Abstract
We describe a new rapid, low cost, and scalable method for purification of various recombinant adeno-associated viruses (rAAVs) from the lysates of producer cells of either mammalian or insect origin. The method takes advantage of two general biochemical properties of all characterized AAV serotypes: (i) low isoelectric point of a capsid and (ii) relative biological stability of the viral particle in the acidic environment. A simple and rapid clarification of cell lysate toremove the bulk of proteins and DNA is accomplished by utilizing inexpensive off-the-shelf reagents such as sodium citrate and citric acid. After the low-speed centrifugation step, the supernatant is subjected to cation exchange chromatography via sulfopropyl (SP) column. The eluted virus may then be further concentrated by either centrifugal spin devices or tangential flow filtration yielding material of high titer and Good Manufacturing Practice (GMP) grade biochemical purity. The protocol is validated for rAAV serotypes 2, 8, and 9. The described method makes rAAV vector technology readily available for the low budget research laboratories and could be easily adapted for a large scale GMP production format.
Introduction
Recombinant adeno-associated virus (rAAV) vectors have emerged as one of the most versatile and successful gene therapy delivery vehicles. A number of recent clinical trials had impressive clinical outcomes1,2,3,4,5,6 and patients diagnosed with lipoprotein lipase deficiency will now have an option to be treated with Glybera, the first rAAV-based drug to win the regulatory approval of the European Medicines Agency. However, even though the industry is poised for the expansion into several application areas represented by orphan diseases, a simple and scalable rAAV production technology is still lacking.
The ever growing rAAV vector toolbox, in addition to many natural AAV serotypes, now includes numerous AAV capsid mutants derived from combinatorial libraries or through rational engineering.5,7 To purify all these divergent AAV variants, buoyant density gradients such as CsCl, or iso-osmotic medium iodixanol discontinuous gradients8 are routinely used. Although quite useful in a laboratory setting, these procedures are neither scalable nor easily adapted for Good Manufacturing Practice (GMP) protocols. In this regard, the more promising approach incorporates chromatography steps, either affinity, hydrophobic, or ion-exchange, depending on the biochemical properties of a particular serotype. For example, heparin affinity chromatography based on interaction with heparan sulfate proteoglycan has been successfully applied to rAAV2,8,9 while mucin affinity chromatography can be used for rAAV5 purification because it binds to sialic acid.10 Many successful examples of one- or two-step ion-exchange chromatography purification have been reported for rAAV serotypes 1, 2, 4, 5, and 8.11,12,13,14,15 More recently, an affinity media incorporating an anti-AAV VHH ligand, a single-domain camelid antibody derivative, was utilized to purify serotypes 1, 2, 3, and 5.16 In spite of these documented successful examples, some AAV serotypes, such as rAAV9, are refractory to conventional chromatography procedures and require significant effort and exceptional laboratory skills for their purification.17
In this report, we describe an efficient and reproducible protocol based on a partial purification of the initial crude lysate by flocculation of cell debris under low pH conditions, followed by one-step cation-exchange chromatography. The flocculation step eliminates the bulk of the contaminating protein and DNA allowing for quantitative AAV binding to, and subsequent elution from the resin. The method could be applied to several serotypes and for vectors purified from both mammalian and insect cell production systems.
Results
Testing vector infectivity and stability over pH range
AAV9 was selected for the development of all the experimental steps since this is one of the most challenging AAV serotypes to purify. Its been observed previously that the unique N-terminus of the AAV capsid viral protein VP1 (VP1u) undergoes a reversible pH-induced unfolding/refolding process, complemented by a loss/gain of α-helical structure which does not disrupt the capsid integrity.18 To test whether these pH-induced structural changes affect vector infectivity, we exposed rAAV9 vector to citrate and phosphate buffers with pH ranging from pH2.5 to pH8 for periods of time of up to 3 weeks followed by an infectivity assay. A physiological solution, Lactated Ringer (pH6.42) was used as a positive control buffer. The in vitro infectivity of the rAAV-GFP was assayed before and after pH exposure for a specified period of time. As shown in Figure 1a, after a short 2-hour exposure, the infectivity of the virus does not change in any of the buffers over the whole pH range tested. After 24-hour incubation, however, there was a tenfold reduction in infectivity which was more pronounced at the range of pH5-6, followed by another log reduction after exposure for 3 weeks. Surprisingly, however, the lower pH range was less deleterious thus providing experimental validation for the low pH-induced flocculation step. Obviously, other components of the buffer provide structural stability as well because Lactated Ringer appears to better sustain higher virus infectivity over 3 weeks, the period of time tested.
Figure 1.
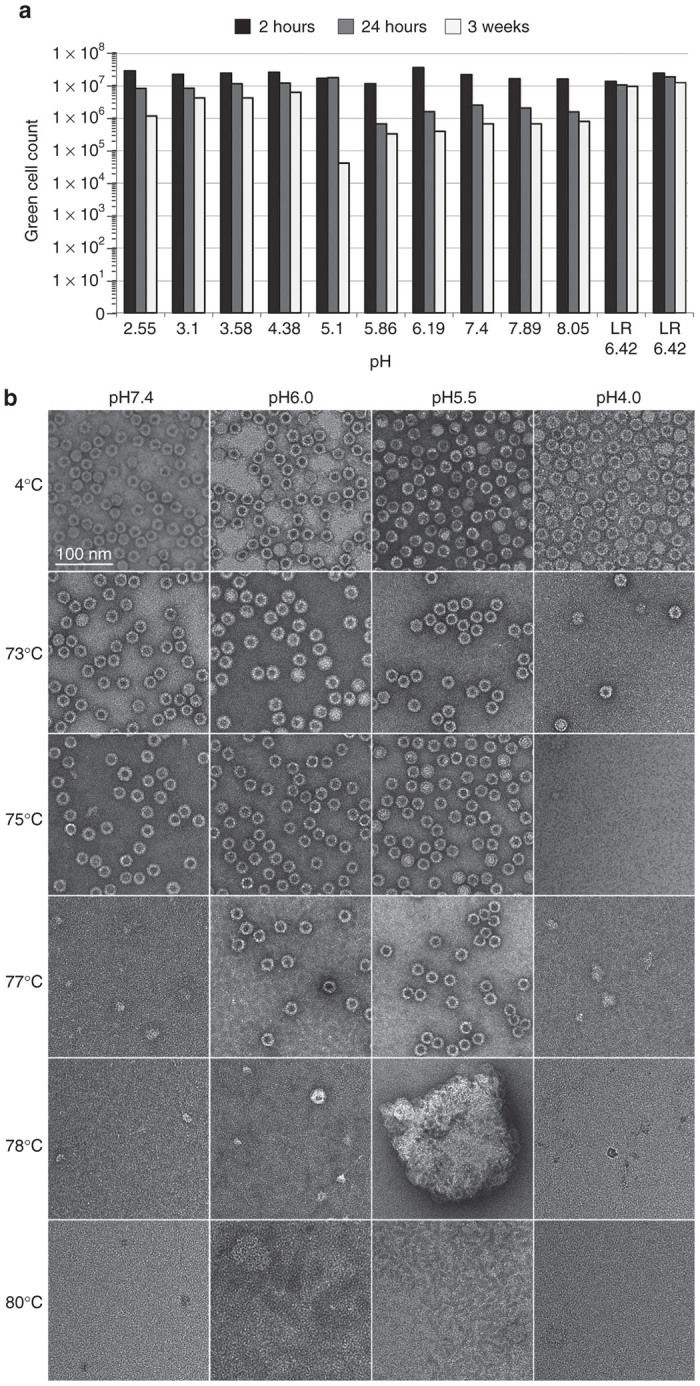
rAAV9 stability testing. (a) Infectious titer assay: iodixanol-purified rAAV9-GFP was exposed to sodium citrate/phosphate buffers in the range of pH2.55–8.05, at room temperature for the time period of 2 hours, 24 hours, or 3 weeks. After incubation, an aliquot was diluted in Lactate Ringer solution and used to infect C12 cells coinfected with Ad5 (multiplicity of infection of 5). GFP (+) cells were visually scored at 48 hours after infection. (b) EM studies: viral capsid were visualized after treatment at different temperatures (rows) in buffers of different pHs (columns).
Independently, the impact of pH on the structural stability of rAAV9-GFP was tested by a dot-blot immunoassay. Iodixanol-purified rAAV9-GFP, in citrate-phosphate buffer at pH 7.4, 6.0, 5.5, or 4.0, was exposed to the range of temperatures 4–100 °C and blotted for antibodies to detect intact capsids (HL-2370), denatured capsids (B1), and VP1u externalization (A1) (data not shown). Based on the observations, the temperature range was narrowed to 70–80 °C to more accurately determine capsid stability and denaturation temperature. Table 1 lists the last temperature at which intact capsids were detected (HL-2370) and the first temperature at which denatured capsids were detected (B1 and A1). At neutral pH (pH 7.4) the capsid is intact at 75 °C but not 78 °C. Low pH (pH 4.0) induced a loss in capsid stability since particles were only intact to 73 °C. However, intermediate acidity (pH 5.5–6.0) increased the stability of the capsid to 78 °C. Interestingly, there appears to be a reverse correlation between increased capsid stability in the pH 5.5–6.0 range and the reduced infectivity (Figure 1a) in the same pH range which may be due to slower uncoating of the capsid within a cell.
Table 1. AAV9 structural stability dot-blot immunoassaya.

The structural stability of the rAAV9-GFP virus was further validated using electron microscopy (EM). As with the dot-blot assay, capsids in citrate-phosphate buffer at pH 7.4, 6.0, 5.5, or 4.0 were exposed to temperatures between 70–80 °C and visualized using negative-stain EM (Figure 1b). Intact capsids were detected up to 73 °C for pH 4.0, 75 °C for pH 7.4, and 78 °C for pH 5.5 and 6.0. However, above these respective temperatures the capsids were denatured and no longer visible. In addition, for capsids at pH 6.0, while stable at 77 °C, when heated to 78 °C there was a decrease in the number of intact particles visible and an increase in broken particles indicating the capsids are not stable and are denaturing at this point. These EM results agree with the dot-blot data and together these observations demonstrate a very sharp transition temperature in capsid stability, which is otherwise very high for the rAAV9-GFP capsids.
SP column chromatography
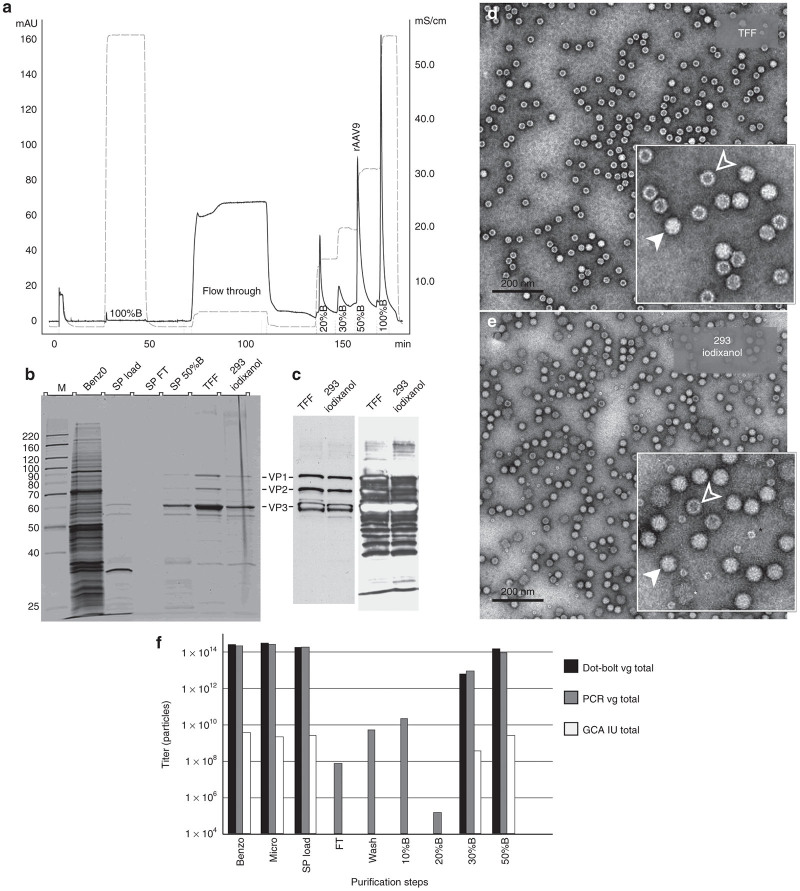
AAV9. AAV9-based vectors exhibit distinctive properties such as delayed blood clearance, ability to cross blood–brain barrier, and targeting cardiac muscle with a higher tropism. This serotype, however, proved to be challenging to purify using standard chromatography procedures. Followed flocculation step, we tested whether AAV9 remaining in the supernatant was capable of binding to an ion-exchange resin (Figure 2a). AKTA FPLC (GE Health Care) system had been utilized. After the microfluidization step, the crude lysate contained 1,288 mg of protein, the amount of which was reduced down to 14.8 mg of total protein in the supernatant fraction applied to the column. Thus, low pH flocculation followed by low-speed centrifugation disposes of 98.85% of total protein in the crude lysate making AAV9 remaining in the supernatant (Figure 2b, lane marked “SP load”) highly amenable to cation-exchange chromatography. AAV9 quantitatively binds to sulfopropyl (SP) resin (Figure 2b, lane “SP FT”), and is eluted with higher pH (measured pH4.6) and sodium ion concentration (0.25 mol/l NaCl) with very little contaminating proteins (Figure 2b, lane “SP 50% B”). These low molecular weight were effectively removed during the subsequent concentration/ultrafiltration step through 150 kDa cut-off tangential flow filtration (TFF) concentrator (Figure 2b, lane “TFF”). There is little difference between the SP-purified/TFF-concentrated fraction and the rAAV9 sample purified by the “standard” protocol using an iodixanol gradient (Figure 2b, lane “293 iodixanol”). The additional faint bands in the purified sample seen on the silver-stained gel are VP-derived peptides (Figure 2c, right panel). These peptides apparently are the products of capsid protein autocleavage;19 therefore, there is no evidence that AAV vectors purified by the current protocol incorporate higher ratios of the cleaved peptides compared to the “standard” iodixanol method. EM examination of rAAV9 capsid purified by SP column chromatography (Figure 2d) reveals a higher degree of purity and integrity of the sample as compared to the one purified by iodixanol gradient (Figure 2e).
Figure 2.
Summary of rAAV9 purification. (a) SP column chromatography profile of rAAV9 purification. Solid line indicates OD280(mAU), dashed line – measured electric conductance (mS/cm). (b) Silver-stain SDS protein gel analysis of rAAV9 purification steps. Fractions are “Benzo”: crude lysate treated with benzonase; “SP load”: supernatant after flocculation/centrifugation step; “SP FT”: SP column flow through; “SP 50%B”: fraction containing rAAV9 eluted from the column with the buffer mixture containing 50% buffer B (see the Materials and Methods section for details); “TFF”: tangential flow filtration fraction (final purified rAAV9 stock); “293 iodixanol”: positive control, rAAV9 purified by “conventional” iodixanol buoyant density gradient. (c) Western blotting analysis of the final purified rAAV9 fraction (TFF), side-by-side with positive control; left panel: adjusted gel load sample volumes; right panel: same sample volumes as in the b. Higher sample loads allow visualizing VP-derived, B1-immunoreactive peptides but result in “burnt out” effect in the X-ray film for some peptides (e.g., VP3).(d,e) Electron microscopy. Negative stain images of rAAV9 purified (d) using the sodium citrate method, or (e) using discontinuous step gradient of iodixanol.8 The inserts in the lower right hand corner are close-up images of the virus capsids. Filled arrowheads point at the DNA-containing particles; empty arrowheads: at the empty capsids; black asterisk indicates proteasome; white asterisk indicates deformed AAV particle. (f) Graphical representation of the rAAV9-GFP yields at all purification steps, as titered for the genome-containing particles (dot-blot assay, black bars, or PCR assay, gray bars); and infectious particles (green cell assay, GCA, white bars). The missing bars for some steps indicate the titers below 10E4, the Y-axis plotting scale. min, minute.
The total yield in the peak SP 50% B fraction constituted 84% of the SP load (by dot-blot assay), or 53% (by PCR assay), or 93% (by green cell assay or GCA) (Figure 2f). Thus, in several simple steps, rAAV9-GFP was purified to a highest degree of purity while retaining more than 90% yield in a TFF-concentrated final stock.
AAV2. AAV2 is one of the most utilized and studied serotypes providing structural platform for rationally designed vectors and combinatorial capsid libraries. Using similar to AAV9 conditions, we have attempted to purify AAV2-GFP produced in HEK 293 cells (Figure 3a). AAV2 capsid proteins appear to constitute one of the major protein components of the supernatant after the flocculation/centrifugation steps since VP3 was readily identifiable on a silver stained gel (Figure 3b, lane “SP load”). However, a significant fraction of the virus was lost in the flow through (Figure 3b, lane “SP FT”). Consistent with weak binding at this pH conditions, the elution of AAV2 was initiated at lower Na+ (50–100 mmol/l). The total yield in the peak SP 20%B fraction constituted 5.2% of the SP load (by dot-blot assay), or 6.2% (by PCR assay), or 11.2% (by GCA) (Figure 3d). The eluted and concentrated vector, however, was remarkably pure (Figure 3b, lane “TFF”), with no detectable contaminating proteins identified at this sample load, with the exception of B1-immunoreactive peptides, the product of capsid autocleavage (Figure 3c, lane “293 iodixanol”).
Figure 3.

Summary of rAAV2 purification. (a) SP column chromatography profile of rAAV2 purification. Solid line: OD280(mAU), dashed line: measured electric conductance (mS/cm). (b) Silver-stain SDS protein gel analysis of rAAV2 purification steps. Fractions are as following: “Benzo”: crude lysate treated with benzonase; “SP load”: supernatant after flocculation/centrifugation step; “SP FT”: SP column flow through; “SP 10%B”: fraction containing rAAV2 eluted from the column with the buffer mixture containing 10% buffer B (see the Materials and Methods section for details); “SP 20%B”: 20% buffer B; “TFF”: tangential flow filtration fraction (final purified rAAV2 stock); “293 iodixanol”: positive control, rAAV2 purified by “conventional” iodixanol buoyant density gradient. (c) Western blotting analysis of the final purified rAAV2 fraction (TFF), side-by-side with positive control; left panel: adjusted gel load sample volumes; right panel: same sample volumes as in b. (d) Graphical representation of the rAAV2-GFP yields at all purification steps, as titered for the genome-containing particles (dot-blot assay, black bars, or PCR assay, gray bars); and infectious particles (green cell assay, GCA, white bars). min, minute.
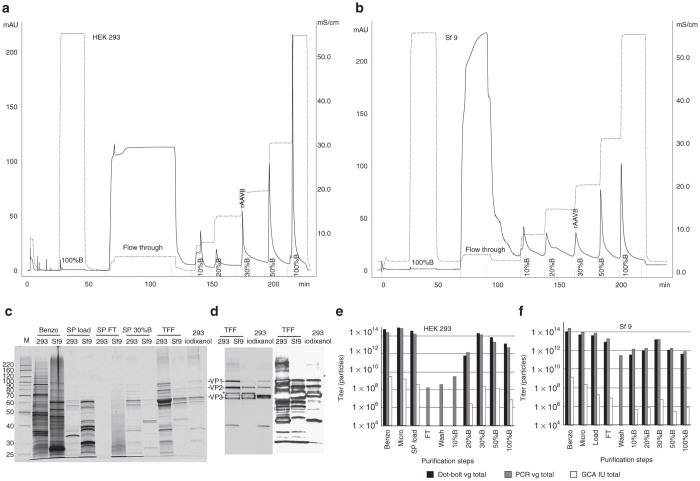
AAV8. AAV8 serotype-based vectors are among the most efficient vehicles for transducing target tissues in vivo, and they are widely utilized both in the laboratory setting and in clinical trials. Moreover, several laboratories have designed insect cell-based systems to scale up production of rAAV8 vectors.20,21 To investigate whether this method could be extended further to purify AAV8 from mammalian, as well as from the insect cell milieu, we have conducted a side-by-side purification using identical conditions for rAAV8 packaged in HEK 293 cells versus Sf9 cells (Figure 4a,b). As expected, the respective crude lysates showed divergent protein patterns (Figure 4c, lanes “Benzo, 293 and Sf9”). Even after flocculation/centrifugation, the SP loads were very much distinct for both samples, although already at this point, VP3 could be easily identified in both samples. The Sf9-derived rAAV8 vector had a lower VP1/VP2 capsid protein content (Figure 4d), consistent with previous reports.20,22 This is a characteristic of the AAV8 produced in Baculovirus expression system and in no way is related to the purification protocol. An extra B1-immureactive minor band present in both 293- and Sf9-derived TFF samples (Figure 4d, marked by the asterisk (*)) was also identified in the iodixanol sample, although only at tenfold higher load. If this peptide is a product of capsid autocleavage, then perhaps it happens at a somewhat higher rate under the low pH conditions used in this protocol. There is no evidence, however, that these minor alterations affect the vector’s infectivity. The total yield in the peak SP 30% B fraction constituted 56% of the SP load (by dot-blot assay), or 22% (by PCR assay), or 62.8% (by GCA) for 293-derived rAAV8 (Figure 4e), with comparable yields for Sf9-derived vector (Figure 4f).
Figure 4.
Summary of rAAV8 purification. (a) SP column chromatography profile of HEK 293-derived rAAV8 purification. Solid line: OD280(mAU), dashed line: measured electric conductance (mS/cm). (b) SP column chromatography profile of Sf9-derived rAAV8 purification. (c) Silver-stain SDS protein gel analysis of rAAV8 purification steps. Fractions are as following: “293”: sample derived from chromatography shown in a; “Sf9”: sample derived from chromatography shown in b; “Benzo”: crude lysate treated with benzonase; “SP load”: supernatant after flocculation/centrifugation step; “SP FT”: SP column flow through; “SP 50%B”: fraction containing rAAV8 eluted from the column with the buffer mixture containing 50% buffer B (see the Materials and Methods section for details); “TFF”: tangential flow filtration fraction (final purified rAAV8 stock); “293 iodixanol”: positive control, rAAV8 purified by “conventional” iodixanol buoyant density gradient. (d) Western blotting analysis of the final purified rAAV8 fraction (TFF), side-by-side with positive control; left panel: adjusted gel load sample volumes; right panel: same sample volumes as in c. (e,f) Graphical representation of the rAAV8-GFP yields (e) from HEK 293 cells or (f) from Sf9 cells at all purification steps, as titered for the genome-containing particles (dot-blot assay, black bars, or PCR assay, gray bars); and infectious particles (green cell assay, GCA, white bars). The missing bars for some steps indicate the titers below 10E4, the Y-axis plotting scale. min, minute.
Discussion
The purpose of the current project was to develop a simple and reproducible purification method applicable to many, if not all, AAV serotypes and variants. In addition, the method should be applicable to AAV vectors produced from either mammalian or Sf9 insect cell cultures, i. e. from very different cellular protein milieus. Previously, we conducted an in-depth study of methods for the recovery of rAAV from bulk cell lysates establishing that no satisfactory method existed among those tested at the time.8 In the current project, we utilized the less explored method of low pH-induced flocculation of colloidal suspensions in a crude cell lysate.23 The relative stability of AAV under low pH conditions has been taken into consideration,18,24 as well as reports that rAAVs can be purified under low pH conditions by AVB Sepharose HP affinity chromatography.16,25 Target flocculation pH was selected in the range of pH3–pH4,23 and the sodium citrate buffer pH3.9 was found to be the most consistent in forming the flocculate without AAV virus loss. Moreover, sodium citrate buffer was selected for the subsequent chromatography step because of its working pH range of 3.0–6.2, pKA = 6.4.26 The analysis of the calculated isoelectric points (pI) mean values of capsids of all characterized AAV serotypes 1–12 showed their slightly acidic character (pI = 6.3).18 This meant that at lower pH, the capsids will be protonated and thus capable of binding to a strong cation exchanger containing, for example, a bonded sulfonic acid group, such as SP. At the buffer pH values approaching the isoelectric target point (pI = 6.3), capsids become zwitterionic (charge-neutral), which would enable their dissociation and elution from the resin. Thus, the flocculation and subsequent binding to SP resin could be carried at pH3.9, while elution from the resin could be conducted at higher pH approaching pH6.2. It was also anticipated that because of the relative uniformity of the capsids’ pI across all characterized serotypes, the purification procedure would be applicable to many AAVs.
Prior to the method development we investigated whether exposing virus to low pH modifies its structure rendering it noninfectious. Consistent with the previous observations,18 acidification of the virus for 2 hours resulted in little if any reduction of the infectivity. Although longer incubations were somewhat unfavorable, the actual time of the purification (flocculation followed by column chromatography) is compatible with short low pH exposure times.
In this report, we describe a simplified purification protocol which, regardless of the upstream production method, yields exceptionally pure vector preparations. We have tested the protocol using three serotypes (AAV2, AAV8, and AAV9) from three different clades (Clade B, E, and F, respectively)27 thus validating its potential applicability to many AAV serotypes and mutants. The yield of the purified virus was significantly different for three serotypes tested ranging from to 11% (AAV2), to 63% (AAV8), to 93% (AAV9) suggesting that for each serotype, optimization of chromatography step may be required.
We have shown the protocol’s utility in the context of rAAV vectors produced by triple transfection in mammalian HEK 293 cells and in insect Sf9 cells produced by infection with baculovirus, providing evidence of the protocol’s potential usefulness in an industrial scale-up production environment. To our knowledge, this is one of the most inexpensive protocols utilizing simple off-the-shelf reagents such as sodium citrate and citric acid. Moreover, substituting SP Sepharose (bulk price ~US$1/ml) for AVB Sepharose HP (US$50/ml) brings the AAV vector methodology within the reach of essentially any research laboratory. The protocol could be simplified even further by substituting microfluidization with sonication, or freeze/thawing of a cell pellet, or even hypotonic lysis in H2O; and tangential flow filtration—with low-speed centrifugation/concentration through 150 kDa cut-off Apollo concentrator.
Separating DNA-containing and empty rAAV particles without ultracentrifugation in buoyant density gradients remains a technical challenge. Curiously, the pI value for virions incorporating packaged DNA is different from those for empty capsids.12 Perhaps modifying chromatography conditions and/or carriers as previously described12 will allow rAAV9 (and other serotypes) preparations to be more enriched for particles incorporating DNA.
In summary, we have developed an affordable protocol for the purification of rAAV using off-the-shelf reagents and easy to follow steps. Because of its overall simplicity, the protocol could be used in a regular research laboratory, as well as further adapted for GMP-grade industrial scale production.
Materials and Methods
Production of rAAV from HEK 293 cells
Typically, rAAV was produced by CaPO4-mediated transfection of plasmid DNA in 293 cells grown in CellStack format (Corning, New York, NY; seeded area of 6,360 cm2 containing ~1 × 109 cells at the time of transfection, ~1.5 × 109 cells at harvest), as previously described.14
Production of rAAV from Sf9 producer cell lines
rAAV was produced from Sf9-based producer stable cell lines upon infection with a single recombinant baculovirus encoding the rAAV expression cassette, as previously described.21,28 Typically, insect cells were grown in 1 l suspension (4 × 250 ml in 2 l Erlenmeyer flasks each) until their densities reached 2 × 106 cells/ml at which point recombinant Bac-rAAV-GFP was added at multiplicity of infection of 5. Cells were harvested 72 hours later and rAAV was purified as described below.
Production of clarified crude lysates
To maintain the reproducibility regardless of the scale of the process the volumes of each of the reagents used in this protocol are based on the approximate wet weight of the harvested cell pellet. An approximation of 1 g cell pellet wet weight being equal to 1 ml is used in lieu of actual weights and volumes that can be determined by direct measure or displacement. Figure 5 shows an overview of the process: cell pellet is resuspended in an appropriate volume of 100 mmol/l sodium citrate followed by the addition of magnesium and benzonase. Following the digestion an appropriate volume of 100 mmol/l citric acid is added to acidify the slurry and to initiate the formation of a protein flocculate. After low-speed centrifugation, the supernatant is subjected to ion exchange chromatography.
Figure 5.
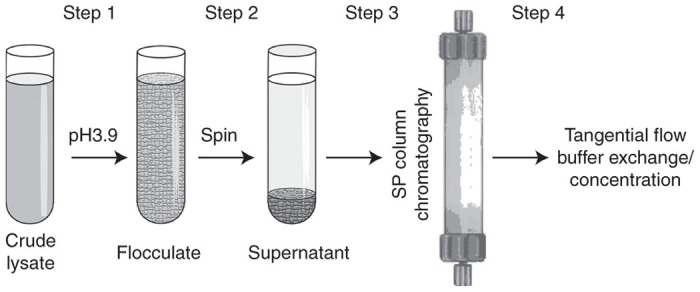
Schematic flowchart of adeno-associated virus (AAV) purification protocol. Step 1: A crude cell lysate is acidified by the addition of citric acid. Step 2: A heavy flocculate is precipitated by a low spin centrifugation. Step 3: A supernatant is subjected to a one-step cation-exchange chromatography. Step 4: Eluted virus is dialyzed/concentrated using tangential flow filtration.
A Citrate Buffer Table26 was used to identify ratios of the buffer pair: 100 mmol/l sodium citrate (Na3C6H5O7) and 100 mmol/l citric acid (C6H8O7) were mixed at the respective ratio of 16:34 (v/v) to derive a buffer of pH3.9. In practice, frozen cell pellets were resuspended in 100 mmol/l sodium citrate pH8.05, 1.44 ml/g wet cell pellet weight, at ~1.9 × 108 cells/g wet cell weight. ddH2O was then added, 2.25 ml/g wet cell weight, followed by MgCl2 to a final concentration of 1.6 mmol/l. DNA contaminants were digested by incubation with Benzonase (200 units/g wet cell weight) for 1 hour at 37 °C. Cells were disrupted by one-pass microfluidization (Microfluidics, Westwood, MA; Model 110S Microfluidizerfitted with “Z” type interaction chamber to produce a colloidal suspension of particles of 100 µm or less) and 100 mmol/l citric acid was added (3.06 ml/g wet cell weight), followed by addition of H2O, 2.25 ml/g wet cell weight. Heavy flocculate, which immediately formed at this point, was precipitated by centrifugation at 4,450g for 10 minutes at room temperature. Supernatant (pH~3.6) was collected and subjected to SP cation exchange chromatography.
SP column chromatography
HiPrep SP HP 16/10 (20 ml bed volume, GE Healthcare Life Sciences, Pittsburgh, PA) was equilibrated with 5 column bed volumes (BV) of Buffer A: 25 mmol/l sodium citrate buffer, target pH3.9 (mixed at the ratios as described above and diluted fourfold), followed by 5 BV of Buffer B: 50 mmol/l sodium citrate buffer, target pH6.2 (sodium citrate to citric acid ratio of 42.8:7.2, diluted twofold), containing 0.5 mol/l NaCl, followed by 5 BV of Buffer A. The clarified supernatant was applied to the column at 5 ml/minute, washed with Buffer A until the absorption at A280 reached background levels, followed by wash with 5 BV of 90% Buffer A and 10% Buffer B mixture (target pH4.65). AAV virus was eluted by the buffer mixture of 80% Buffer A and 20% Buffer B (for AAV2, target pH4.96), or 70% Buffer A and 30% Buffer B (for AAV8, target pH5.22), or 50% Buffer A and 50% Buffer B (for AAV9, target pH5.57).
rAAV titering
Dot-blot assay, PCR assay, and green cell fluorescent assays (GCA) were described earlier.14,29 For the GCA, 2 × 104 C12 cells30 per well in 96-well plates were infected with serial dilutions of a rAAV-GFP vector and coinfected with Ad5 (multiplicity of infection of 5) to increase the sensitivity. Forty-eight hours later, cells infected with rAAV-GFP were visually scored using a fluorescence microscope and the titer was calculated according to the dilution factor.
Dot-blot immunoassay
rAAV9-GFP was diluted to 2 ng/µl in citrate-phosphate buffer at pH 7.4, 6.0, 5.5, or 4.0 containing 150 mmol/l NaCl. Samples were incubated in a BioRad (Hercules, CA) C1000 Touch Thermal Cycler at a temperature range of 4–100 °C for initial experiments to broadly determine capsid stability (data not shown) then a narrower temperature range of 70–80 °C for subsequent experiments to more accurately assess capsid stability, for 5 minutes, then cooled to 4 °C. Twenty ng of a treated sample was immobilized onto a nitrocellulose membrane using a dot-blot apparatus (Bio-Rad). Blots were blocked in 5% milk in 0.05% Tween-PBS and probed for intact capsids, denatured capsids, or VP1u externalization using anti-AAV9 capsid monoclonal antibodies HL-2370 at 1:1,000, B1 (American Research Products, Waltham, MA) at 1:3,000, or A1 (American Research Products) at 1:20, respectively. Detection was carried out using HRP-linked anti-mouse monoclonal antibody at 1:5,000 (GE Healthcare) and Immobilon chemiluminescent substrate (Millipore, Billerica, MA)
Electron microscopy
rAAV9-GFP was diluted to 50 ng/µl in citrate-phosphate buffer at pH 7.4, 6.0, 5.5, or 4.0 containing 150 mmol/l NaCl and heated to 73 °C, 75 °C, 77 °C, 78 °C, or 80 °C for 5 minutes then cooled to 4 °C using a BioRad C1000 Touch Thermal Cycler. Samples were adhered to glow-discharged carbon-coated copper grids (TED Pella, Redding, CA) and stained with 2% uranyl acetate. Images were collected using a Tecnai G2 Spirit transmission electron microscope (FEI, Eindhoven, The Netherlands).
Acknowledgments
This work was funded by NIH NHLBI ROI HL097088.
The authors declare no conflict of interest.
References
- Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- High KA, Aubourg P. rAAV human trial experience. Methods Mol Biol. 2011;807:429–457. doi: 10.1007/978-1-61779-370-7_18. [DOI] [PubMed] [Google Scholar]
- High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost. 2011;9 (suppl. 1):2–11. doi: 10.1111/j.1538-7836.2011.04369.x. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2011;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- Auricchio A, O“Connor E, Hildinger M, Wilson JM. A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther. 2001;4:372–374. doi: 10.1006/mthe.2001.0462. [DOI] [PubMed] [Google Scholar]
- Brument N, Morenweiser R, Blouin V, Toublanc E, Raimbaud I, Chérel Y. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol Ther. 2002;6:678–686. doi: 10.1006/mthe.2002.0719. [DOI] [PubMed] [Google Scholar]
- Okada T, Nonaka-Sarukawa M, Uchibori R, Kinoshita K, Hayashita-Kinoh H, Nitahara-Kasahara Y. Scalable purification of adeno-associated virus serotype 1 (AAV1) and AAV8 vectors, using dual ion-exchange adsorptive membranes. Hum Gene Ther. 2009;20:1013–1021. doi: 10.1089/hum.2009.006. [DOI] [PubMed] [Google Scholar]
- Kaludov N, Handelman B, Chiorini JA. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum Gene Ther. 2002;13:1235–1243. doi: 10.1089/104303402320139014. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ., Jr Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Sleep S, Gray J, Azam S, Zhao Y. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J Virol Methods. 2004;121:209–215. doi: 10.1016/j.jviromet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Hellström M, Ruitenberg MJ, Pollett MA, Ehlert EM, Twisk J, Verhaagen J. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. doi: 10.1038/gt.2008.178. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yang X, Wright JF, High KA, Couto L, Qu G. PEG-modulated column chromatography for purification of recombinant adeno-associated virus serotype 9. J Virol Methods. 2011;173:99–107. doi: 10.1016/j.jviromet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan B, Yarbrough J, Domsic J, Bennett A, Bothner B, Kozyreva OG. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J Virol. 2013;87:4974–4984. doi: 10.1128/JVI.02524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salganik M, Venkatakrishnan B, Bennett A, Lins B, Yarbrough J, Muzyczka N. Evidence for pH-dependent protease activity in the adeno-associated virus capsid. J Virol. 2012;86:11877–11885. doi: 10.1128/JVI.01717-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlbrenner E, Aslanidi G, Nash K, Shklyaev S, Campbell-Thompson M, Byrne BJ. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzsch M, Grasse S, Zurawski C, Weger S, Bennett A, Agbandje-McKenna M. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1-12 vectors for gene therapy. Hum Gene Ther. 2014;25:212–222. doi: 10.1089/hum.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Gellan E, Martin GF, Cumming RH. Flocculation of cell debris from ultrasonicated Escherichia coli. Biotechnol Bioeng. 1991;37:697–702. doi: 10.1002/bit.260370802. [DOI] [PubMed] [Google Scholar]
- Nam HJ, Gurda BL, McKenna R, Potter M, Byrne B, Salganik M. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J Virol. 2011;85:11791–11799. doi: 10.1128/JVI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RH, Levy JR, Kotin RM. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori G.1955Preparation of Buffers for Use in Enzyme Studies, vol. 1 Academic Press; New York. [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidi G, Lamb K, Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc Natl Acad Sci USA. 2009;106:5059–5064. doi: 10.1073/pnas.0810614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltner N, Kohlbrenner E, Clément N, Weber T, Linden RM. Near-perfect infectivity of wild-type AAV as benchmark for infectivity of recombinant AAV vectors. Gene Ther. 2010;17:872–879. doi: 10.1038/gt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KR, Voulgaropoulou F, Johnson PR. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996;3:1124–1132. [PubMed] [Google Scholar]