Abstract
We previously detected and investigated nine altered microRNAs in small intestinal neuroendocrine tumor (SI-NET) tissues at different stages of disease. The aims of this study are to: 1) analyze whether SI-NET tissue microRNAs can be also detected in patient serum samples, 2) investigate a potential somatostatin analogs (SSAs) role on microRNA levels regulation in SSA-treated patient samples and 3) elucidate whether the serum microRNA levels in samples collected in different hospitals are predictable and steady. Our results show that tissue microRNAs are detectable in patient serum samples, and miR-96, -182, -183, -196a and -200a levels are lower in SI-NET untreated patients than in SSA-treated patients at all different stages. Conversely, miR-31, -129-5p, -133a and -215 levels do not show any difference in untreated SI-NET patients and SSA-treated patients at all different stages. Our findings also show that miR-200a exhibits an atypical behavior with high levels in both untreated and SSA-treated patients at liver metastasis stage, and unequivocally never at the earlier stages. Serum samples collected in two hospitals keep alike microRNA level pattern, elucidating that the results are not dependent on samples handling. In conclusion, SI-NET tissue microRNAs are always detectable in untreated and SSA-treated patient serum samples, SSAs play an unknown role in eliciting SSA-treated patients’ microRNA levels higher than in untreated patients, and this study enlightens that miR-200a might be involved in the liver metastasis during SI-NET progression.
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of malignancies in terms of their biological and clinical features, which cause NETs difficulties in being early diagnosed [1]. NETs are rare malignancies that mainly develop in the gastrointestinal and bronchopulmonary tract [2]. Small intestinal NETs (SI-NETs) are the most common gastrointestinal NETs, and derive from serotonin-producing enterochromaffin cells, which are distributed throughout the human body [3].
The majority of SI-NETs have an indolent course. Surgical resection of primary SI-NETs is the only efficient method for curing patients. However, delayed diagnosis mainly shows that metastases spread to the liver producing inoperable SI-NETs, becoming mainly fatal. Biotherapies using somatostatin analogs (SSAs) are palliative therapies of major clinical importance to reduce symptoms and control tumor growth [4].
MicroRNAs (miRNAs) are short non-coding RNA sequences of 18 to 25 nucleotides involved in a variety of biological processes, such as cell proliferation, differentiation, apoptosis, development and metabolism. They have a role as post-transcriptional regulators, inducing either translational repression of target mRNAs or their target mRNAs degradation [5]. Abnormal miRNA expression in cancer was initially detected in B-cell chronic lymphocytic leukemia using blood samples [6]; however, miRNAs altered regulation has been associated with many solid tumor types as well [7]. Several studies, which include our recent SI-NET miRNA expression profile [8–11], have reported a number of unexpected miRNAs regulation in NETs.
MiRNAs have recently been detected in human body fluids, mainly serum and plasma, and patient blood is easier to collect than tumor tissues [12,13]. Circulating miRNAs have already been used to perform miRNA profiles, and this supports the scientific possibility of miRNAs becoming potential cancer biomarkers [14–17]. Nevertheless, challenges are involved in circulating miRNA detection, such as biofluids contain a lower amount of miRNAs, having more inhibitors of reverse transcriptase than tissues, and there is lack of reliable method to quantitate miRNAs and normalize quantitative real time PCR (QRT-PCR) analysis using serum and plasma samples [18,19]. To outdistance these challenges, adding carrier RNA, such as bacteriophage MS2, during RNA isolation procedure, including synthetic spike-in RNA to monitor sample quality and selecting miR-16 as reliable reference for normalization of QRT-PCR analysis are mainly the best systems to use [20].
The importance of cancer metabolism became an important hall mark [21]. Cancer-relevant pathways and metabolic phenotype of tumors newly addressed the importance of the Warburg effect [22] throughout molecular aspects and therapeutic possibilities. Although normal cells undergo glycolysis and oxidative phosphorylation in the presence of oxygen, proliferating and cancer cells exhibit an increased uptake of glucose and increased rate of glycolysis, predominantly undergo lactic acid fermentation. Today, it is a challenge to understand whether this phenomenon is either the consequence of altered genetic regulation in cancer or it is one cause of cancer development. Hence, it is of importance to remember that miRNAs regulate the aerobic glycolysis through a variety of established mechanisms and pathways as properly described [23]. Because altered regulated miRNAs has already turned out being correlated with diagnosis, staging, progression, prognosis and response to clinical therapies in several malignancies [24], establishing the miRNA roles in tumor biology and cancer metabolism might be important for SI-NETs.
We recently provided a genome-wide miRNA profiling by using SI-NET tissue specimens. Bioinformatics and experimental analyses showed that five tissue miRNAs (miR-96, -182, -183, -196a and -200a) were upregulated, whereas four (miR-31, -129-5p, -133a and -215) were downregulated at all different stages [8]. We first extended our analyses from tissue specimens to serum samples to show that biological fluids are ideal for monitoring SI-NET patients at different stages. We also investigated the nine tissue miRNAs in patient serum samples at different stages, with two other major aims. The second one focused on whether SSAs might play a control role on serum miRNA levels. The third one investigated serum miRNA level patterns, using samples collected in different hospitals, to determine whether the patterns were not dependent on sample handling.
Materials and Methods
Serum Samples
A total of 70 serum samples were collected at Uppsala University Hospital (UU) and University College of London (UCL), 49 out of 70 were from UU, 21 out of 70 from UCL (Tables 1 and 2). Seven healthy donor (HD) samples and 42 SI-NET patient samples at different stages: 14 primary tumors (PT), 14 lymph node metastases (LNM) and 14 liver metastases (LM), in summary 49 samples collected at UU (Table 1). We included a total of 21 samples collected at UCL, 7 HD serum samples and 14 SI-NET serum samples at the LM stage, collected at UCL (Table 2). A drug regimen, metastatic site and grade unbiased selection to match UCL samples with the ones from UU resulted in nine samples 3 HD and 6 LM, which were used in the study. The nine bolded samples from UCL, matching the bolded ones from UU in Table 1, are shown in Table 2. In conclusion, 58 samples (49 from UU and 9 from UCL) were used in the study upon unbiased selection of matched samples. Permission to collect serum samples at Uppsala University Hospital was approved by the Regional Ethics Committee Uppsala [Dnr 2011/426], whereas permission to collect serum samples at the University College of London was approved by The East London and The City Alpha Research Ethics Committee [09/H0704/44] with written informed consent provided by all the donors. Eligible patients had pathologically confirmed NETs categorized according to their primary site of origin with metastatic disease measurable by Response Evaluation Criteria in Solid Tumors (RECIST).
Table 1. Serum samples collected at Uppsala University Hospital.
| ID | Sex/Age* | Treatment | Type | Metastatic site | Grade |
|---|---|---|---|---|---|
| HD1 | F/42 | ||||
| HD2 | F/51 | ||||
| HD3 | M/35 | ||||
| HD4 | F/61 | ||||
| HD5 | M/59 | ||||
| HD6 | M41 | ||||
| HD7 | M/41 | ||||
| PT1 | M/46 | Untreated | Primary | G1 | |
| PT2 | F/66 | Untreated | Primary | G1 | |
| PT3 | M/64 | Untreated | Primary | G1 | |
| PT4 | M/76 | Untreated | Primary | G2 | |
| PT5 | F/59 | Untreated | Primary | G1 | |
| PT6 | M/74 | Untreated | Primary | G1 | |
| PT7 | F/47 | Untreated | Primary | G1 | |
| PT8 | M/85 | SSA | Primary | G1 | |
| PT9 | M/79 | SSA | Primary | G1 | |
| PT10 | M/70 | SSA | Primary | G1 | |
| PT11 | M/60 | SSA | Primary | G1 | |
| PT12 | F/76 | SSA | Primary | G1 | |
| PT13 | M/79 | SSA | Primary | G1 | |
| PT14 | F/68 | SSA | Primary | G1 | |
| LNM1 | M/70 | Untreated | Metastases | Lymph Node | G2 |
| LNM2 | F/52 | Untreated | Metastases | Lymph Node | G1 |
| LNM3 | F/76 | Untreated | Metastases | Lymph Node | G1 |
| LNM4 | F/76 | Untreated | Metastases | Lymph Node | G1 |
| LNM5 | M/65 | Untreated | Metastases | Lymph Node | G1 |
| LNM6 | F/65 | Untreated | Metastases | Lymph Node | G1 |
| LNM7 | M/64 | Untreated | Metastases | Lymph Node | G1 |
| LNM8 | F/66 | SSA | Metastases | Lymph Node | G1 |
| LNM9 | M/75 | SSA | Metastases | Lymph Node | G1 |
| LNM10 | F/70 | SSA | Metastases | Lymph Node | G1 |
| LNM11 | F/72 | SSA | Metastases | Lymph Node | G1 |
| LNM12 | F/50 | SSA | Metastases | Lymph Node | G1 |
| LNM13 | M/77 | SSA | Metastases | Lymph Node | G1 |
| LNM14 | M/62 | SSA | Metastases | Lymph Node | G1 |
| LM1 | M/46 | Untreated | Metastases | Liver | G1 |
| LM2 | F/51 | Untreated | Metastases | Liver | G1 |
| LM3 | F/57 | Untreated | Metastases | Liver | G1 |
| LM4 | M/45 | Untreated | Metastases | Liver | G1 |
| LM5 | F/63 | Untreated | Metastases | Liver | G1 |
| LM6 | F/40 | Untreated | Metastases | Liver | G1 |
| LM7 | M/43 | Untreated | Metastases | Liver | G1 |
| LM8 | F/70 | SSA | Metastases | Liver | G1 |
| LM9 | F/71 | SSA | Metastases | Liver | G1 |
| LM10 | M/57 | SSA | Metastases | Liver | G1 |
| LM11 | M/72 | SSA | Metastases | Liver | G2 |
| LM12 | M/69 | SSA | Metastases | Liver | G2 |
| LM13 | F/51 | SSA | Metastases | Liver | G2 |
| LM14 | M/65 | SSA | Metastases | Liver | G2 |
Age at the time of sampling (Age*); Healthy donor (HD); Female (F); Male (M); Somatostatin Analogs (SSAs)
Bolded samples were selected to match with UCL samples
Table 2. Serum samples collected at University College London, UK.
| ID | Sex/Age* | Treatment | Type | Metastatic site | Grade |
|---|---|---|---|---|---|
| HD8 | M/31 | ||||
| HD9 | M/31 | ||||
| HD10 | F/30 | ||||
| HD11 | M/63 | ||||
| HD12 | M/48 | ||||
| HD13 | M/55 | ||||
| HD14 | F/56 | ||||
| LM15 | F/41 | Untreated | Metastases | Liver | G3 |
| LM16 | M/83 | Untreated | Metastases | Liver | G1 |
| LM17 | M/55 | Untreated | Metastases | Liver | G1 |
| LM18 | F/66 | Untreated | Metastases | Liver | G1 |
| LM19 | F/65 | Untreated | Metastases | Liver | G1 |
| LM20 | F/66 | Untreated | Metastases | Liver | G2 |
| LM21 | M/65 | Untreated | Metastases | Liver | G2 |
| LM22 | F/75 | Untreated | Metastases | Liver | G2 |
| LM23 | F/57 | Surgery | Metastases | Liver | G1 |
| LM24 | F/67 | Y90 | Metastases | Liver | G1 |
| LM25 | F/71 | Y90 | Metastases | Liver | G2 |
| LM26 | M/74 | SSA | Metastases | Liver | G2 |
| LM27 | M/69 | SSA | Metastases | Liver | G2 |
| LM28 | F/74 | SSA | Metastases | Liver | G2 |
Age at the time of sampling (Age*); Healthy donor (HD); Female (F); Male (M); Yttrium (Y90); Somatostatin Analogs (SSA)
Bolded samples were selected to match with UU samples
RNA Extraction
Total RNA containing small RNA, including miRNAs, was isolated by using the mirVana PARIS Kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions, with a few added modifications, as reported in the next paragraph. We added 250 μL serum from each sample to a 1.5 mL RNase-free tube mixed with Denaturing Solution Master Mix, including 2x Denaturing Solution, 1 μg carrier MS2 bacteriophage RNA (Roche Applied Science, Basel, Switzerland) and 25 fmol cel-miR-39 spike-in oligos (Life Technologies). The purified serum RNA was eluted with RNAse-free water (Life Technologies) and was stored at -70°C until further experiments. The RNA concentration was measured by using the NanoDrop 1000 (NanoDrop, Wilmington, DE, USA). The efficiency of RNA recovery among the samples was evaluated by measuring cel-miR-39 expression, by using quantitative real-time PCR (QRT-PCR) analysis.
Reverse transcription (RT) and Pre-amplification of RT Products
Total RNA (5 μL) was reverse transcribed to cDNA using the TaqMan miRNA Reverse Transcription (RT) Kit (Life Technologies) and miRNA specific stem-loop primers (Life Technologies), according to the manufacturer’s instructions. Pre-amplification was performed following the RT process to increase serum miRNA quantity. Each pre-amplified reaction was carried out in 20 μL comprising 13 μL Platinum PCR SuperMix (Life Technologies), 5 μL 0.2x TaqMan Small RNA Assay (Life Technologies) and 2 μL RT products. The reaction mixture was incubated in the FlexCycler instrument (Analytik Jean AG, Jean, Germany) at 94°C for 2 minutes, and then at 94°C for 15 seconds and 60°C for 1 minute for 15 cycles.
Quantitative Real-Time PCR
The QRT-PCR reaction was run on the Stratagene Mx3005P real-time PCR System (Agilent Technologies, Santa Clara, CA, USA). Each QRT-PCR reaction was carried out in 20 μL comprising 2x TaqMan Universal PCR Master Mix II without UNG (Life Technologies), 20x TaqMan Small RNA Assay (1 μL) and 10x diluted pre-amplified RT product (5 μL). The reaction mixture was incubated for 2 minutes at 50°C and 10 minutes at 95°C. Then, 40 cycles of 15 seconds at 94°C, and 1 minute at 60°C, were run. The relative miRNA expression was evaluated by using the comparative cycle threshold method (2-ΔΔCT) [25] and hsa-miR-16, set to 1, as an endogenous control [20].
Statistical analysis
The statistical significance of the difference among groups was evaluated by using a one-way ANOVA test followed by either Dunnett’s test or the Bonferroni test, using GraphPad Prism 5 (Graph Pad, Software, La Jolla CA, USA); p value < 0.05 was considered significant.
Results
QRT-PCR analyses of serum samples from untreated SI-NET patients
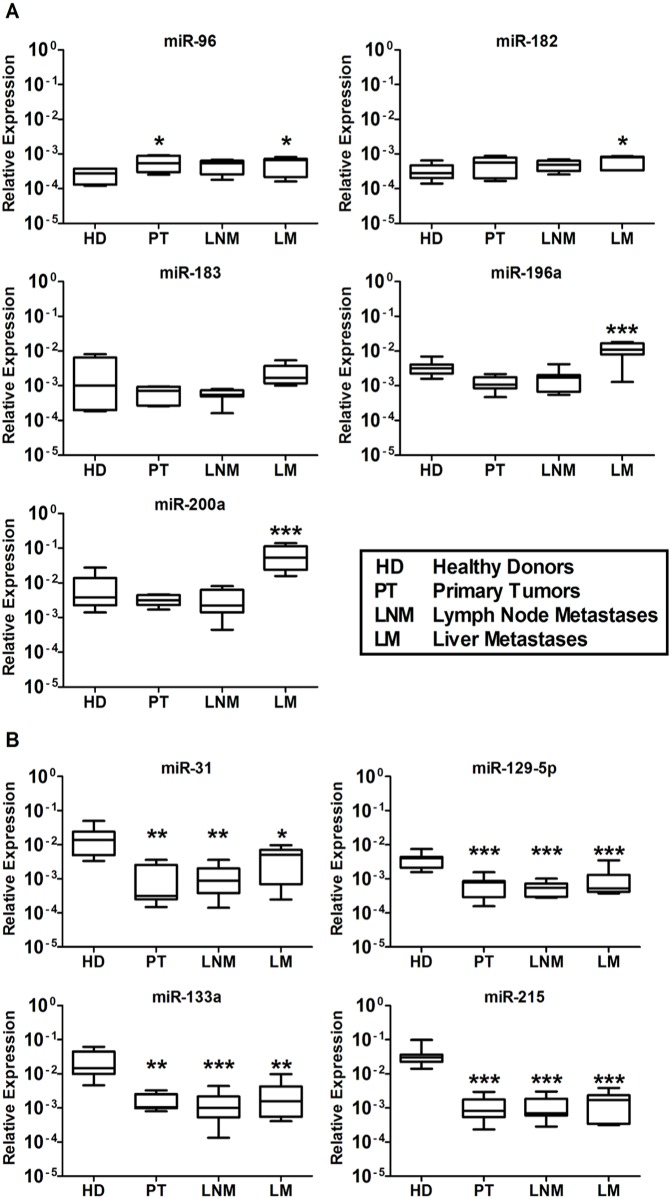
The nine tissue miRNAs were detected from serum samples of seven HD and 21 untreated SI-NET patients (7 PT, 7 LNM, and 7 LM), using TaqMan miRNA array (Life Technologies). The level of miR-96 was significantly higher at the stage of PT and LM, whereas miR-182, -196a and -200a levels were exclusively and significantly higher in LM patients versus (vs) HD. Oddly, miR-183 level was never significantly higher in any SI-NET patient group vs HD (Fig 1A). The level of miR-31, -129-5p, -133a and -215 was significantly lower in SI-NET patients at all different stages vs HD (Fig 1B).
Fig 1. QRT-PCR analysis of untreated SI-NETs at different stages of disease to detect previously analyzed SI-NET tissue miRNAs.
Total RNA was isolated from serum samples of seven healthy donors (HD 1–7), seven primary SI-NET (PT 1–7), seven lymph node metastases (LNM 1–7) and seven liver metastases (LM 1–7). (A) MiR-96 was significantly higher in PT and LM patients vs HD, whereas miR-182, -196a and -200a levels were exclusively and significantly higher in LM patients vs HD. Conversely, miR-183 level was never significantly higher in any SI-NET patient group vs HD (B) MiR-31, -129-5p, -133a and -215 levels were significantly lower in untreated patients at different stages vs HD. Results were plotted using the 2-ΔΔCt method with miR-16 expression (set to 1) from each individual sample for normalization. Significance was calculated by One-Way ANOVA followed by Dunnett’s test. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001.
QRT-PCR analyses of serum samples from SSA-treated SI-NET patients
The nine tissue miRNAs were clearly detected in serum samples of seven HD and 21 SSA-treated SI-NET patients at different stages (7 SSA-PT, 7 SSA-LNM, and 7 SSA-LM). The level of miR-96, -182, -183, -196a and -200a was significantly higher in SSA-treated patients at all different stages vs HD (Fig 2A). Conversely, the level of miR-31, -129-5p, -133a and -215 was significantly lower in SSA-treated patients at all different stages vs HD (Fig 2B).
Fig 2. QRT-PCR analysis of SSA-treated SI-NETs at different stages of disease to detect previously analyzed SI-NET tissue miRNAs.
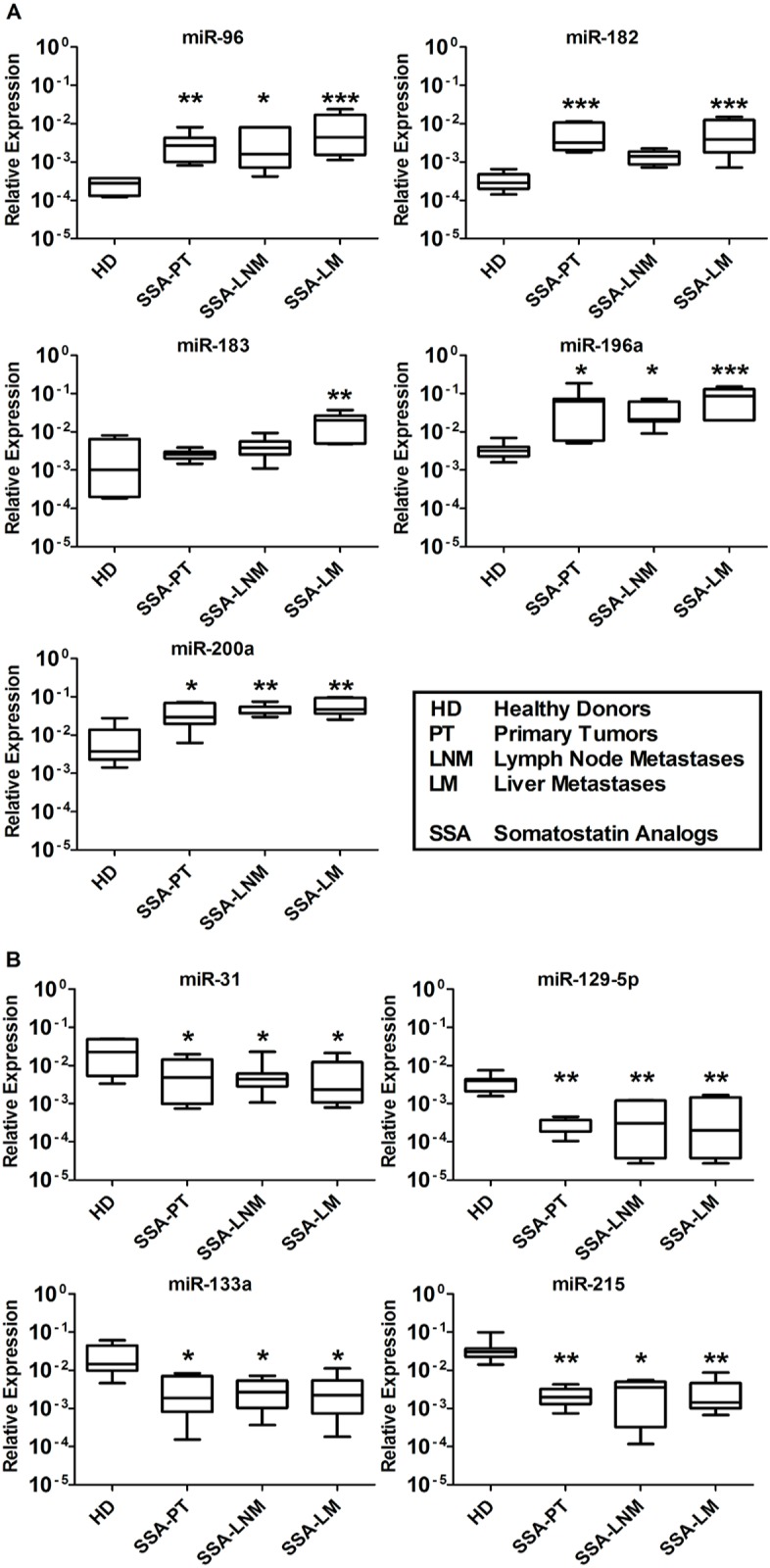
Total RNA was isolated from serum samples of seven healthy donors (HD 1–7), seven primary SI-NET (PT 8–14), seven lymph node metastases (LNM 8–14) and seven liver metastases (LM 8–14). (A) MiR-96, -182, -183, -196a and -200a levels were significantly higher at all stages of SSA-treated patients vs HD. (B) MiR-31, -129-5p, -133a and -215 levels were significantly lower at all different stages of SSA-treated patients vs HD. Results were plotted using the 2-ΔΔCt method with miR-16 expression (set to 1) from each individual sample for normalization. Significance was calculated by One-Way ANOVA followed by Dunnett’s test. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001.
MiRNA expression analysis of untreated and SSA-treated SI-NET patient serum
We further explored the nine serum miRNAs level in untreated and SSA-treated SI-NET patients. This investigation aimed at evaluating whether SSAs might have played any role in the miRNA level regulation. QRT-PCR analysis results show that the level of miR-96, -182, -183, -196a and -200a was higher in SSA-treated patients vs untreated SI-NET patients at all different stages. Oddly, miR-200a shows a different level pattern with no significant difference between untreated LM patients and SSA-treated LM patients (Fig 3). Instead, the four miRNAs, miR-31, -129-5p, -133a and -215, characterized by low levels in serum always show a similar level pattern in untreated SI-NET patients and in SSA-treated patients at all different stages (Fig 4).
Fig 3. Five upregulated tissue miRNAs were detected in serum samples of healthy donors, untreated and SSA-treated SI-NET patients at different stages.
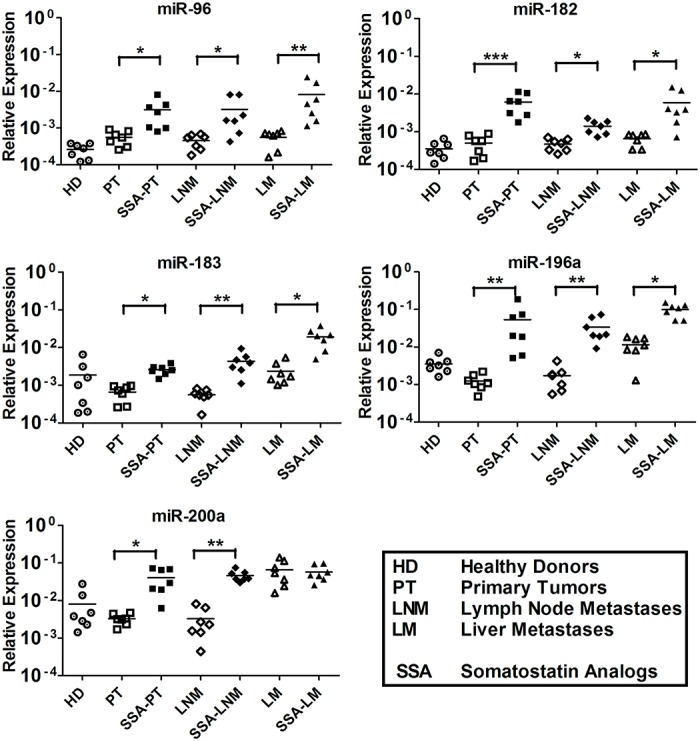
MiR-96, -182, -183, -196a and -200a levels were significantly higher at all stages of SSA-treated patients vs untreated patients. Remarkably, miR-200a showed an atypical behavior because its level was higher in both untreated LM patients and SSA-treated LM patients. The QRT-PCR analysis included seven healthy donors (HD 1–7), 21 untreated patients (PT 1–7, LNM 1–7 and LM 1–7) and 21 SSA-treated patients (SSA-PT 8–14, SSA-LNM 8–14 and SSA-LM 8–14). Results were plotted using the 2-ΔΔCt method with miR-16 expression (set to 1) from each individual sample for normalization. Significance was calculated by One-Way ANOVA followed by the Dunnett’s test. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001.
Fig 4. Four downregulated tissue miRNAs were detected in serum samples of untreated and SSA-treated SI-NET patients at all stages.
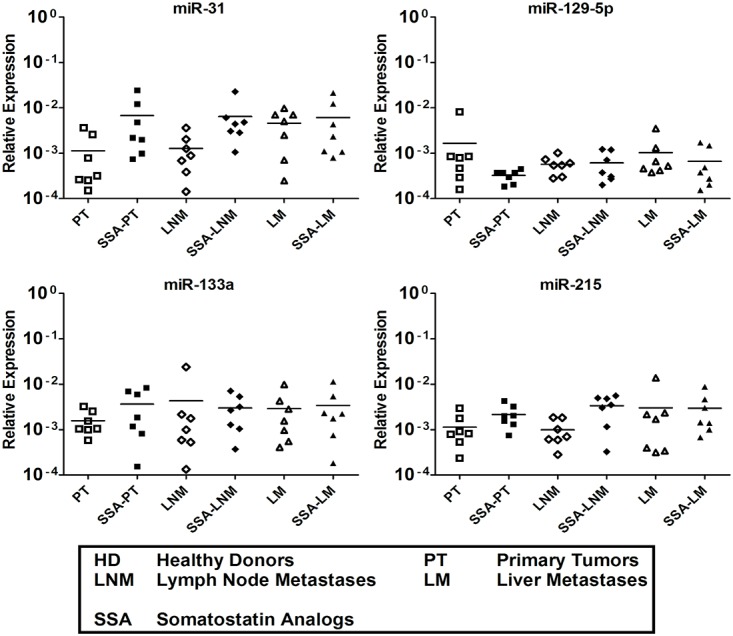
MiR-31, -129-5p, -133a and -215 levels did not show any different expression at all different stages of SSA-treated SI-NET patients vs untreated patients. The QRT-PCR analysis included 21 untreated patients (PT 1–7, LNM 1–7 and LM 1–7) and 21 SSA-treated patients (SSA-PT 8–14, SSA-LNM 8–14 and SSA-LM 8–14). Results were plotted using the 2-ΔΔCt method with miR-16 expression (set to 1) from each individual sample for normalization. Significance was calculated by One-Way ANOVA followed by the Dunnett’s test. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001.
Serum miRNA level patterns are alike in samples collected at UU and UCL
Nine out of 49 serum samples collected at UU, which match drug regimen, metastatic site and grade to nine out of 21 serum samples collected at UCL, were used to run independent experiments (Tables 1 and 2). The level of miR-96, -182, -183 and -196a was significantly higher in six SSA-treated LM patients vs six untreated LM patients and six HD (Fig 5A). Oddly, miR-200a was high level in both untreated LM patients and SSA-treated LM patients, as previously shown in Fig 3 (Fig 5B). Conversely, the level of miR-31, -129-5p, -133a and -215 was significantly lower in both untreated and SSA-treated at LM stage vs HD (Fig 6).
Fig 5. Five upregulated tissue miRNAs were detected in untreated and SSA-treated SI-NET patients at LM stage in serum samples collected at UU and UCL.
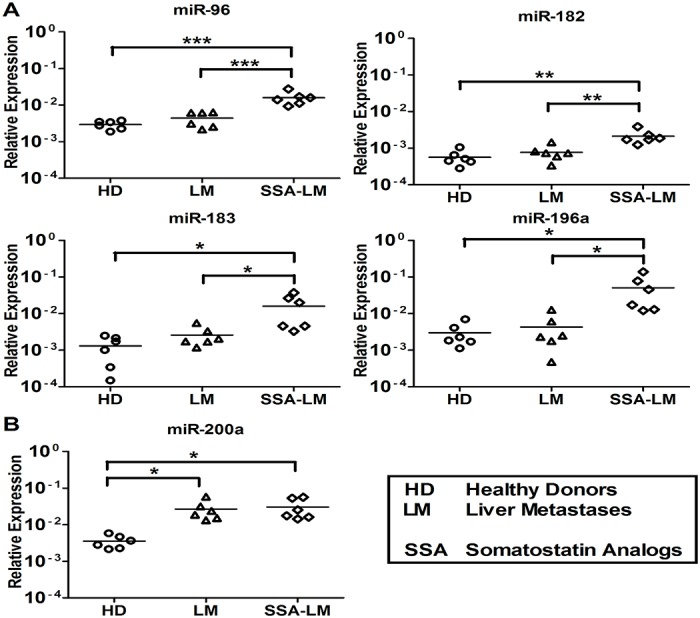
Total RNA was isolated from UU collection, including three healthy donors (HD 4–6), three untreated liver metastases (LM 3–5) and three SSA-treated liver metastases (SSA-LM 11–13). Total RNA was also isolated from UCL collection, including three healthy donors (HD 12–14), three untreated liver metastases (LM 17–19) and three SSA-treated liver metastases (SSA-LM 26–28). (A) MiR-96, -182, -183 and -196a levels were significantly higher in 6 SSA-treated LM patients vs 6 untreated LM patients and 6 HD. (B) MiR-200a showed the same atypical behavior previously shown in Fig 3. Results were plotted using the 2-ΔΔCt method with miR-16 expression (set to 1) from each individual sample for normalization. Significance was calculated by One-Way ANOVA followed by the Bonferroni test. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001.
Fig 6. Four downregulated tissue miRNAs were detected in untreated and SSA-treated SI-NET patients at LM stage in serum samples collected at UU and UCL.
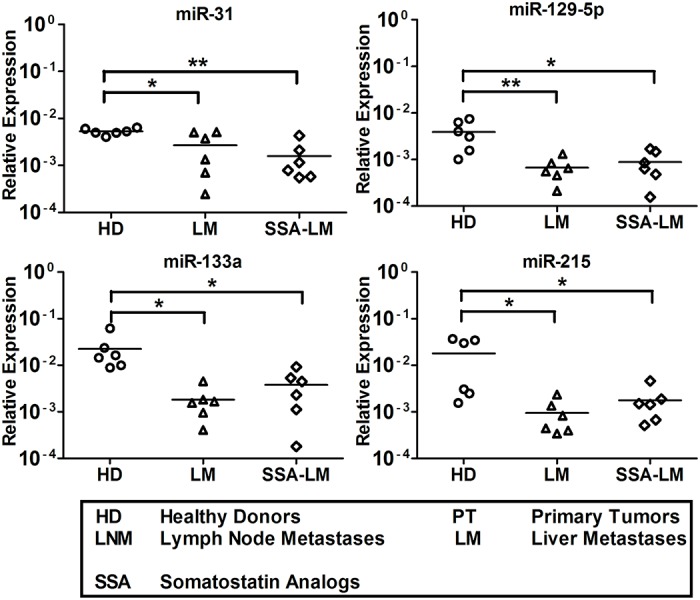
Total RNA was isolated from UU collection, including three healthy donors (HD 4–6), three untreated liver metastases (LM 3–5) and three SSA-treated liver metastases (SSA-LM 11–13). Total RNA was also isolated from UCL collection, including three healthy donors (HD 12–14), three untreated liver metastases (LM 17–19) and three SSA-treated liver metastases (SSA-LM 26–28). MiR-31, -129-5p, -133a and -215 levels were significantly lower in 6 untreated LM patients and 6 SSA-treated LM patients vs 6 HD. Results were plotted using the 2-ΔΔCt method with miR-16 expression (set to 1) from each individual sample for normalization. Significance was calculated by One-Way ANOVA followed by the Bonferroni test. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001.
Discussion
Most SI-NET patients receive a delayed diagnosis. Although early tumor detection might lead to curative surgery highlighting the unmet need for novel SI-NET biomarkers to identify and treat such rare tumors, novel SI-NET late biomarkers are also needed to have better SI-NET patient follow-up, and to develop therapeutic drugs to potentially reprogram the cancer glycolytic metabolism. Over recent decades, a variety of gene regulatory mechanisms have been unveiled. Nevertheless miRNAs, which are post-transcriptional regulators, able to control one third of the entire human genome became of major interest. They are correlated to a number of diseases, such as hematological and solid malignancies [6,26], and have been addressed as pivotal regulators of cancer glycolytic metabolism lately [23].
We identified differentially expressed miRNAs in SI-NET tissue at different stages showing how to develop SI-NET miRNAs as novel biomarkers and therapeutic molecular targets [8]. Scientists have been exploring miRNA expression in biological fluids since 2008 [12,13], because body fluid collection, such as serum and plasma, which are liquid biopsies, is much easier than tissue collection, and is advantageous to study rare diseases, such as SI-NETs, to improve prognostics and follow-up markers to improve patient clinical outcome.
Evaluation of tissue SI-NET miRNA in patient serum samples was one of the major aims of this study, to extend the scientific value of our previous study [8]. We thus, first investigated the levels of previously selected tissue miRNAs by using SI-NET patient serum samples. The possible detection triggered the interest to investigate whether SSAs, often used to improve the clinical management of SI-NET patients, might have regulating miRNA levels in serum. Results show that the five upregulated tissue miRNAs (miR-96, -182, -183, 196a and -200a), are present at high level in serum samples from SSA-treated patients, whereas the four downregulated tissue miRNAs (miR-31, -129-5p, -133a and -215) at low level in serum samples from SSA-treated patients.
Different miRNA levels in untreated and SSA-treated patient samples were analyzed in serum for the first time, showing a previously unveiled SSAs regulative role on miRNA levels. This might be investigated in patient tumor tissue in a retrospective manner to confirm the importance of these findings next future. Although five out of five miRNAs show high levels in patients’ serum, they always show higher levels when patients’ are under specific drug regimen, i.e. SSAs. Nevertheless, miR-200a showed an oddly phenomenon, showing that the high miR-200a level at the LM stage is not dependent on SSAs, because miR-200a is at high level in the untreated samples as well. Unexpectedly, the four miRNAs at low levels did not change in expression at any stage of disease in the presence of SSAs treatment.
SI-NET samples collected at Uppsala University showed that the SSA drug regimen has an unknown regulatory miRNA level effect in SI-NET patient serum samples. This finding suggested investigating whether the regulatory effect was relevant and not dependent on different hospitals sample handling. This last investigation turned out showing that the results of UU serum samples were alike to the ones using serum samples collected at UCL, characterized by unbiased identical drug regimen, metastatic site and grade. The remarkable consistency level patterns of the UU and UCL serum samples strengthened the relevant importance of SSAs regulatory effect.
One of the most unexpected findings is that miR-200a at the LM stage shows an oddly pattern level compared to the other four miRNAs at high levels included in the analyses both using serum samples collected UU and UCL. The miR-200a expression pattern level is also independently elevated in the absence of SSAs drug regimen in patients at LM stage, as previously shown by the UU samples analysis. Conversely, the four miRNAs with low levels showed no significant difference between untreated and SSA-treated samples in the experiment carried out with the samples collected in UU and UCL, in agreement with our hypothesis.
Metformin is currently the first-line drug treatment for type 2 diabetes. Besides its glucose-lowering effect, there is a growing interest for the actions of this drug as a relevant potential anti-cancer drug [27]. Over the last few years scientific evidences have shown that metformin exerts its cancer effects through miRNAs modulation to reprogram cancer metabolism on many different tumor types, such as breast, lung and pancreatic cancers [28]. Metformin recently started being evaluated for its role in pancreatic adenocarcinomas and neuroendocrine tumors as well [29]. Growing evidences have highlighted the role of miRNAs as pivotal regulators of metabolic process, such as lipid and cholesterol-synthesis. Although alteration in these processes represents important steps in tumor development, they also offer strategic opportunity to block the activity of specific miRNAs by using synthetic antagomir, also known as anti-miRNAs, as we discussed above. Several studies summarize a variety of miRNAs and their more relevant target genes in cancer, which are modulated by metformin, such as in the mentioned references [28,30].
Some of the most important miRNAs involved in anticancer metformin action are the miR-200 family. Several evidences have shown that cancer progression has similarities with the process of epithelial-to-mesenchimal transition (EMT) [31], and miR-200a is pivotal in this process [28]. Park et al., described that the entire miR-200 family, containing five miRNAs: miR-200a/miR-141; miR-200b/miR200c/miR-429, might become a powerful marker determining the epithelial phenotype of cancer cells by targeting the E-cadherin repressors. Although the miR-200 family members target a similar and highly overlapping set of genes, the relevance of miR-200a in controlling this phenomenon is clearly elucidated [32]. Furthermore, Eades et al. established the effects of the miR-200 family on transformation of the normal mammary epithelial cells showing that miR-200a plays a pivotal role in this [33].
Because very little is known on SI-NETs at the stage of liver metastases and their glycolytic metabolism, depicting miR-200a as the most important miRNA of this last study, revealing an unknown role of SSAs in regulating miRNAs levels is opening a new window of investigation, after miR-200a description in a recent published papers [8]. Thus, the unknown role of SSAs in regulating miRNAs levels might elucidate weather miR-200a regulate the SI-NET metastatic process, controlling target genes involved in the cancer glycolytic metabolism reprogramming.
In conclusion, our study shows that using body fluids instead of tumor tissue is possible to follow up SI-NET patients at different stage of diseases because miRNAs can be evaluated by QRT-PCR analysis. This might offer potential novel drug tests, which are less invasive for the patient and less expensive for the hospital. In addition, SSAs drug regimen has an unknown regulative effect on high level miRNAs in SI-NET patients, whereas this capacity is absent in the ones characterized by low level in patients serum. We have also shown that miR-200a is always at higher level during the liver metastasis stage during tumor progression in SI-NET patients, and that SSAs treatment cannot control miR-200a at this stage of disease at all, differently from the other four high levels miRNAs. This is the most novel and challenging finding for miR-200a in serum samples in our opinion, and it requires further studies to elucidate its role in both tumor progression and potentially in glycolytic cancer metabolism.
Acknowledgments
We appreciate Prof. M. Essand scientific support. We thank R. Naboulsi for technical support. We also thank N. Shabbir at the Royal Free London NHS Foundation Trust and J. North at University College London for collecting and transporting the serum samples.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by funding from the Lions Cancer Research, and Erik, Karin and Gösta Selander Foundation. The funders had no role in study design, data collection analysis and interpretation of data, decision to publish or preparation of the manuscript.
References
- 1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. (2008) One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26: 3063–3072. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 2. Taal BG, Visser O (2004) Epidemiology of neuroendocrine tumours. J Neuroendocrinol 80 Suppl 1: 3–7. [DOI] [PubMed] [Google Scholar]
- 3. Ahlman H, Nilsson (2001) The gut as the largest endocrine organ in the body. Ann Oncol 12 Suppl 2: S63–68. [DOI] [PubMed] [Google Scholar]
- 4. Oberg KE (2010) Gastrointestinal neuroendocrine tumors. Ann Oncol 21 Suppl 7: vii72–80. 10.1093/annonc/mdq290 [DOI] [PubMed] [Google Scholar]
- 5. He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5: 522–531. [DOI] [PubMed] [Google Scholar]
- 6. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. (2002) Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 99: 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen J, Stass SA, Jiang F (2013) MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett 329: 125–136. 10.1016/j.canlet.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li SC, Essaghir A, Martijn C, Lloyd RV, Demoulin JB, Oberg K, et al. (2013) Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod Pathol 26: 685–696. 10.1038/modpathol.2012.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luzi E, Brandi ML (2011) Are microRNAs involved in the endocrine-specific pattern of tumorigenesis in multiple endocrine neoplasia type 1? Endocr Pract 17 Suppl 3: 58–63. 10.4158/EP11062.RA [DOI] [PubMed] [Google Scholar]
- 10. Ruebel K, Leontovich AA, Stilling GA, Zhang S, Righi A, Jin L, et al. (2010) MicroRNA expression in ileal carcinoid tumors: downregulation of microRNA-133a with tumor progression. Mod Pathol 23: 367–375. 10.1038/modpathol.2009.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. (2006) MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 24: 4677–4684. [DOI] [PubMed] [Google Scholar]
- 12. Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, et al. (2008) Serum microRNAs are promising novel biomarkers. PLoS One 3: e3148 10.1371/journal.pone.0003148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18: 997–1006. 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 14. Weiland M, Gao XH, Zhou L, Mi QS (2012) Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol 9: 850–859. 10.4161/rna.20378 [DOI] [PubMed] [Google Scholar]
- 15. Cortez MA, Calin GA (2009) MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther 9: 703–711. 10.1517/14712590902932889 [DOI] [PubMed] [Google Scholar]
- 16. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105: 10513–10518. 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vicentini C, Fassan M, D'Angelo E, Corbo V, Silvestris N, Nuovo GJ, et al. (2014) Clinical application of microRNA testing in neuroendocrine tumors of the gastrointestinal tract. Molecules 19: 2458–2468. 10.3390/molecules19022458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A (2011) Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem 57: 833–840. 10.1373/clinchem.2010.157198 [DOI] [PubMed] [Google Scholar]
- 19. Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M (2014) Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med 18: 371–390. 10.1111/jcmm.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song J, Bai Z, Han W, Zhang J, Meng H, Bi J, et al. (2012) Identification of suitable reference genes for qPCR analysis of serum microRNA in gastric cancer patients. Dig Dis Sci 57: 897–904. 10.1007/s10620-011-1981-7 [DOI] [PubMed] [Google Scholar]
- 21. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 22. Warburg O (1956) On the origin of cancer cells. Science 123: 309–314. [DOI] [PubMed] [Google Scholar]
- 23. Singh PK, Mehla K, Hollingsworth MA, Johnson KR (2011) Regulation of Aerobic Glycolysis by microRNAs in Cancer. Mol Cell Pharmacol 3: 125–134. [PMC free article] [PubMed] [Google Scholar]
- 24. Iorio MV, Croce CM (2012) Causes and consequences of microRNA dysregulation. Cancer J 18: 215–222. 10.1097/PPO.0b013e318250c001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 26. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. (2005) MicroRNA expression profiles classify human cancers. Nature 435: 834–838. [DOI] [PubMed] [Google Scholar]
- 27. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies. Cell Metab 20: 953–966. 10.1016/j.cmet.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 28. Pulito C, Donzelli S, Muti P, Puzzo L, Strano S, Blandino G (2014) microRNAs and cancer metabolism reprogramming: the paradigm of metformin. Ann Transl Med 2: 58 10.3978/j.issn.2305-5839.2014.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burney S, Khawaja KI, Saif MW, Masud F (2014) Chemotherapy and metformin in pancreatic adenocarcinoma and neuroendocrine tumors. JOP 15: 313–316. 10.6092/1590-8577/2677 [DOI] [PubMed] [Google Scholar]
- 30. Bao B, Azmi AS, Ali S, Zaiem F, Sarkar FH (2014) Metformin may function as anti-cancer agent via targeting cancer stem cells: the potential biological significance of tumor-associated miRNAs in breast and pancreatic cancers. Ann Transl Med 2: 59 10.3978/j.issn.2305-5839.2014.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang AI, Schwertschkow AH, Nolta JA, Wu J (2015) Involvement of Mesenchymal Stem Cells in Cancer Progression and Metastases. Curr Cancer Drug Targets. [DOI] [PubMed] [Google Scholar]
- 32. Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22: 894–907. 10.1101/gad.1640608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q (2011) miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem 286: 25992–26002. 10.1074/jbc.M111.229401 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.