Renal impairment is a common presenting feature in multiple myeloma (MM) and an estimated 50% of patients are affected during the course of their disease.1 Moderate and/or severe renal impairment/failure is associated with poorer survival.2–4 Subanalyses of phase III studies in patients with previously untreated or relapsed/refractory MM, along with other studies and analyses, have demonstrated that bortezomib-based treatment is active and well tolerated in patients with renal impairment, and also appears to partly or fully overcome the poor prognosis associated with renal impairment.5–11 In addition, bortezomib has been shown to result in renal impairment reversal in a proportion of patients.6,8–12 These previous bortezomib studies all used the intravenous (IV) route of administration.
Bortezomib is approved for both subcutaneous (SC) and IV administration. Approval of SC administration was based on the phase III MMY-3021 study in 222 patients with relapsed MM, which demonstrated non-inferiority of SC versus IV bortezomib in terms of response rates after four cycles, similar efficacy, and an improved systemic safety profile.13,14 To confirm these findings in the setting of renal impairment, we conducted a post hoc subanalysis to compare activity, response kinetics, long-term outcomes, and safety with SC versus IV bortezomib in patients with moderate-to-severe (CrCl 20–50 mL/min) or mild/no renal impairment (base-line CrCl >50 mL/min).
The design of MMY-3021 has been reported previously.13,14 Patients were randomized 2:1 to receive up to eight 21-day cycles of SC or IV bortezomib 1.3 mg/m2 on days 1, 4, 8, and 21. Two additional cycles were permitted for patients with evolving response. Dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 could be added from cycle 5 onwards. All patients required adequate hematologic, hepatic, and renal function (base-line CrCl ≥20 mL/min). Institutional review boards or independent ethics committees at all participating institutions approved the study, which was conducted in accordance with the provisions of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients provided written informed consent. This study is registered at clinicaltrials.gov identifier: 00722566 and EudraCT (2008-000952-28).
Base-line demographics and disease characteristics of patients in each arm according to renal subgroup are summarized in Table 1. Among patients with CrCl more than 50 mL/min, median number of cycles was eight (range 1–10) in both arms; 65 (57%) and 37 (61%) of patients in the SC and IV subgroups, respectively, received added dexamethasone. However, among patients with CrCl 20–50 mL/min, those in the SC arm received a substantially longer median duration of treatment than those in the IV arm. Median number of cycles of bortezomib received was eight (range 1–10) in the SC arm and three (range 1–9) in the IV arm; 17 (52%) and 2 (15%) patients, respectively, received added dexamethasone. Among patients with CrCl 20–50 mL/min, in the SC subgroup, 16 of 33 patients (48%) discontinued treatment early, prior to receiving eight treatment cycles (4, 1, 2, 3, 4, 1, and 1 patient(s) received 1, 2, 3, 4, 5, 6, and 7 cycles, respectively); discontinuations were due to adverse events (AEs) in 8 patients [24%; 4 peripheral neuropathy (PN)/neuropathic pain, 2 pneumonia, 2 renal impairment/failure], progressive disease in 5 patients (15%), death in 2 patients (6%; pneumonia, sudden death), and patient choice in one patient (3%). In the IV subgroup, 9 of 13 patients (69%) discontinued early (2, 2, 3, 1, and 1 patient(s) received 1, 2, 3, 4, and 5 cycles, respectively); discontinuations were due to AEs in 2 patients (15%; diarrhea, viral conjunctivitis), progressive disease in 4 patients (31%), death in 2 patients (15%; coronary artery disease, myocardial infarction), and patient choice in one patient (8%).
Table 1.
Base-line demographics and disease characteristics of patients receiving SC and IV bortezomib according to renal subgroup (CrCl 20–50 or >50 mL/min).
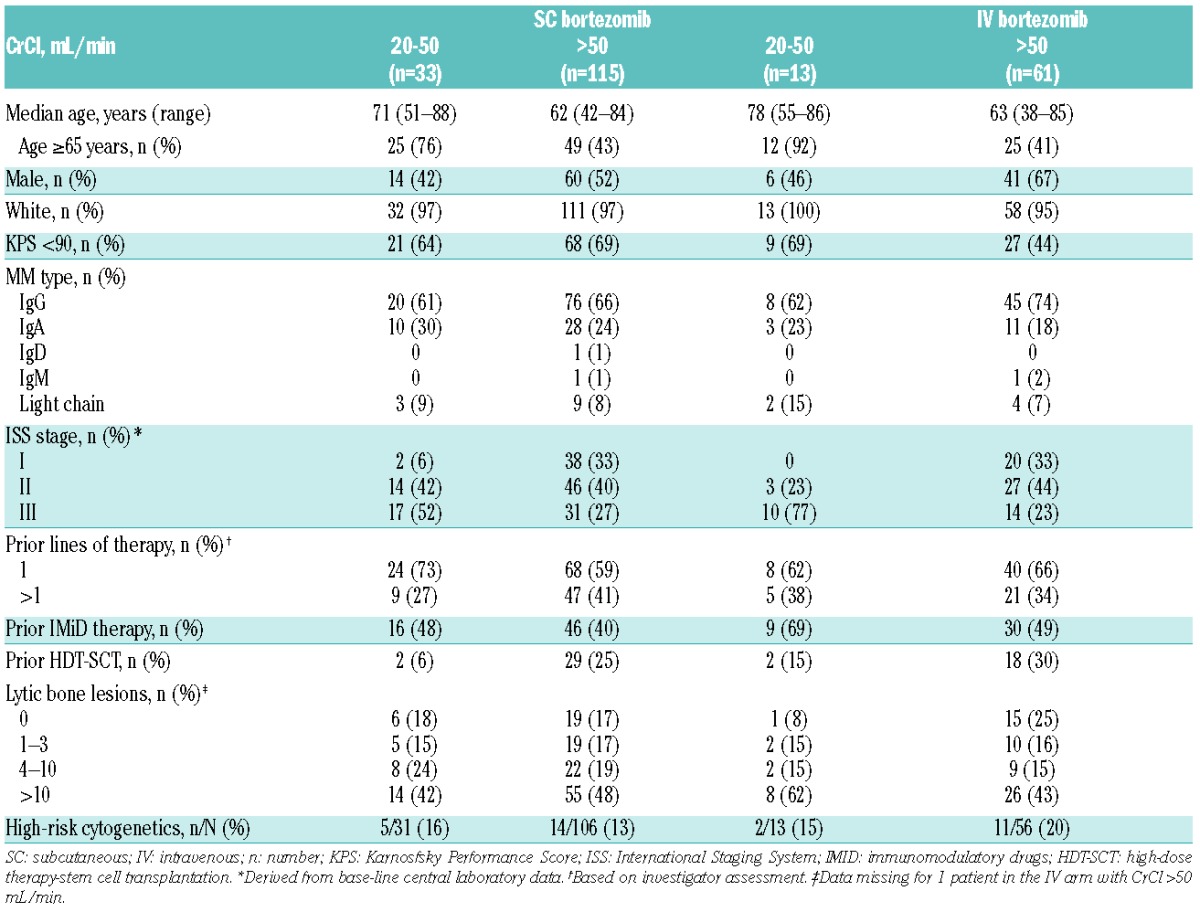
Due to the confounding factors of the substantially shorter duration of treatment, and the very small number of patients (n = 13) in the IV bortezomib subgroup with CrCl 20–50 mL/min, no comparisons of efficacy and safety can be made between SC and IV bortezomib in these patients. The shorter treatment exposure may have been associated with limited efficacy but also a substantially reduced safety profile, and the small number also prevents meaningful statistical comparison.
Response rates (across all treatment with bortezomib ± dexamethasone) and outcomes across renal subgroups are summarized in Table 2. Response rates were generally similar between SC and IV bortezomib in patients with CrCl more than 50 mL/min; the relative risk of response was 0.92 (95%CI: 0.69, 1.22; unstratified Mantel-Haenszel estimate). Overall response rate also appeared to be similar with SC bortezomib in patients with CrCl 20–50 mL/min and CrCl more than 50 mL/min (53% and 52%, respectively). Of importance in the patient population with CrCl 20–50 mL/min, time to response was rapid via both routes of bortezomib administration; a finding also seen in the subgroups of patients with CrCl more than 50 mL/min (Table 2). In addition, among patients with base-line CrCl 20–50 mL/min, clinical benefit in terms of renal impairment reversal (to CrCl >60 mL/min) was reported in 10 (30%) SC bortezomib patients and 2 (15%) IV bortezomib patients.
Table 2.
Best confirmed response rates and time-to-event outcomes with SC and IV bortezomib (± dexamethasone) according to renal subgroup (CrCl 20–50 or >50 mL/min), in response-evaluable patients (those with measurable, secretory multiple myeloma who received ≥1 dose of bortezomib; response rates and time to response) or the intent-to-treat population (other outcomes).
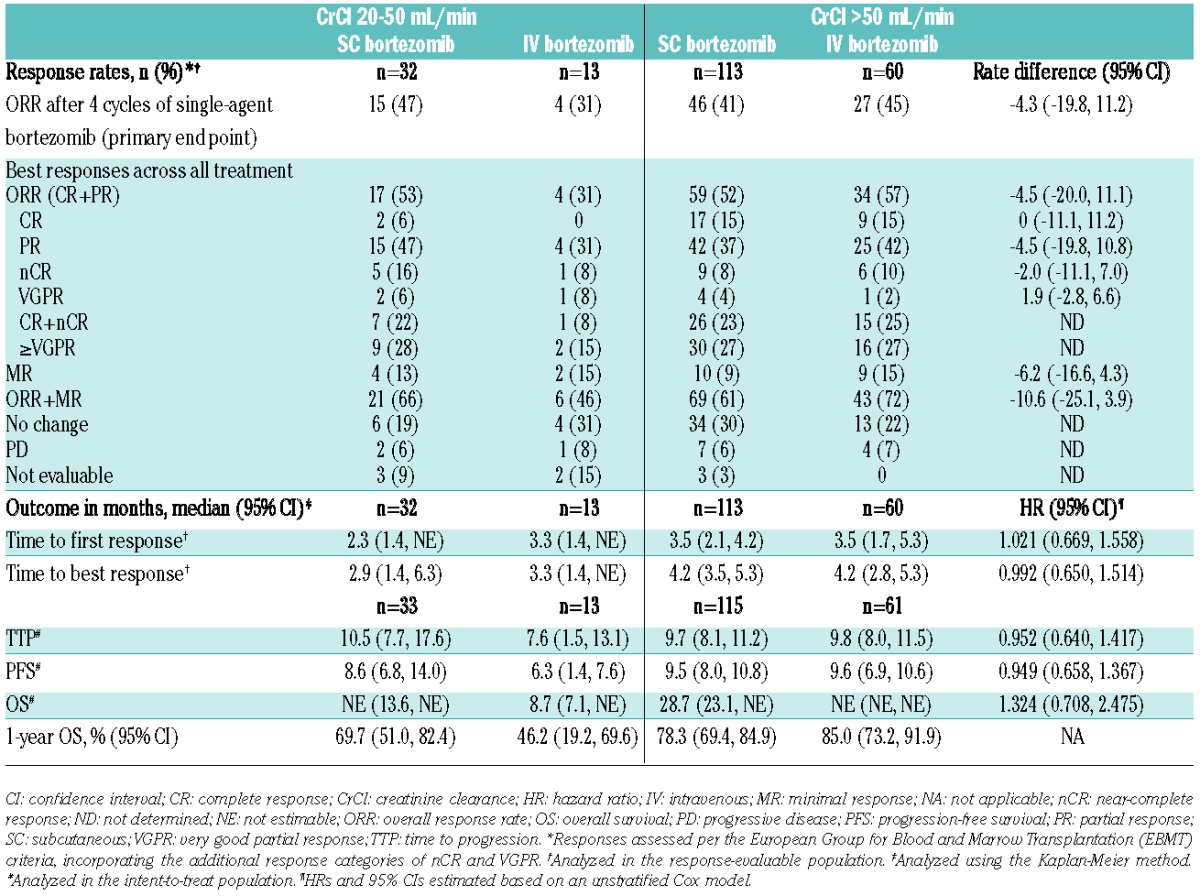
Overall median follow up was 17.3 months in the SC arm and 17.8 months in IV arm. In the subgroups of patients with CrCl more than 50 mL/min, long-term outcomes including time to progression (TTP), progression-free survival (PFS), and overall survival (OS) were generally similar between SC and IV bortezomib (Table 2). Median TTP (10.5 and 9.7 months) and median PFS (8.6 and 9.5 months) also appeared to be similar between patients with CrCl 20–50 mL/min and CrCl more than 50 mL/min who received SC bortezomib. However, OS with SC bortezomib appeared somewhat shorter in patients with CrCl 20–50 mL/min compared with patients with CrCl more than 50 mL/min (1-year OS: 69.7% and 78.3%, respectively). These data suggest that SC bortezomib may possibly partly overcome the negative prognostic impact of renal impairment, while the apparent trend to shorter OS may be influenced by the use of subsequent therapies that do not sustain the effect of SC bortezomib in renally impaired patients.
Safety profiles according to renal subgroup are summarized in Table 3. The safety profiles of SC and IV bortezomib (± dexamethasone) in patients with CrCl more than 50 mL/min reflected the previously reported findings for the overall study population,13,14 with the same relative differences between arms. Findings showed an improved systemic safety profile with SC versus IV bortezomib in patients with CrCl more than 50 mL/min, with the most common AEs including PN not elsewhere classified (NEC; high-level term) (36% vs. 57%), anemia (33% vs. 38%), and thrombocytopenia (33% vs. 41%). Overall, 54% versus 69% of patients with CrCl more than 50 mL/min receiving SC versus IV bortezomib reported grade 3 or more AEs, which included 5% versus 18% PN NEC. Serious AEs were reported in 35% versus 33% of patients, 22% versus 28% of patients discontinued due to AEs, and 3% versus 7% of patients died due to AEs with SC versus IV bortezomib. As noted, in patients with CrCl 20–50 mL/min, comparisons of the safety profiles of SC and IV bortezomib are confounded; the relative safety profiles differed compared with in the overall study population, likely associated with the difference in treatment exposure.
Table 3.
Safety profiles of SC and IV bortezomib (± dexamethasone) by renal subgroup, including common adverse events (all grades, ≥10% of patients overall in either arm; grade ≥3, ≥5% overall in either arm), in the safety population (all patients who received ≥1 dose of bortezomib), assessed per the National Cancer Institute’s Common Terminology Criteria for Adverse Events v.3.0.
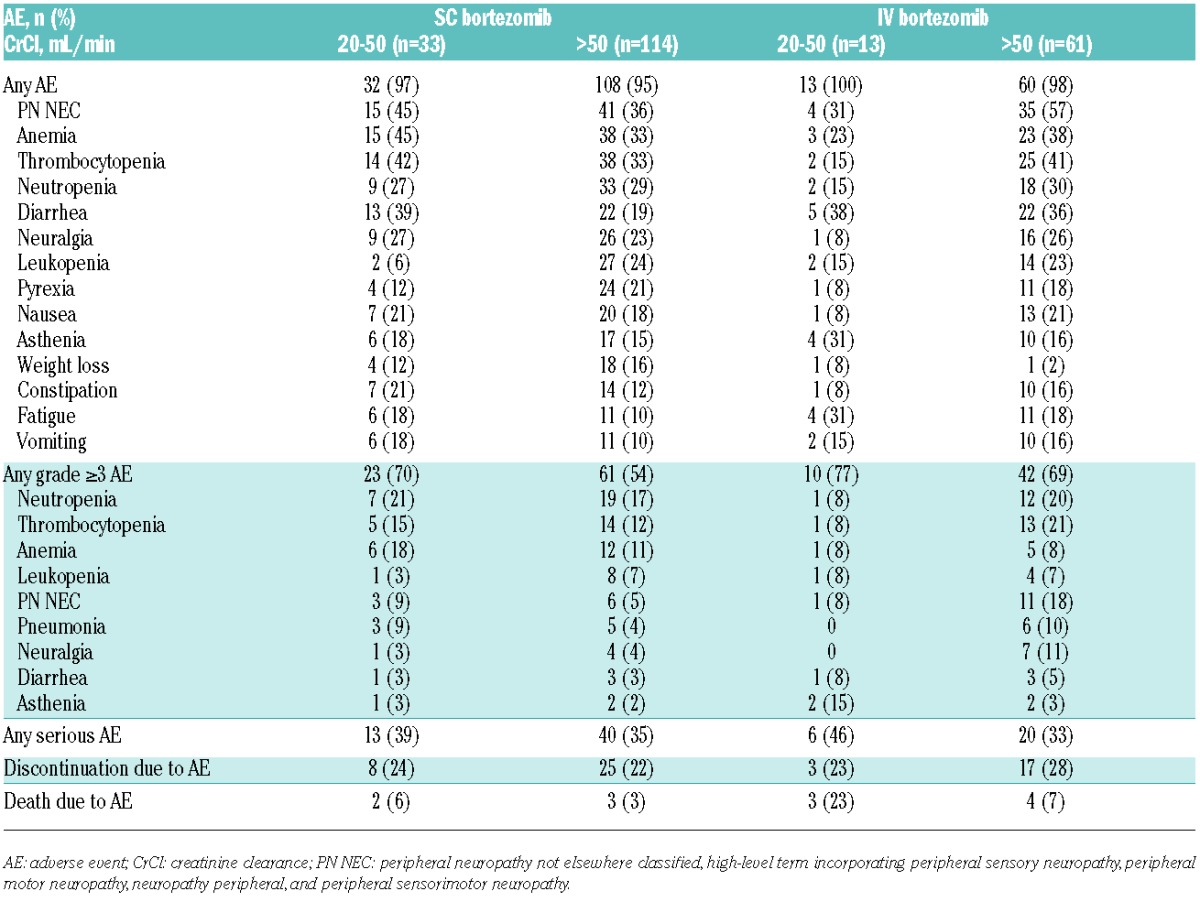
Incidences of common AEs with SC bortezomib appeared generally similar or numerically higher in patients with CrCl 20–50 mL/min versus more than 50 mL/min, with PN NEC, anemia, thrombocytopenia, and diarrhea appearing more frequent (Table 3). In addition, there appeared a higher overall rate of grade 3 or more AEs in patients with CrCl 20–50 mL/min. Within the SC arm, 3 (9%) patients with CrCl 20–50 mL/min and 6 (5%) patients with CrCl more than 50 mL/min had local reactions to SC administration that were reported as AEs. Rates of serious AEs (39% and 35%) and discontinuations due to AEs (24% and 22%) with SC bortezomib appeared similar between CrCl 20–50 mL/min and CrCl more than 50 mL/min subgroups.
The findings of this subanalysis indicate that SC bortezomib appears effective in patients with renal impairment, resulting in a beneficial impact in terms of response rates and long-term outcomes, including TTP, PFS, and OS. Of clinical importance, SC bortezomib resulted in a 30% rate of renal impairment reversal in this patient population. Moreover, across renal subgroups, SC bortezomib resulted in a rapid onset of response. Notably, the activity and safety of SC bortezomib in MM patients with renal impairment demonstrated in these analyses appear to reflect previously reported data on IV bortezomib-based therapy in this setting.5–11 Furthermore, the findings from this post hoc subgroup analysis of the MMY-3021 study add to the increasing level of recent data on SC bortezomib in previously untreated and relapsed/refractory MM that support the feasibility and utility of this route of administration. For example, similar efficacy and a substantially lower rate of PN were seen with SC compared with IV bortezomib in the bortezomib-thalidomide-dexamethasone regimen when used as pre-transplant induction therapy in previously untreated patients.15
In conclusion, the findings from this subgroup analysis, combined with data from other recent studies, support the use of SC bortezomib across the MM treatment algorithm in settings in which bortezomib use is established, including as a feasible and effective treatment that can enable renal function reversal in relapsed MM patients with moderate-to-severe renal impairment.
Acknowledgments
The authors would like to acknowledge the writing assistance of Steve Hill of FireKite, part of KnowledgePoint360, an Ashfield Company, during the development of this publication, which was funded by Millennium: The Takeda Oncology Company, and Janssen Global Services, LLC, and complied with Good Publication Practice 2 ethical guidelines (Graf et al., BMJ 2009).
Footnotes
Trial registrations: the ClinicalTrials.gov registration number of the MMY-3021 study is NCT00722566.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res. 2012;18(8):2145–2163. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25(1):195–200. [DOI] [PubMed] [Google Scholar]
- 3.Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48(2):337–341. [DOI] [PubMed] [Google Scholar]
- 5.Blade J, Sonneveld P, San Miguel JF, et al. Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clin Lymphoma Myeloma. 2008;8(6):352–355. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36):6086–6093. [DOI] [PubMed] [Google Scholar]
- 7.San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;22(4):842–849. [DOI] [PubMed] [Google Scholar]
- 8.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9(4):302–306. [DOI] [PubMed] [Google Scholar]
- 10.Chanan-Khan AA, Kaufman JL, Mehta J, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109(6):2604–2606. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010;28(30):4635–4641. [DOI] [PubMed] [Google Scholar]
- 12.Roussou M, Kastritis E, Christoulas D, et al. Reversibility of renal failure in newly diagnosed patients with multiple myeloma and the role of novel agents. Leuk Res. 2010;34(10):1395–1397. [DOI] [PubMed] [Google Scholar]
- 13.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]
- 14.Arnulf B, Pylypenko H, Grosicki S, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97(12):1925–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok A, Mocquard J, Bourcier J, et al. Subcutaneous bortezomib incorporated into the bortezomib-thalidomide-dexamethasone regimen as part of front-line therapy in the context of autologous stem cell transplantation for multiple myeloma. Haematologica. 2014; 99(3):e33–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]


