Abstract
The yellow octocoral Eunicella cavolini is one of the most common gorgonians thriving in Mediterranean hard-bottom communities. However, information regarding its distribution and ecology in several parts of the Mediterranean is lacking, while population trends and conservation status remain largely unknown. We investigated 19 populations of E. cavolini over three representative geographic regions: the NW Mediterranean, CE Adriatic, and N Aegean. Focusing on the upper bathymetric range of the species (<40 m), data were collected on the populations’ upper depth limit, density, colony height, and extent of injury. A three-level hierarchical sampling design was applied to assess the existence of spatial patterns, using: a) regions (located thousands of km apart), b) localities within regions (tens to hundreds of km apart), and c) sites within localities (hundreds of m to a few km apart). In the NW Mediterranean and CE Adriatic, the upper distribution limit was at depths ≤15 m, whereas in the N Aegean most populations were found deeper than 30 m. Population density ranged between 4.46-62 colonies per m2, while mean colony height was 15.6±8.9 SD cm with a maximum of 62 cm. The NW Mediterranean sites were characterized by dense populations dominated by small colonies (<20 cm), periodic recruitment, and low proportion of large gorgonians (>30 cm). The CE Adriatic displayed intermediate densities, with well-structured populations, and continuous recruitment. In the N Aegean, most populations presented low densities, high proportion of large colonies, but low number of small colonies, signifying limited recruitment. Disturbance levels, as a function of extent and type of injury, are discussed in relation to past or present human-induced threats. This work represents geographically the most wide ranging demographic study of a Mediterranean octocoral to date. The quantitative information obtained provides a basis for future monitoring at a Mediterranean scale.
Introduction
Demographic studies provide valuable information regarding the ecology of long-lived benthic octocorals (e.g. [1–4]). The size structure of a population reflects how key life history parameters, such as growth, reproduction and mortality, have been shaped through its interaction with the surrounding environment and the local stressors [5–8]. Similarly, knowledge on the distribution and population dynamics of a species over large spatial scales enables a more comprehensive understanding of its ability to persist under variable environmental conditions [9]. Such information may further reveal relationships between populations and their environment, enabling the evaluation of habitat stability and suitability, and the monitoring of ecosystem change over time [10, 11].
Gorgonian octocorals represent one of the most important benthic taxonomic groups in the Mediterranean [12]. Given the colonies’ three dimensional growth, gorgonian populations greatly modulate benthic habitats and enhance the overall structural complexity, biomass, and species diversity [13, 14]. Furthermore, they act as ecosystem engineers (sensu [15]) by modifying current flow, sedimentation rates and shading levels [16–18]. As several other coral species, Mediterranean gorgonians are long-lived, with slow growth, delayed maturity, low or infrequent recruitment success and reduced post-settlement survival [19–22]. The slow population dynamics of gorgonians render them susceptible to a wide range of direct or indirect anthropogenic stressors. Mechanical damage (mainly caused by fishing and unregulated recreational diving), pollution and mucilagenous algal aggregates represent localized types of disturbance [23–25], whereas biological invasions [26] and mass mortality outbreaks, related to climate induced temperature anomalies, constitute basin-scale threats [27, 28].
The yellow octocoral Eunicella cavolini (Koch, 1887) is one of the most common gorgonian species of the Mediterranean hard-bottom communities, and forms distinct facies within the emblematic coralligenous assemblages [14, 29]. Its distribution range is wide, although patchy in terms of abundance, and it is known to occur from the W Mediterranean and Tunisian coasts to the Aegean Sea, and the Sea of Marmara [30, 31]. Still, information regarding its distribution and ecological characteristics in different parts of the Mediterranean is lacking, while population trends and conservation status remain largely unknown. The limited number of studies on the ecology of this species are restricted in space and time [11, 29, 32–39], while more recent information comes primarily from research regarding the effects of mass mortality events (MMEs) on gorgonian species [27, 28, 40, 41]. These studies commonly underline the limitations posed by the absence of pre-disturbance data as critical baselines for the rigorous evaluation of gorgonian populations under stress [42, 43].
Given the rapid alteration of the marine environment due to direct and indirect human induced pressures [44, 45], assessment of ongoing threats and effective management decisions must be based on a thorough understanding of the natural spatial and temporal variability exhibited by species and their populations living in different geographic areas. In response to these requirements, E. cavolini populations were studied in three distinct biogeographical regions of the Mediterranean, namely the NW Mediterranean, the CE Adriatic and the N Aegean seas. Aiming to provide comparable, quantitative reference data for future monitoring and assessment of future impacts or threats, we focused on the upper depth distribution range of the species (<40 m), where populations are exposed to greater variability of environmental conditions [14, 46] and higher level of human induced disturbances [47, 48]. This study is the first to assess the population structure and disturbance levels of E. cavolini populations across most parts of its known distribution in the Mediterranean, and geographically represents the widest ranging demographic study of an octocoral in the basin.
Materials and Methods
Field survey
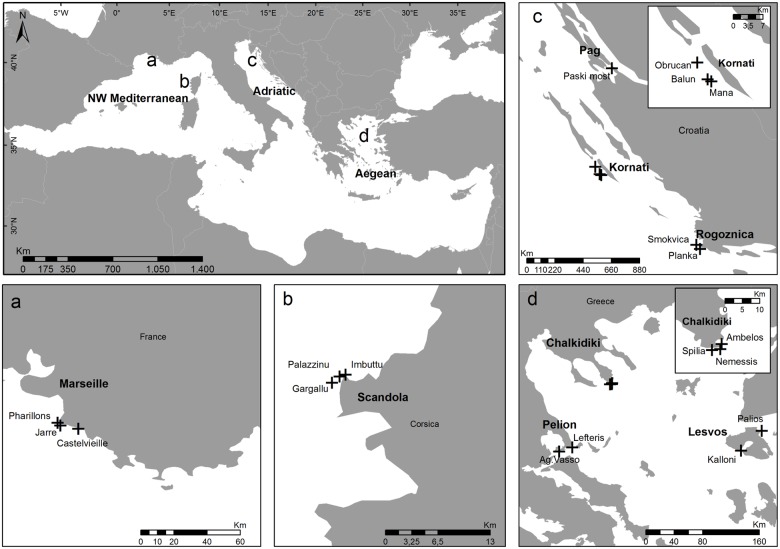
A three-level hierarchical sampling design was applied to assess the spatial patterns of E. cavolini populations, including: a) regions (located thousands of km apart), b) localities within regions (tens to hundreds of km apart), and c) sites within localities (hundreds of m to a few km apart). Three regions of the Mediterranean Sea were considered: the NW Mediterranean, CE Adriatic and N Aegean. Within each region, 2 to 3 localities were chosen, and within each locality 1 to 3 random sites were investigated. A total of 19 sites with well-developed E. cavolini populations were studied within 2 localities of the NW Mediterranean (Marseille, Scandola), 3 localities of the CE Adriatic (Kornati, Pag, Rogoznica), and 3 localities of the N Aegean (Pelio, Chalkidiki, Lesvos, Fig 1, Table 1). In situ underwater demographic surveys were conducted within the upper distribution depth range of the species (<40 m). Data for each population were collected once, during the period 2005–2013. Field surveys in the locality of Scandola were conducted under the authorization of the Scientific Comittee of Réserve Marine de Scandola—Parc Regional de Corse. In the case of Kornati National Park a special permission was issued by the Croatian Ministry of Culture—Department for Nature Protection. For the remaining sites, no specific permits were required at the time of field work for the sampling protocols described herein. Locations were not privately owned and the study did not involve endangered or protected species. Our study was based exclusively on non-destructive methods, and no plant or animal material was collected.
Fig 1. Map of the Mediterranean showing the investigated regions of the NW Mediterranean, CE Adriatic and N Aegean.
Inset figures a—d present localities (in bold). Crosses mark the study sites of the yellow gorgonian Eunicella cavolini.
Table 1. Characteristics of studied sites.
| Region | Locality | Site | Depth range Min—Max (m) | Coordinates | Habitat | Inclination | Protection level | |
|---|---|---|---|---|---|---|---|---|
| NW Mediterranean | Marseille | Jarre | 5–20 | 43°11'47"N | 05°21'45"E | Wall | Vertical | MPA |
| Castelvieille | 5–35 | 43°12'01"N | 05°29'39"E | Wall | Vertical | MPA | ||
| Pharillons | 10–45 | 43°12'27"N | 05°20'18"E | Wall | Vertical | MPA | ||
| Scandola | Imbuttu | 15–45 | 42°22'41"N | 08°33'05"E | Wall | Vertical | MPA | |
| Palazzinu | 15–40 | 42°22'48"N | 08°33'00"E | Wall | Vertical | MPA | ||
| Gargallu | 15–40 | 42°22'21"N | 08°32'05"E | Wall | Sub-vertical | MPA | ||
| CE Adriatic | Kornati | Mana | 5–60 | 43°48'01''N | 15°15'59''E | Wall | Vertical | MPA; Natura 2000 |
| Balun | 5–60 | 43°48'14''N | 15°15'18''E | Wall | Vertical | MPA; Natura 2000 | ||
| Obrucan | 5–55 | 43°50'11''N | 15°13'12''E | Wall | Vertical | MPA; Natura 2000 | ||
| Pag | Paski most | 12–35 | 44°19'07''N | 15°15'38''E | Wall | Cascading | unprotected | |
| Rogoznica | Smokvica | 15–50 | 43°30'38"N | 15°56'32"E | Wall | Cascading | unprotected | |
| Planka | 15–50 | 43°29'36''N | 15°58'09''E | Wall | Cascading | unprotected | ||
| Aegean | Pelio | Lefteris | 20–30 | 39°08'31"N | 23°20'39"E | Rocky outcrop | Vertical | unprotected |
| Ag.Vasso | 30–50 | 39°05'08"N | 23°07'48"E | Wall | Sub vertical | unprotected | ||
| Chalkidiki | Ambelos | 30–45 | 39°57'33"N | 23°59'29"E | Wall | Sub-vertical | unprotected | |
| Nemessis | 30–45 | 39°56'46"N | 23°59'10"E | Wall | Sub-vertical | unprotected | ||
| Spilia | 32–50 | 39°56'38"N | 23°57'31"E | Rocky outcrop | Sub-vertical | unprotected | ||
| Lesvos | Kalloni | 10–27 | 39°04'56"N | 26°05'25"E | Rocky outcrop | Sub-vertical | Natura 2000 | |
| Palios | 30–44 | 39°19'42"N | 26°26'10"E | Wall | Sub-vertical | unprotected | ||
Depth range refers to the recorded depth distribution of the yellow gorgonian Eunicella cavolini population at each site. Inclination characterized as “cascading” refers to slopes intercepted by smaller vertical walls.
Assessment of demographic characteristics and disturbance levels
For the assessment of the main population characteristics we followed the methodology proposed by Linares et al. [3]. Colonies’ density, height, proportion of injured surface and type of injury were chosen as the main population descriptors, and measurements were taken using 50×50 cm haphazardly placed quadrats within the E. cavolini populations.
Density was determined based on the number of colonies present within 50×50 cm quadrats, averaged and recalculated for 1 m2 surface. For each colony found, maximum height was measured as the distance from the colony base to the tip of the furthest apical branch. At each site, the aforementioned parameters were assessed over an area of more than 3 m2 and for more than 45 colonies, thus satisfying previously determined minimum sample size criteria [3].
Three descriptors were used to assess the impact of potential disturbances: extent of injury per colony, type of injury, and proportion of healthy colonies. The combined investigation of the type and extent of injury may provide insights regarding past disturbance events, and allow for an estimation of the approximate time of their occurrence [25, 42]. Extent of injury was estimated as the proportion of the colony’s total surface that appeared devoid of coenenchyma tissue (i.e. denuded axis) and/or that was overgrown by other organisms. Based on the presence/absence of different epibionts, and the time it takes for their development, three types of injury were identified; Type A: denuded colony axis, indicating a new injury up to 1 month; Type B: colony overgrowth by pioneer species, such as filamentous algae and hydrozoans, representing injuries of approximately 1–12 months old; Type C: colony overgrowth mostly by bryozoans, sponges and algae, reflecting an old injury of approximately ≥12 months [42]. Overall, out of the 3188 colonies measured, injury data were collected for 3045 colonies and where used to calculate mean extent of injury and percentage of healthy or affected colonies. Colonies with less than 10% of injured surface were considered as healthy, colonies with injuries ≥10% of total surface where classified as affected, whereas 100% of injury corresponded to death [3, 28]. Type of injury was quantitatively described only for colonies with ≥10% of injured surface (i.e. affected colonies); their proportion was calculated against the total number of colonies for which extent of injury was assessed per population.
Data analysis
To assess the height frequency distribution per site, height measurements of colonies displaying <100% of injured surface were grouped into five classes: 1–10, 11–20, 21–30, 31–40, and >41 cm, and the descriptive distribution parameters of skewness (g1) and kurtosis (g2) were estimated. Coefficients of g1 and g2 were considered significant if the ratio to their standard error was >2 [49]. The relation between mean height and density was explored using a Spearman rank order correlation.
A non-metric multidimensional scaling (MDS) ordination [50] was performed to visualize patterns of population structure based on the following parameters after normalization of data: mean height, max height, proportion of the smaller (0–20 cm) and larger (>30 cm) height classes, density and upper depth distribution limit. One-way, non-parametric analysis of variance PERMANOVA [51], based on square root transformed data and Euclidean distances, was used to test for spatial variability in colony height and density. A three-factor hierarchical design was applied using "region" (3 levels) as a fixed factor, "locality" (8 levels) as a random factor nested within region, and "site" (19 levels) as a random factor nested within locality. Pair-wise comparisons were performed to determine specific inter or intra-regional differences when the main test indicated significant differences. Significance was confirmed based on 9999 permutations. Analyses were performed using the PRIMER v6 software with PERMANOVA+ add-on package [52, 53].
Results
Upper depth distribution range
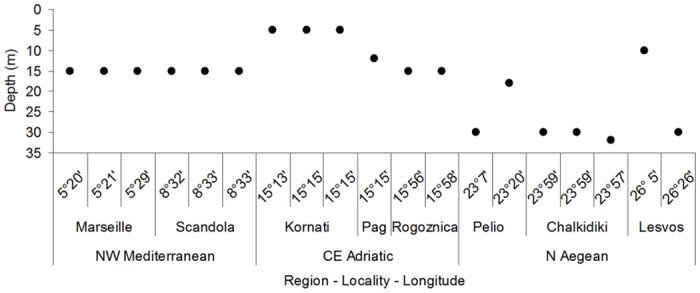
The upper depth distribution limit varied considerably along the longitudinal gradient (Fig 2). In all localities of the NW Mediterranean and CE Adriatic, the upper distribution limit of E. cavolini populations was found at 15 m depth or shallower, with some populations appearing at 5 m depth in the locality of Kornati (CE Adriatic). On the contrary, most populations of the N Aegean were found in waters deeper than 30 meters, with the exception of two populations that were located at 10 and 20 m depth.
Fig 2. Upper depth distribution limits for the investigated Eunicella cavolini populations along a longitudinal gradient.
Demographic characteristics
Mean population density of E. cavolini was 14.9 ± 14.6 SD (standard deviation) per m2. The minimum density of 4.5 colonies per m2 was observed at Ambellos (N Aegean), while the maximum density of 62 colonies per m2 was recorded at Jarre (NW Mediterranean, Table 2). Among localities (Fig 3), higher densities were recorded in Scandola (29.5 ± 12.9 SD per m2) and Marseille (24.1 ± 27 SD per m2) within the NW Mediterranean, followed by Rogoznica (17.8 ± 10.9 SD per m2), Kornati (15.2 ± 12.8 SD per m2), and Pag (12.9 ± 12.6 SD per m2) within the CE Adriatic, while the lowest densities were observed in the localities of the N Aegean, namely Lesvos (10.5 ± 13.1 SD per m2), Pelio (9.03 ± 7.1 SD per m2), and Chalkidiki (7.5 ± 6.6 SD per m2). PERMANOVA results suggest that a significant variability in density exists among regions and sites (p < 0.05), but not between localities (Table 3). Furthermore, the greatest variation in density, indicated by the components of variation, was observed among regions. The pair-wise tests show that a significant difference in population density exists among all regions (p < 0.05, Table 4) and between sites in 4 of the 7 localities analysed (Table A in S1 File).
Table 2. Population characteristics of Eunicella cavolini per region, locality and site.
| Region | Locality | Site | Sampling depth (m) | Area (m-2) | Density (m-2) | Height (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | N | Mean | Min | Max | SD | SE | Skewness | Kurtosis | |||||
| NW Mediterranean | Marseille | Jarre | 15–20 | 3.50 | 62.00 | 217 | 12.21 | 1.0 | 38.0 | 7.22 | 0.49 | 0.69* | 0.20 |
| Castelvieille | 15–20 | 6.00 | 12.83 | 76 | 18.28 | 3.0 | 45.0 | 9.21 | 1.06 | 0.49 | -0.05 | ||
| Pharillons | 15–20 | 4.75 | 10.32 | 49 | 14.20 | 2.0 | 35.0 | 7.79 | 1.11 | 1.02* | 0.73 | ||
| Scandola | Imbuttu | 15–20 | 9.50 | 29.47 | 280 | 13.76 | 1.5 | 28.5 | 5.32 | 0.32 | 0.07 | -0.47 | |
| Palazzinu | 20–23 | 9.00 | 30.78 | 270 | 12.33 | 0.5 | 37.0 | 5.96 | 0.36 | 0.68* | 0.73* | ||
| Gargallu | 20–25 | 8.75 | 28.34 | 245 | 15.12 | 2.0 | 33.0 | 5.72 | 0.36 | 0.29 | -0.01 | ||
| CE Adriatic | Kornati | Mana | 9–28 | 16.75 | 12.24 | 198 | 10.15 | 1.5 | 43.0 | 4.64 | 0.33 | 1.89* | 11.67* |
| Balun | 13–28 | 20.25 | 17.43 | 345 | 10.92 | 1.0 | 28.0 | 5.03 | 0.27 | 0.39* | 0.08 | ||
| Obrucan | 15–28 | 23.50 | 15.45 | 360 | 15.29 | 2.0 | 45.0 | 8.27 | 0.43 | 0.45* | -0.27 | ||
| Pag | Paski most | 20–30 | 7.50 | 12.93 | 89 | 19.47 | 3.0 | 50.0 | 10.04 | 1.06 | 0.85* | 0.18 | |
| Rogoznica | Smokvica | 20–30 | 7.50 | 17.06 | 126 | 20.66 | 5.0 | 50.0 | 8.43 | 0.75 | 0.72* | 0.79 | |
| Planka | 23–30 | 5.50 | 18.91 | 102 | 17.40 | 4.0 | 50.0 | 10.12 | 1.00 | 1.08* | 0.66 | ||
| N Aegean | Pelio | Lefteris | 24–30 | 11.00 | 10.36 | 113 | 17.13 | 4.0 | 53.0 | 10.29 | 0.97 | 1.13* | 1.09* |
| Ag.Vasso | 32–35 | 12.25 | 7.84 | 95 | 23.66 | 2.0 | 62.0 | 12.08 | 1.24 | 0.37 | -0.25 | ||
| Chalkidiki | Ambelos | 30–40 | 26.25 | 4.46 | 114 | 25.06 | 5.0 | 50.0 | 9.16 | 0.86 | 0.21 | 0.03 | |
| Nemessis | 30–38 | 9.25 | 11.35 | 105 | 13.81 | 2.0 | 34.0 | 6.84 | 0.67 | 0.44 | -0.44 | ||
| Spilia | 35–40 | 7.75 | 13.29 | 103 | 23.17 | 3.0 | 51.0 | 10.93 | 1.08 | 0.21 | -0.56 | ||
| Lesvos | Kalloni | 13–27 | 6.25 | 23.52 | 127 | 16.71 | 1.0 | 46.0 | 11.11 | 0.98 | 0.49* | -0.45 | |
| Palios | 33–40 | 18.00 | 6.00 | 108 | 23.86 | 3.0 | 48.0 | 10.62 | 1.02 | 0.07 | -0.88 | ||
*statistically significant results.
Fig 3. Mean density (colonies per m2) of Eunicella cavolini populations, tabulated by locality.
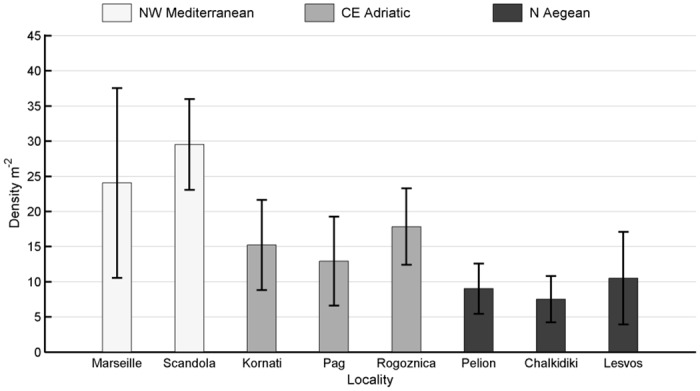
Whisker span indicates standard deviation. Colors denote geographic regions.
Table 3. Summary of PERMANOVA results for Eunicella cavolini population density.
| Source of variation | ||||||
|---|---|---|---|---|---|---|
| df | SS | MS | Pseudo-F | P(perm.) | Unique perm. | |
| Region | 2 | 726.65 | 363.33 | 21.237 | 0.0008* | 9968 |
| Locality(Region) | 5 | 57.722 | 11.544 | 0.31125 | 0.8763 | 9960 |
| Site(Locality(Region)) | 11 | 409.2 | 37.2 | 14.303 | 0.0001* | 9944 |
| Residuals | 834 | 2169.1 | 2.6008 | |||
| Total | 852 | 3362.7 | ||||
Tests of significance were run based on Euclidean distances for square root transformed data.
*statistically significant differences (p<0.05).
Table 4. Summary of PERMANOVA pairwise comparisons for Eunicella cavolini population density among regions.
| Pairwise test for regions’ density | t | P(perm.) | Unique perm. |
|---|---|---|---|
| NW Mediterranean, CE Adriatic | 2.9077 | 0.0183* | 9970 |
| NW Mediterranean, Aegean | 7.0213 | 0.0019* | 9965 |
| CE Adriatic, Aegean | 4.263 | 0.0114* | 9965 |
Tests of significance were run based on Euclidean distances for square root transformed data.
*statistically significant differences (p<0.05).
Overall, mean colony height was 15.6 ± 8.9 SD cm, while the maximum recorded height was 62 cm (Table 2). Among localities (Fig 4), mean colony height was greater at Chalkidiki (20.8 ± 10.3 SD cm), Pelio (20.1 ± 11.6 SD cm) and Lesvos (19.9 ± 11.4 SD cm) in the N Aegean, while equally high values were recorded at Pag (19.5 ± 10 SD cm) and Rogoznica (19.2 ± 9.3 SD cm) in the CE Adriatic. Lower height values were observed at Marseille (13.8 ± 8.1 SD cm) and Scandola (13.7 ± 5.8 SD cm) in the NW Mediterranean, as well as Kornati (12.5 ± 6.8 SD cm) in the CE Adriatic. Significant differences in height were only found at the level of sites within localities (PERMANOVA test, Table 5; Table B in S1 File).
Fig 4. Mean height of Eunicella cavolini colonies per locality.
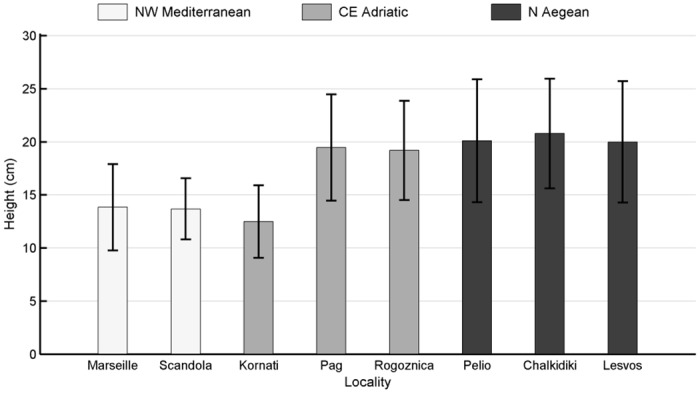
Whisker span indicates standard deviation. Colors denote geographic regions.
Table 5. Summary of PERMANOVA results for Eunicella cavolini colony height.
| Colony height | Source of variation | |||||
|---|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | P(perm.) | Unique perm. |
| Region | 2 | 295.97 | 147.98 | 2.4786 | 0.1657 | 9955 |
| Locality(Region) | 5 | 176.86 | 35.371 | 1.4701 | 0.2791 | 9955 |
| Site(Locality(Region)) | 11 | 326.65 | 29.695 | 29.73 | 0.0001* | 9932 |
| Residuals | 3103 | 3099.4 | 0.99884 | |||
| Total | 3121 | 3898.9 | ||||
Tests of significance were run based on Euclidean distances for square root transformed data.
*statistically significant differences (p<0.05).
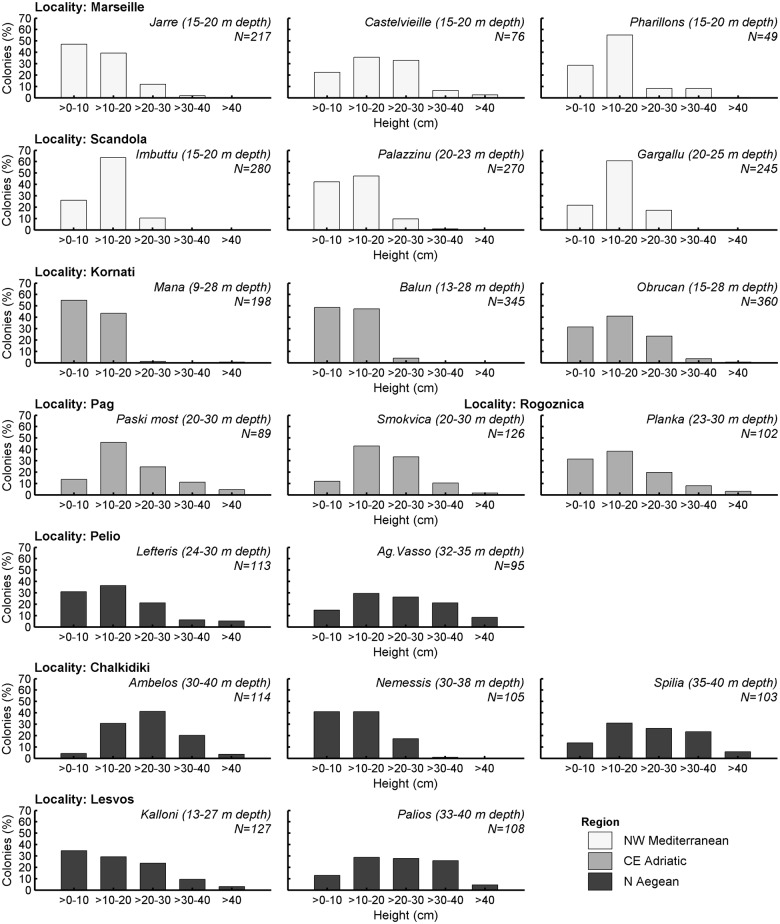
Height frequency distribution of E. cavolini populations appeared to be either positively skewed or relatively symmetric depending on site (Fig 5). A significant positive skewness, indicating a prevalence of the smaller height classes, was found for the populations of Jarre, Pharillons, and Palazzinu in NW Mediterranean, all populations of the CE Adriatic, and for the shallower sites in the N Aegean, namely Lefteris and Kalloni (Table 2). Of these positively skewed populations, Palazzinu, Mana, and Lefteris displayed additionally a significant positive kurtosis value, suggesting a dominance of either one or both of the two smaller height classes (>0–10 and >10–20 cm). The majority of the N Aegean populations presented a non-significant negative kurtosis value. Although an important proportion of large colonies (20–30 cm) appeared in most sites, the number of colonies with height >30 cm was generally low, and more pronounced in certain sites of the CE Adriatic and N Aegean.
Fig 5. Height frequency distribution of Eunicella cavolini populations per site, grouped by locality (in bold) and region (different colors).
Italics indicate site name, numbers in parentheses denote sampling depth range, and (N) corresponds to the number of colonies used.
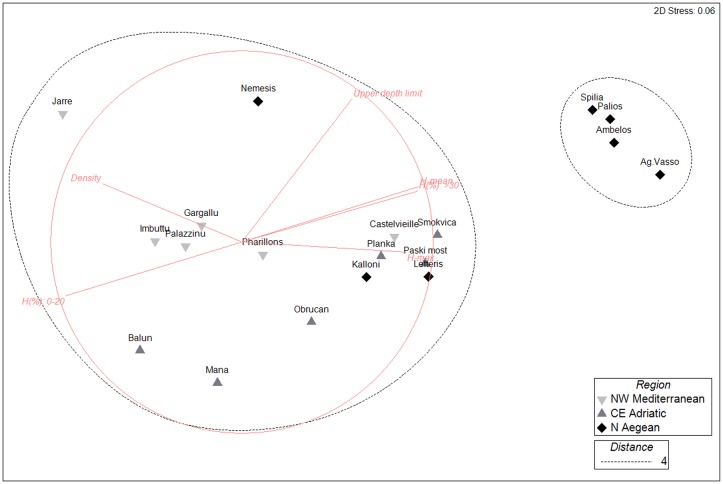
According to the Spearman rank order correlation values of density and mean height across sites displayed a significant inverse relation (rs = -0.58, p = 0.009). The MDS plot of E. cavolini populations according to basic demographic characteristics (Fig 6) produced two main clusters at a stress level of 0.06, indicating good ordination; one cluster encompassing all NW Mediteranean and CE Adriatic populations, as well as the shallower populations of the N Aegean, and a second one including the deeper populations of the N Aegean.
Fig 6. Non-metric multidimensional scaling (MDS) plot of Eunicella cavolini population structure per site.
Different markers indicate different regions. Black dotted lines signify clusters formed at Euclidean distance equal to 4. The superimposed red lines denote the Euclidean distance coefficients used, after data normalization: mean height, max height, H% (proportion of height classes ≤20 cm and >30 cm), height skewness, height kurtosis, density, and upper depth distribution.
Disturbance levels
Among populations, mean extent of injury of gorgonian tissue varied substantially, ranging from 0.8% to 38.3% (Table 6). Most populations (14 out of 19) presented a mean extent of injury less than 10%, while higher values were observed at Planka (13%), Smokvica (16.5%) and Paski most (25.8%) in the CE Adriatic, and at Ambelos (18.6%) and Kalloni (38.3%) in the N Aegean. The proportion of injured colonies (i.e. ≥10% to ≤99% of injured surface) ranged between 0–64%, with highest values at Paski most (37.1%) and Smokvica (39.8%) in the CE Adriatic, and Ambellos (64.1%) and Kalloni (52.4%) in the N Aegean (Table 6). The percentage of dead colonies was generally below 3.5% for the majority of sites, with the exception of Paski most (8.25%) and Kalloni (13.6%). Overall, the greatest proportions of healthy colonies (<10% of injured surface) were recorded in the localities of Marseille (75.3–100%), Scandola (83–91%) and Kornati (78.5–82.6%) (Table 6).
Table 6. Summary data on injury characteristics of Eunicella cavolini colonies per site.
| Extent of colony injury (%) | Proportion of uninjured, injured and dead colonies | Proportion of coloniesper type of injury | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Locality | Site | Year | N | Mean | SD | <10% | ≥10–≤99% | 100% | A | B | C |
| NW Mediterranean | Marseille | Jarre | 2005 | 75 | 6.7 | 10.5 | 85.33 | 14.67 | 0.00 | 10.66 | 2.67 | 9.33 |
| Castelvieille | 2005 | 77 | 9.6 | 22.4 | 75.32 | 23.38 | 1.30 | 0.00 | 1.30 | 23.38 | ||
| Pharillons | 2005 | 49 | 0.8 | 1.9 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Scandola | Imbuttu | 2013 | 280 | 3.1 | 10.3 | 89.29 | 10.71 | 0.00 | 0.00 | 0.00 | 10.71 | |
| Palazzinu | 2013 | 277 | 7.9 | 21.7 | 83.03 | 14.44 | 2.53 | 0.00 | 0.00 | 16.97 | ||
| Gargallu | 2013 | 248 | 3.3 | 14.2 | 91.94 | 6.85 | 1.21 | 0.00 | 0.00 | 8.06 | ||
| CE Adriatic | Kornati | Mana | 2009 | 205 | 7.9 | 20.7 | 78.54 | 18.05 | 3.41 | 1.46 | 8.78 | 11.71 |
| Balun | 2009 | 353 | 5.5 | 18.4 | 89.52 | 8.22 | 2.27 | 0.28 | 1.13 | 9.35 | ||
| Obrucan | 2009 | 362 | 5.7 | 15.8 | 82.60 | 16.57 | 0.83 | 0.28 | 3.31 | 13.81 | ||
| Pag | Paski most | 2011 | 97 | 25.8 | 36 | 54.64 | 37.11 | 8.25 | 1.03 | 10.31 | 37.11 | |
| Rogoznica | Smokvica | 2009 | 128 | 16.5 | 26.3 | 58.59 | 39.84 | 1.56 | 0.00 | 1.56 | 38.28 | |
| Planka | 2009 | 104 | 13.3 | 24.2 | 66.35 | 31.73 | 1.92 | 0.00 | 0.00 | 33.65 | ||
| N Aegean | Pelio | Lefteris | 2011 | 114 | 8.5 | 16 | 64.91 | 34.21 | 0.85 | 0.00 | 26.32 | 6.14 |
| Ag.Vasso | 2011 | 96 | 6.7 | 16.3 | 77.1 | 21.87 | 1.04 | 0.00 | 17.71 | 3.13 | ||
| Chalkidiki | Ambelos | 2011 | 117 | 18.6 | 24.9 | 33.33 | 64.1 | 2.56 | 46.15 | 11.97 | 5.98 | |
| Nemessis | 2011 | 105 | 4.1 | 12.1 | 85.71 | 14.29 | 0.00 | 0.00 | 14.29 | 0.00 | ||
| Spilia | 2011 | 103 | 6.9 | 15.7 | 78.6 | 21.4 | 0.00 | 0.00 | 19.42 | 1.91 | ||
| Lesvos | Kalloni | 2013 | 147 | 38.3 | 39.1 | 34.01 | 52.38 | 13.61 | 12.24 | 57.82 | 49.66 | |
| Palios | 2011 | 108 | 2.5 | 8.3 | 89.81 | 10.18 | 0.00 | 0.93 | 9.26 | 0.93 | ||
Proportion of colonies per type of injury was estimated using only colonies displaying ≥10% of injured surface against the total number of colonies per site.
The majority of affected colonies (≥10% of injured surface) in the NW Mediterranean and the CE Adriatic presented type C (old) injuries. In the N Aegean, populations mainly presented overgrowth by pioneer species (type B injury); exceptions were Ambelos, which displayed high proportion of recent tissue necrosis (type A injury: 46.15%), and Kalloni which had high levels of all types of injury. As the time of sampling varied among localities, no statistical comparisons regarding disturbance parameters were attempted.
Discussion
The extensive geographic distribution of E. cavolini and its relatively wide bathymetric range (<10–220 m [30, 35, 54, 55]) reflect its ability to adapt and survive over highly variable abiotic conditions. In this study, we carried out a comprehensive analysis of E. cavolini population structure and dynamics at three distinct Mediterranean regions, in order to facilitate a better understanding of the patterns observed across contrasting environmental gradients.
Upper depth distribution range
One of the most robust findings is the deeper upper bathymetric limit of populations in the N Aegean, compared to those thriving in the NW Mediterranean and CE Adriatic. In the latter regions, the upper bathymetric limit of E. cavolini was at 5–15 m. These observations are in line with the minimum depths reported in other localities of the western Mediterranean basin (e.g. [29, 35, 38, 56]) or the Adriatic Sea (e.g. [57]), and the fact that the species can withstand a fairly wide range of light intensity (1–44% of surface light according to [29]). Within the N Aegean, most populations were located at depths below 30 m. According to previous reports, gorgonian species in the Aegean Sea are generally observed in waters deeper than 40 m [58, 59] and are rarely found at depths shallower than 20 m [60, 61].
We hypothesize that the observed distribution patterns are related to the variability of abiotic factors that predominate in the distinct regions under study, putatively coupled with biotic interactions [62, 63]. In fact, Zabala & Ballesteros [62] suggested that in oligotrophic areas suspension feeders are restricted to deeper waters or in areas were strong currents prevail, while in shallow waters algal species can outcompete long-lived suspension feeders, such as gorgonian species. Moreover, Mediterranean gorgonian species are particularly vulnerable to temperature anomalies, which are more likely to occur in shallow waters. According to the above, the downward shift of the upper bathymetric limit observed in the N Aegean may be due to the more oligotrophic conditions and higher water temperatures characterizing this region compared to those of the NW Mediterranean and CE Adriatic [64, 65]. The only exceptions to the bathymetric pattern of the N Aegean were the sites of Kalloni (10 m depth) and Lefteris (20 m depth), which are characterized by the presence of strong currents that are known to promote the growth of suspension feeders such as E. cavolini [16].
Population structure and dynamics
E. cavolini population density ranged from 4.46 to 62 colonies per m2, although the species may attain much greater densities [39]. Density was highly variable across regions, and displayed a decreasing trend from west to east. Highest values were observed in the NW Mediterranean, intermediate in the CE Adriatic, and lowest in the N Aegean, while in all regions significant differences were also detected at the level of sites within localities. Variability in density among sites within the same depth range (i.e. <40 m) has also been observed in other localities of the NW Mediterranean (e.g. for E. cavolini [35, 39, 40]), as well as for other Mediterranean gorgonians (e.g. for E. singularis [3, 66], Paramuricea clavata [3, 67], and Corallium rubrum [7, 68]). According to these studies, density is usually related to factors that affect reproduction and recruitment success. For example, Weinbauer & Velimirov [11, 39] justified the wide density differences observed in E. cavolini populations (15–180 colonies per m2) among nearby sites at the Bay of Calvi—Corsica, on the basis of substrate availability, turbulence, abundance of large colonies and degree of colony overgrowth by other organisms. They further related these factors to the successful reproduction, settlement and survival of new colonies. On the other hand, increased densities have also been observed during periods of population recovery from MMEs, once detachment of dead colonies created free space for new recruits (e.g. [69–71]). In the present study, density was overall inversly related to height, supporting the idea that recruitment is driven by intra-specific competition mechanisms, where the lack of large colonies enhances recruitment success.
With regard to colony height, mean value for all examined populations was 15.7 ± 8.87 cm, with a maximum of 62 cm. Similar height values have been reported for both shallow [35] and deep water populations of E. cavolini (i.e. >70 m, [54]). As in several other octocoral species, E. cavolini is known to diplay a high level of phenotypic plasticity (Fig 7A and 7B) by modulating its structural characteristics and growth form (i.e. including fan size, shape, orientation, and polyp number) in response to water movement [32, 34, 37, 72]. It is therefore possible that the direction and velocity of prevailing water currents are the main factors determining colony height in several sites of the present study. In this respect, the greater proportion of larger colonies (>30 cm) found in the deeper sites of the CE Adriatic and N Aegean may be due to the greater environmental stability of deeper waters [4, 46], and the decrease of hydrodynamic forces that are known to affect colony morphology. At the same time, the greater proportion of small colonies (<20 cm) observed in sites characterised by higher densities (mainly in the NW Mediterranean) may be indicative of a more dynamic environment (in terms of water flow and productivity), which reduces the optimal size of colonies, but retains highly reproductive, small sized colonies belonging to a wide range of age classes [73].
Fig 7. Colonies of the yellow gorgonian Eunicella cavolini exposed to different orientations and disturbances.
a) in a large overhang; b) on open rock; c) under stress by fishing gear and mucilagenous algae, d) heavily overgrown during a mass mortality event. Photos a and b are courtesy of Thanos Dalianis and Panagiotis Papadelis, respectively.
Although morphological plasticity may partly explain differences in population structure among sites, no significant differences were detected in colony height at the level of localities or regions. The overall patterns of height frequency distribution suggest that E. cavolini populations were at varying stages of development, driven by different recruitment and mortality dynamics. NW Mediterranean and CE Adriatic populations were mainly characterised by the predominance of one or both of the smaller size classes (0–10, >10–20 cm), indicating either continuous or pulse recruitment episodes. In the majority of the N Aegean populations, all size classes were almost equally represented, while the number of small colonies (especially of the 0–10 cm height class) was typically low, suggesting limited recruitment. Still, the presumably low recruitment dynamics of the N Aegean populations may be compensated by the presence of a large number of mature colonies during sporadic reproductive events, since gamete production, and hence reproductive output, increase exponentially with colony size [22, 74–76]. On the contrary, the low abundance of large colonies, or even the lack of them, observed in several studied sites across regions, may be indicative either of newly formed and expanding populations, or of the existence of additional natural and/or human-induced pressures (e.g. MMEs, mechanical damage especially caused by unregulated fisheries and recreational marine activities, or extreme wave action) which particularly affect the survivorship of larger height classes [7, 10, 39, 42, 43, 69, 77].
Patterns of height frequency distribution similar to those observed herein have been documented for several gorgonian populations in other localities of NW Mediterranean and E Adriatic. A unimodal peak at the second size class—implying periodic recruitment—has been observed in populations of E. cavolini dwelling both shallow (<30 m, NW Corsica [11]) and deep waters (>70 m, S Tyrrhenian Sea [54]), in shallow P. clavata populations off the Spanish and French coasts (<40 m [2, 3]), as well as in deep E. singularis populations (>50 m, off the Spanish coasts [4]). Positively skewed populations, displaying prevalence of new recruits (0–10 cm) and low proportion of larger gorgonians, have been described for shallow E. singularis populations along the Spanish coasts (<25 m [3, 4]). Yet again, several authors [77, 78] have reported similar population structure for P. clavata in the Ligurian Sea following MMEs. On the other hand, populations characterised by a predominance of the smallest height class along with a high proportion of large colonies—reflecting well structured populations with continuous recruitment—were not observed during the present study, regardless the wide distribution range covered. Occasional reports of the latter pattern at depths <50 m include a single population of E. cavolini in NW Corsica [11] and P. clavata populations in the E Adriatic [79].
Extent of injuries: baseline for future monitoring
Injuries of gorgonian colonies are either caused by mechanical abrasion and predation or through necrosis of living tissue under stress conditions (e.g. persistence of thermal anomalies) causing partial and/or total mortality of colonies (Fig 7C and 7D). Injured parts may break, regenerate or become colonised by overgrowing organisms [36, 67]. E. cavolini is particularly vulnerable to such injuries, and has been affected by several MMEs related to temperature anomalies in the NW Mediterranean, some of which have led to substantial population reductions (e.g. [27, 28, 41]). Although injury estimates were taken over different years in this study, thus not allowing for direct quantitative comparisons among regions, they do however provide insights regarding conservation status at the time of assessment, as well as baseline data for future assessments. Our assessment in Marseille and Scandola was realized two and ten years respectively, after one of the largest and best documented MME in the NW Mediterranean, which took place during late summer of 2003 [28]. At the time of the MME, the estimated proportion of affected E. cavolini colonies ranged between 3–50.8% (mean: 14.5 ± 14.5) in Marseille and 4.9–34.2% (mean 17.1 ± 10.6) in Scandola [28]. It is therefore possible that the number of affected colonies displaying old overgrowth (type C injury) in Marseille reflects the effects of the past MME given the slow recovery capacity of gorgonian species [22, 42]. This hypothesis is further supported by the high densities observed in the majority of the NW Mediterranean sites, and the low number of large colonies (>30 cm), as observed in other populations during recovery (e.g. [69–71]).
Within the CE Adriatic, E. cavolini populations found in the relative pristine conditions of Kornati presented a smaller mean extent of injury, as well as smaller proportion of affected colonies, compared to the populations of Pag and Rogoznica which are located more closely to the mainland and are potentially more exposed to human-induced stressors. As overgrowth by epibionts in all sites of the CE Adriatic was mostly old (injury type C), no recent disturbances were indicated at the time of assessment. Our observations are in agreement with reported disturbance values for populations of the red gorgonian P. clavata in the region [79].
Regardless locality, the majority of N Aegean populations displayed a relatively low mean extent of injury, but overall high proportion of affected colonies. The injury values observed, combined with the generally low density and recruitment success recorded, render gorgonian populations of the N Aegean more prone to potential threats. Furthermore, in the sites of Ambelos and Kalloni, proportion of injured colonies (64.1% and 52.4% respectively) suggest increased levels of stress, while the high number of dead colonies (13.6%) in Kalloni is indicative of a strong impact. Although the reasons of the observed disturbance cannot be readily addressed through the present study, the increased levels of fishing activities (especially recreational and artisanal), and recurrent periods of high nutrient loads in the wider area [80–81], constitute some of the potential contributing stressors, especially given the shallow depth range of this population.
Closing remarks
Quantifying the demographic characteristics and disturbance levels of E. cavolini populations enabled the assessment of their conservation status and the acquisition of comparative information over a wide range of the species’ known spatial distribution [30]. The patterns observed provide insights as to how biotic, abiotic, and anthropogenic factors may affect the structure and dynamics of populations at the spatial scales addressed in this study, and subsequently influence their adaptive capacity to environmental change. Given the widespread distribution of E. cavolini, further research will allow a more comprehensive view of the population trends presented herein, and enhance understanding and mitigation of potential future impacts. Extending the same kind of approaches to include other areas and species would provide key information that will help develop effective management plans for the conservation of valuable hard-bottom communities, including coralligenous assemblages, across the highly heterogeneous Mediterranean basin [82].
Supporting Information
(PDF)
(XLS)
Acknowledgments
We are grateful to Emma Cebrian, Kike Ballesteros, Jean-Baptiste Ledoux, Diego K. Kersting, Jeremiah McCurdy, Aleksandar Popijač, Dumica Klarić, Donat Petricioli, Panagiotis Papadelis, Elina Samara, Vasilis Gerovasileiou, Hector Giourgis, and Elena Akritopoulou for assistance during the field work. Paris Sofos, Elias Papadopoulos, Vladislav Mihelčić and Kornati National Park staff are acknowledged for providing logistic assistance. Special thanks to Vasilis Trygonis for his constructive comments on the manuscript and programming skills, and to Charis Dimitriadis for providing statistical advice. Prof. G. Bavestrello and an anonymous reviewer are acknowledged for their valluable comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
MS research activity has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds (Ministry of Education and Religious Affairs managing authority) through the Operational Program "Education and Lifelong Learning" of the National Strategic Reference Framework (NSRF) - Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. SK received funding provided by the Croatian Ministry of Culture through the grant allocated to Marine Explorers Society –“20,000 leagues” in 2009 and by the Croatian Ministry of Science and Education (project 119-0362975-1226). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bak RPM, Meesters EH. Coral population structure: the hidden information of colony size-frequency distributions. Mar Ecol Prog Ser. 1998; 162: 301–306. [Google Scholar]
- 2. Harmelin JG, Garrabou J. Suivi d’une population de Paramuricea clavata (Risso, 1826) (Cnidaria, Octocorallia, Gorgonacea) dans le parc national de Port-Cros (Me′diterraneé, France): comparaison des e′tats 1992 et 2004 sur le site de la Gale`re. Sci Rep Port-Cros Natl Park. 2005; 21: 175–191. [Google Scholar]
- 3. Linares C, Coma R, Garrabou J, Diaz D, Zabala M. Size distribution, density and disturbance of two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis . J Appl Ecol. 2008; 45: 688–699. [Google Scholar]
- 4. Gori A, Rossi S, Linares C, Berganzo E, Orejas C, Dale MRT, et al. Size and spatial structure in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Mar Biol. 2011; 158: 1721–1732. [Google Scholar]
- 5. Grigg RW. Population dynamics of two gorgonian corals. Ecology. 1977; 57:278–290. [Google Scholar]
- 6. Gilmour JP. Size-structures of populations of the mushroom coral Fungia fungites: the role of disturbance. Coral Reefs. 2004; 23: 493–504. [Google Scholar]
- 7. Tsounis G, Rossi S, Gili JM, Arntz W. Population structure of an exploited benthic cnidarian: the red coral case study. Mar Biol. 2006; 149: 1059–1070. [Google Scholar]
- 8. Alvarado-Chácon EM, Acosta A. Population size-structure of the reef-coral Montastraea annularis in two contrasting reefs of a marine protected area in the southern Caribbean Sea. Bull Mar Sci. 2009; 85: 61–76. [Google Scholar]
- 9. Bak RPM, Meesters EH. Population structure as a response of coral communities to global change. Am Zool. 1999; 39: 56–65. [Google Scholar]
- 10. Grigg RW. Age structure of a longevous coral: A relative index of habitat suitability and stability. Am Nat. 1975; 109: 647–657. [Google Scholar]
- 11. Weinbauer MG, Velimirov B. Relative habitat suitability and stability of the Mediterranean gorgonian coral Eunicella cavolini (Coelenterata: Octocorallia). Bull Mar Sci. 1996; 58: 786–791. [Google Scholar]
- 12. Pérès JM, Picard J. Nouveau manuel de bionomie benthique de la Méditerranée. Rec. Trav Stat Mar Endoume. 1964; 31: 1–37. [Google Scholar]
- 13. True MA. Étude quantitative de quatre peuplements sciaphiles sur substrat rocheurs dans la région marseillaise. Bull Inst Océanogr Monaco. 1970; 60: 1–41. [Google Scholar]
- 14. Ballesteros E. Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr Mar Biol Ann Rev. 2006; 44: 123–195. [Google Scholar]
- 15. Jones CJ, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994; 69: 373–386. [Google Scholar]
- 16. Gili JM, Coma R. Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol. 1998; 13: 316–321. [DOI] [PubMed] [Google Scholar]
- 17. Cerrano C, Danovaro R, Gambi C, Pusceddu A, Riva A, Schiaparelli S. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv. 2010; 19: 153–167. [Google Scholar]
- 18. Ponti M, Perlini RA, Ventra V, Grech D, Abbiati M, Cerrano C. Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PLOS ONE. 2014; 9: e102782 10.1371/journal.pone.0102782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coma R, Ribes M, Zabala M, Gili J M. Growth in a modular colonial marine invertebrate. Estuar Coast Shelf Sci. 1998; 47: 459–470. [Google Scholar]
- 20. Garrabou J, Harmelin G. A 20-year study on life-history traits of a harvested long-lived temperate coral in the NW Mediterranean: insights into conservation and management needs. J Anim Ecol. 2002; 71: 966–978. [Google Scholar]
- 21. Torrents O, Garrabou J, Marschal C, Harmelin JG. Age and size at first reproduction in the commercially exploited red coral Corallium rubrum (L.) in the Marseilles area (France, NW Mediterranean). Biol Conserv. 2005; 121: 391–397 [Google Scholar]
- 22. Linares C, Doak D, Coma R, Diaz D, Zabala M. Life history and population viability of a long-lived marine invertebrate: the octocoral Paramuricea clavata . Ecology. 2007; 88: 918–928. [DOI] [PubMed] [Google Scholar]
- 23. Mistri M, Ceccherelli VU. Effects of a mucilage event on the Mediterranean gorgonian Paramuricea clavata. Short term impacts at the population and colony levels. Ital J Zool. 1996; 63: 221–230. [Google Scholar]
- 24. Bavestrello G, Cerrano C, Zanzi D, Cattaneo-Vietti R. Damage by fishing activities in the Gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquat Conserv. 1997; 7: 253–262. [Google Scholar]
- 25. Coma R, Pola E, Ribes M, Zabala M. Long-term assessment of the patterns of mortality of a temperate octocoral in protected and unprotected areas: a contribution to conservation and management needs. Ecol Appl. 2004; 14: 1466–1478. [Google Scholar]
- 26. Cebrian E, Linares C, Marschal C, Garrabou J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol Invasions. 2012; 14: 2647–2656. 10.1128/MCB.00573-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerrano C, Bavestrello G, Bianchi N, Cattano-Vietti R, Bava S, Morganti C, et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol Lett. 2000; 3: 284–293. [Google Scholar]
- 28. Garrabou J, Coma R, Benssoussan N, Bally M, Chevaldonné P, Cigliano M, et al. Mass mortality in NW Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Change Biol. 2009; 15: 1090–1103. [Google Scholar]
- 29. Weinberg S. Autoecology of shallow-water octocorallia from Mediterranean rocky substrata. II. Marseille, Cote d’Azur and Corsica. Bijdr Dierkd. 1980; 50: 73–86. [Google Scholar]
- 30. Sini M, Kipson S, Linares C, Garrabou J, Koutsoubas D. Distribution of Eunicella cavolini (Koch, 1887) across the Mediterranean In: Bouafif C, Langar H, Ouerghi A, editors. Proceedings of the 2nd Mediterranean Symposium on the conservation of coralligenous and other calcareous bio-concretions (Portorož, Slovenia, October 29–30). Tunis: UNEP-MAP-RAC/SPA; 2014. pp. 235–236. [Google Scholar]
- 31. Topçu EN, Öztürk B. Octocoral diversity of Balıkçı Island, the Marmara Sea. J Black Sea/Medit Environ. 2013; 19: 46–57. [Google Scholar]
- 32. Velimirov B. Orientation in the sea fan Eunicella cavolinii related to water movement. Helgol Wiss Meeres. 1973; 24: 163–173. [Google Scholar]
- 33. Velimirov B. Wachstum und altersbestimmung der gorgonie Eunicella cavolinii . Oecologia. 1975; 19: 259–1272. [DOI] [PubMed] [Google Scholar]
- 34. Velimirov B. Variations in growth forms of Eunicella cavolinii Koch (Octocorallia) related to intensity of water movement. J Exp Mar Biol Ecol. 1976; 21: 109–117. [Google Scholar]
- 35. Russo A. Ecological observations on the gorgonian sea fan Eunicella cavolinii in the Bay of Naples. Mar Ecol Prog Ser. 1985; 24: 155–159. [Google Scholar]
- 36. Bavestrello G, Boero F. Necrosi e rigenerazione in Eunicella cavolinii in Mar Ligure. Boll Mus Ist Biol Univ Genova. 1986; 52: 295–300. [Google Scholar]
- 37. Weinbauer MG, Velimirov B. Morphological variations in the Mediterranean sea fan Eunicella cavolini (Coelenterata: Gorgonacea) in relation to exposure, colony size and colony region. Bull Mar Sci. 1995; 56: 283–295. [Google Scholar]
- 38. Weinbauer MG, Velimirov B. Biomass and secondary production of the temperate gorgonian coral Eunicella cavolini (Coelenterata: Octocorallia). Mar Ecol Prog Ser. 1995; 12: 211–216. [Google Scholar]
- 39. Weinbauer MG, Velimirov B. Population dynamics and overgrowth of the sea fan Eunicella cavolinii (Coelenterata: Octocorallia). Estuar Coast Shelf Sci. 1996; 42: 583–595. [Google Scholar]
- 40. Cigliano M, Gambi MC. The long hot summer: Further mortality event of gorgonians along the Phlaegrean Islands (Tyrrhenian Sea). Biol Mar Mediterr. 2007; 14: 292–293. [Google Scholar]
- 41. Gambi MC, Barbieri F, Singorelli S, Saggiomo V. Mortality events along the Campania coast (Tyrrhenian Sea) in summers 2008 and 2009 and relation to thermal conditions. Biol Mar Mediterr. 2010; 17: 126–127. [Google Scholar]
- 42. Linares C, Coma R, Diaz D, Zabala M, Hereu B, Dantart L. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Mar Ecol Prog Ser. 2005; 305: 127–137. [Google Scholar]
- 43. Coma R, Linares C, Ribes M, Diaz D, Garrabou J, Ballesteros E. Consequences of a mass mortality event in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean). Mar Ecol Prog Ser. 2006; 327: 51–60. [Google Scholar]
- 44. Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D'Agrosa C, et al. A global map of human impact on marine ecosystems. Science. 2008; 319: 948–952. 10.1126/science.1149345 [DOI] [PubMed] [Google Scholar]
- 45. Lejeusne C, Chevaldonne P, Pergent-Martini C, Boudouresque CF, Pérez T. Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol Evol. 2010; 25: 250–260. 10.1016/j.tree.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 46. Garrabou J, Ballesteros E, Zabala M. Structure and dynamics of north-western Mediterranean rocky benthic communities along a depth gradient. Estuar Coast Shelf Sci. 2002; 55: 493–508. [Google Scholar]
- 47. Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, Aguzzi J, et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLOS ONE. 2010; 5: e11842 10.1371/journal.pone.0011842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Micheli F, Halpern BS, Walbridge S, Ciriaco S, Ferretti F, Fraschetti S, et al. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLOS ONE. 2013; 8: e79889 10.1371/journal.pone.0079889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sokal R, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd edition New York: Freeman; 1995. [Google Scholar]
- 50. Kruskal JB, Wish M. Multidimensional Scaling. Beverly Hills: Sage Publications; 1978. [Google Scholar]
- 51. Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001; 26: 32–46. 11469182 [Google Scholar]
- 52. Clarke KR, Gorley RN. PRIMER v6: User Manual/Tutorial. Plymouth: PRIMER-E Ltd; 2006. [Google Scholar]
- 53. Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: PRIMER-E Ltd; 2008. [Google Scholar]
- 54. Bo M, Canese S, Spaggiari C, Pusceddu A, Bertolino M, Angiolillo M, et al. Deep Coral Oases in the South Tyrrhenian Sea. PLOS ONE. 2012; 7: e49870 10.1371/journal.pone.0049870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watremez P. Canyon heads in the French Mediterranean Overview of results from the MEDSEACAN and CORSEACAN campaings (2008–2010) In: Würtz M, editor. Mediterranean Submarine Canyons: Ecology and Governance. Gland and Málaga: IUCN; 2012. pp. 105–112. [Google Scholar]
- 56. Bianchi CN, Morri C, Navone A. I popolamenti delle scogliere rocciose sommerse dell’Area Marina Protetta di Tavolara Punta Coda Cavallo (Sardegna nord-orientale). Tr scient Parc Nat Port-Cros. 2010; 24: 39–85. [Google Scholar]
- 57. Zavodnik D, Pallaoro A, Jaklin A, Kovačić, Arko-Pijevac M. A benthos survey of the Senj Archipelago (North Adriatic Sea, Croatia). Acta Adriat. 2005; 46: 3–68. [Google Scholar]
- 58. Dounas C, Koutsoubas D, Salomidi M, Koulouri P, Gerovasileiou V, Sini M. Distribution and fisheries of the red coral Corallium rubrum (Linnaeus, 1758) in the Greek seas: an overview In: Bussoletti E, Cottingham D, Bruckner A, Roberts G, Sandulli R, editors. Proceedings of the International Workshop on Red Coral Science, Management, and Trade: Lessons from the Mediterranean (Naples, Italy, September 23–26). NOAA Technical Memorandum CRCP-13. Silver Spring: NOAA; 2009. pp. 106–114. [Google Scholar]
- 59. Salomidi M, Smith C, Katsanevakis S, Panayotidis P, Papathanassiou V. Some observations on the structure and distribution of gorgonian assemblages in the eastern Mediterranean Sea In: Pergent-Martini C, Brichet M, editors. Proceedings of the 1st symposium on conservation of the coralligenous and other calcareous bio-concretions (Tabarka, Tunisia, January 15–16). Tunis: UNEP-MAP-RAC/SPA; 2009. pp. 242–245. [Google Scholar]
- 60. Skoufas G. Comparative biometry of Eunicella singularis (Gorgonian) sclerites at East Mediterranean Sea (North Aegean Sea, Greece). Mar Biol. 2006; 149: 1365–1370. [Google Scholar]
- 61. Ioannou E, Abdel-Razika AF, Zervou M, Christofidis D, Alexi X, Vagias C, et al. 5α,8α-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: Isolation and evaluation of their antiproliferative activity. Steroids. 2009; 74: 73–80. 10.1016/j.steroids.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 62. Zabala M, Ballesteros E. Surface-dependent strategies and energy flux in benthic marine communities or, why corals do not exist in the Mediterranean. Sci Mar. 1989; 53: 3–17. [Google Scholar]
- 63. Gili J, Murillo J, Ros J. The distribution pattern of benthic Cnidarians in the Western Mediterranean. Sci Mar. 1989; 53:19–35 [Google Scholar]
- 64. Ignatiadis L. Scaling the trophic status of the Aegean Sea, eastern Mediterranean. J Sea Res. 2005; 54: 51–57. [Google Scholar]
- 65. Bosc E, Bricaud A, Antoine D. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Global Biogeochem Cy. 2004; 18: GB1005, 10.1029/2003GB002034 [DOI] [Google Scholar]
- 66. Weinberg S. Autoecology of shallow-water Octocorallia from Mediterranean rocky substrata. I. The Banyuls area. Bijdr Dierkd. 1979; 49: 1–15. [Google Scholar]
- 67. Harmelin JG, Marinopoulos J. Population structure and partial mortality of the gorgonian Paramuricea clavata (Risso) in the North-Western Mediterranean (France, Port-Cros Island). Marine Life. 1994; 4: 5–13. [Google Scholar]
- 68. Linares C, Bianchimani O, Torrents O, Marschal C, Drap P, Garrabou J. Marine protected areas and the conservation of long-lived invertebrates: the Mediterranean red coral. Mar Ecol Prog Ser. 2010; 402: 69–79. [Google Scholar]
- 69. Cupido R, Cocito S, Barsanti M, Sgorbini S, Peirano A, Santangelo G. Unexpected long-term population dynamics in a canopy-forming gorgonian following mass mortality. Mar Ecol Prog Ser. 2009; 394: 195–200. [Google Scholar]
- 70. Linares C, Zabala M, Garrabou J, Coma R, Diaz D, Hereu B, et al. Assessing the impact of diving in coralligenous communities: the usefulness of demographic studies of red gorgonian populations. Sci Rep Port-Cros Natl Park. 2010; 24:161–184. [Google Scholar]
- 71. Gambi MC, Barbieri F. Population structure of the gorgonian Eunicella cavolinii in the “Grotta Azzurra” cave off Palinuro, after the mass mortality event in 2008. Biol Mar Mediterr. 2012; 19:174–175. [Google Scholar]
- 72. Gori A, Bramanti L, López-González P, Thoma J, Gili JM, Grinyó J, et al. Characterization of the zooxanthellate and azooxanthellate morphotypes of the Mediterranean gorgonian Eunicella singularis . Mar Biol. 2012; 159: 1485–1496. [Google Scholar]
- 73. Sebens KP. Energetic constraints, size gradients, and size limits in benthic marine invertebrates. Integ Comp Biol. 2002; 42:853–861. 10.1093/icb/42.4.853 [DOI] [PubMed] [Google Scholar]
- 74. Yoshioka PM. Size-specific life history pattern of a shallow-water gorgonian. J Exp Mar Biol Ecol. 1994; 184: 111–122. [Google Scholar]
- 75. Yoshioka PM. Are large colonies a “key factor” in the dynamics of gorgonian populations? Rev Biol Trop. 1998; 446: 137–143. [Google Scholar]
- 76. Cupido R, Cocito S, Manno V, Ferrando S, Peirano A, Iannelli M, et al. Sexual structure of a highly reproductive, recovering gorgonian population: quantifying reproductive output. Mar Ecol Prog Ser. 2012; 469: 25–36. [Google Scholar]
- 77. Cerrano C, Arillo A, Azzini F, Calcinai B, Castellano L, Muti C, et al. Gorgonian population recovery after a mass mortality event. Aquat Conserv. 2005; 15: 147–157. [Google Scholar]
- 78. Cupido R, Cocito S, Sgorbini S, Bordone A, Santangelo G. Response of a gorgonian (Paramuricea clavata) population to mortality events: recovery or loss? Aquatic Conserv: Mar Freshw Ecosyst. 2008; 18: 984–992. [Google Scholar]
- 79. Kipson S, Linares C, Čižmek H, Cebrián E, Ballesteros E, Bakran-Petricioli T, et al. Population structure and conservation status of the red gorgonian Paramuricea clavata (Risso, 1826) in the Eastern Adriatic Sea. Mar Ecol. 2014; 10.1111/maec.12195 [DOI] [Google Scholar]
- 80. Spatharis S, Tsirtsis G, Danielidis DB, Do Chi T, Mouillot D. Effects of pulsed nutrient inputs on phytoplankton assemblage structure and blooms in an enclosed coastal area. Coast Estuar Sci. 2007; 73: 807–815. [Google Scholar]
- 81. Spatharis S, Dolapsakis NP, Economou-Amilli A, Tsirtsis G, Danielidis DB. Dynamics of potentially harmful microalgae in a confined Mediterranean Gulf-Assessing the risk of bloom formation. Harmful Algae. 2009; 8: 736–743. [Google Scholar]
- 82. Giakoumi S, Sini M, Gerovasileiou V, Mazor T, Bejer J, Possingham HP, et al. Ecoregion-Based Conservation Planning in the Mediterranean: Dealing with Large-Scale Heterogeneity. PLOS ONE. 2013; 8: e76449 10.1371/journal.pone.0076449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.