14-year follow-up. Bone Marrow Transplant. 2014;49:1036–1041. [DOI] [PubMed] [Google Scholar]
- 5.Cordes S, Dispenzieri A, Lacy MQ, et al. Ten-Year Survival After Autologous Stem Cell Transplantationfor Immunoglobulin Light Chain Amyloidosis. Cancer. 2012; 24:6105–6109. [DOI] [PubMed] [Google Scholar]
- 6.Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002;99:4276–4282. [DOI] [PubMed] [Google Scholar]
- 7.Mhaskar R, Kumar A, Behera M, Kharfan-Dabaja MA, Djulbegovic B. Role of high-dose chemotherapy and autologous hematopoietic cell transplantation in primary systemic amyloidosis: a systematic review. Biol Blood Marrow Transplant. 2009;15:893–902. [DOI] [PubMed] [Google Scholar]
- 8.Jaccard A, Moreau P, Leblond V, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–1093. [DOI] [PubMed] [Google Scholar]
- 9.Segeren CM, Sonneveld P, van der Holt B, et al. Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma. Br J Haematol. 1999;105:127–130. [PubMed] [Google Scholar]
- 10.van Gameren II, Hazenberg BP, Jager PL, Smit JW, Vellenga E. AL amyloidosis treated with induction chemotherapy with VAD followed by high dose melphalan and autologous stem cell transplantation. Amyloid. 2002;9:165–174. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th in ternational Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. [DOI] [PubMed] [Google Scholar]
- 12.Schultz JR, Nichol FR, Elfring GL, Weed SD. Multiple-stage procedures for drug screening, Biometrics. 1973;29:293–300. [PubMed] [Google Scholar]
- 13.Dispenzieri A, Lacy MQ, Kyle RA, et al. Eligibility for hematopoietic stem-cell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. J Clin Oncol. 2001;19:3350–3356. [DOI] [PubMed] [Google Scholar]
- 14.Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–3757. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Barassi A, Klersy C, Pacciolla R, Milani P, Sarais G, et al. The combination of high-sensitivity cardiac troponin T (hscTnT) at presentation and changes in N-terminal natriuretic peptide type B (NTproBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116:3426–3430. [DOI] [PubMed] [Google Scholar]
- 16.Palladini G, Dispenzieri A, Gertz MA, et al. New Criteria for Response to Treatment in Immunoglobulin Light Chain Amyloidosis Based on Free Light Chain Measurement and Cardiac Biomarkers: Impact on Survival Outcomes. J Clin Oncol. 2012; 30:4541–4549. [DOI] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Seenithamby K, Lacy MQ, et al. Patients with immunoglobulin light chain amyloidosis undergoing autologous stem cell transplantation have superior outcomes compared with patients with multiple myeloma: a retrospective review from a tertiary referral center. Bone Marrow Transplant. 2013;48:1302–1307. [DOI] [PubMed] [Google Scholar]
- 18.Cibeira MT, Sanchorawala V, Seldin DC, et al. Outcome of AL amyloidosis after high dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;1184346–1184352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar SK, Hayman SR, Buadi FK, et al. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: longterm results from a phase 2 trial. Blood. 2012;119:4860–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastritis E, Terpos E, Roussou M, et al. A phase 1/2 study of lenalidomide with low-dose oral cyclophosphamide and low-dose dexamethasone (RdC) in AL amyloidosis. Blood. 2012;119:5384–5390. [DOI] [PubMed] [Google Scholar]
- 21.Palladini G, Russo P, Milani P, Foli A, Lavatelli F, Nuvolone M, et al. A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica. 2013;98:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinner S, Witteles W, Afghahi A, et al. lenalidomide, melphalan and dexamethasone in an immunoglobulin light chain amyloidosis patient population with high rates of advanced cardiac involvement Haematologica. 2013;98:1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venner CP, Lane T, Foard D, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119:4387–4390. [DOI] [PubMed] [Google Scholar]
- 24.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012; 119:4391–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;20:2946–2955. [DOI] [PubMed] [Google Scholar]
- 26.Roussel M, Lauwers-Cances V, Robillard N, et al. Front-Line Transplantation Program With Lenalidomide, Bortezomib, and Dexamethasone Combination As Induction and Consolidation Followed by Lenalidomide Maintenance in Patients With Multiple Myeloma: A Phase II Study by the Intergroupe Francophone du Myélome. J Clin Oncol. 2014:32;2712–2717. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Wang Q, Chen W, et al. Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC Med. 2014;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]


