Abstract
Mussel (Mytilus californianus) adhesion to marine surfaces involves an intricate and adaptive synergy of molecules and spatio-temporal processes. Although the molecules, such as mussel foot proteins (mfps), are well characterized, deposition details remain vague and speculative. Developing methods for the precise surveillance of conditions that apply during mfp deposition would aid both in understanding mussel adhesion and translating this adhesion into useful technologies. To probe the interfacial pH at which mussels buffer the local environment during mfp deposition, a lipid bilayer with tethered pH-sensitive fluorochromes was assembled on mica. The interfacial pH during foot contact with modified mica ranged from 2.2−3.3, which is well below the seawater pH of ~8. The acidic pH serves multiple functions: it limits mfp-Dopa oxidation, thereby enabling the catecholic functionalities to adsorb to surface oxides by H-bonding and metal ion coordination, and provides a solubility switch for mfps, most of which aggregate at pH ≥ 7-8.
Keywords: Dopa, mussel interfacial pH, pH sensitive surface, Oregon Green 488 DHPE
Introduction
All bivalve molluscs (Class Bivalvia: Phylum Mollusca) produce a tethering thread or bundle of threads known as a byssus for attachment to solid surfaces during the settlement of post-larval forms (Yonge 1962). In clams, cockles, oysters and scallops, the byssus is largely lost as juveniles adopt the buried, cemented, or free-swimming adult modes of life (Yonge & Thompson 1976). In others such as mussels, the byssus is neotenously retained in adult forms and continuously renewed to maintain holdfast tenacity in the high-energy intertidal zone (Waite 1983). As such, byssus formation is an essential step for development in all bivalves and, more particularly, in species that impact fouling, mariculture and intertidal ecosystems (Yonge &Thompson 1976; Carrington et al. 2015).
Despite the importance of the byssus, substantive progress in understanding byssal biochemistry did not occur until the relatively recent focus on biomimetic adhesion ( Waite et al. 2005; Lee et al. 2011). Constituent proteins of byssus, also known as mussel foot proteins (mfps) have inspired the design of a variety underwater adhesives, hydrogels, and coatings (Lee et al. 2007, 2011; Fullenkamp et al. 2012; Ahn et al. 2014). The interfacial chemistry between native mfps, mussel-inspired polymers and well-characterized solid surfaces has received much scrutiny. However, investigations into the specific conditions imposed by mussels during plaque formation have not kept pace. Without reliable knowledge on the pH, ionic strength, redox and cleaning conditions that prevail during plaque formation, insights drawn solely from the chemistry and in vitro behavior of mfps have limited meaning. The pH-sensitivity of 3,4-dihydroxyphenylalanine (Dopa) oxidation is an excellent case in point. Mfps such as mfp-3 and mfp-5 contain 20-30 mol% Dopa and are highly adhesive (eg Eadh = -14 mJ m−2 on mica) within narrowly defined solution conditions (Danner et al. 2012, Nicklisch et al. 2013; Martinez Rodriguez et al. 2015). Increasing the pH of mfp deposition in vitro from ~3 to 7.5, for example, typically causes significant Dopa oxidation and abolishes mfp-3 and -5 adhesion to mica, which is counterintuitive given that the ambient seawater around natural mussel clusters has a pH of ~8.
The first hint of important processing during byssus formation was obtained by inserting a microelectrode into the distal depression of an adult mussel foot in order to measure the pH and ionic strength of foot fluid collected after the KCl-induced secretion of adhesive proteins, ie pH 5.5 and 0.15 M (Yu et al. 2011). As many invertebrates, especially molluscs, secrete acids in response to irritation (Thompson 1969), a completely convincing demonstration that mussel adhesion is pH-dependent would contrive to measure the pH under a mussel foot during natural plaque formation. This is a challenging undertaking as mussels are far from being compliant participants. What is known about byssus formation is that the mussel uses its foot to reconnoiter its surroundings and, having done so, makes snug contact with a target surface prior to depositing adhesive mfps in a fashion resembling injection molding (Waite 1987). The dimpled distal depression of the foot is positioned over a surface like an inverted rubber cup and compressed, thereby pushing out bulk water (see Supporting video). Mfps are then secreted into the remaining gap from 8-10 pores in the depression ceiling (Tamarin et al. 1976). Strong and durable adhesion is achieved despite the surrounding seawater at pH 8.2, high salt and saturating levels of dissolved O2, which will tend to undermine the strength of protein adhesion to mica and titania surfaces in vitro (Nicklisch et al. 2013).
By affixing live mussels to predetermined positions on an inert backing, then presenting them to surfaces coated with pH-sensitive dyes, it has been possible to monitor the pH conditions accompanying byssal plaque formation.
Materials and methods
Oregon Green® 488 DHPE/DMPC mica surface is reversible to pH change
A mixture of Oregon Green® 488 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (Oregon Green® 488 DHPE, 1 mol% of DMPC) and 1,2-dimyristoyl-sn-glycero-3- phosphocholine (DMPC) was added to 150 mM NaCl buffer and injected onto a freshly cleaved mica surface at 40°C for 10 min (Figure S1). The lipid bi-layer coated mica surface was then rinsed with 150 mM NaCl and kept under wet conditions.
A pH-sensitive lipid-bilayer membrane was prepared on mica using 488 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (Oregon Green® 488 DHPE) / 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) with a responsive pH range of pH 4 – 6 (Figure S1) and selected for its proximity to the previously observed pH of 5.5 for induced plaques (Yu et al. 2011). The pH-coupled fluorescence (λem = 526 nm) arises upon ionizing the carboxyl group (pKa ~ 4.7) in the tethered fluorochrome. At pH below the pKa, the carboxyl spontaneously esterifies to a non-fluorescent lactone (Figure S1).
To measure the effect of pH change of Oregon Green® 488 DHPE/DMPC on the mica surface (pKa ~ 4.7), the lipid bi-layer surface was exposed to citrate buffers ranging from pH 2.6 to 7.6. An inverted confocal microscope was used to image the underside of the surface for the mean integrated intensity or the mean fluorescence intensity of a randomly selected area at 10X (A = 1.6 × 10 −6 μm2). Each buffer change was allowed to equilibrate for ~ 5 min before imaging.
The substratum was first rinsed with 150 mM NaCl and then allowed to equilibrate to pH 7.6. The black line shows a decrease in the normalized fluorescence from pH 7.6 to 2.6. The pH was then increased from 2.6 to 7.6 (Figure S2) and shows an increase in intensity. The pKa of the Oregon Green® 488 DHPE/DMPC bi-layer is between pH 5 and 6 which is above the published pKa of 4.7. The shift in pKa can be attributed to the bulk of the solution at 1.5 pH units higher than the pH at the interface (Longo et al. 2012). The reversal from pH 2.6 to 3 does not recover to the original intensity of the first titration and shows hysteresis attributed to the charging properties of the lipid bilayer (O'Reilly et al. 2005). The reversibility of the lipid bilayer remained consistent after three additional cycles of buffer changes. The results show that the Oregon Green® 488 DHPE/DMPC lipid bi-layer is responsive to pH changes between pH 7.6 and 2.6.
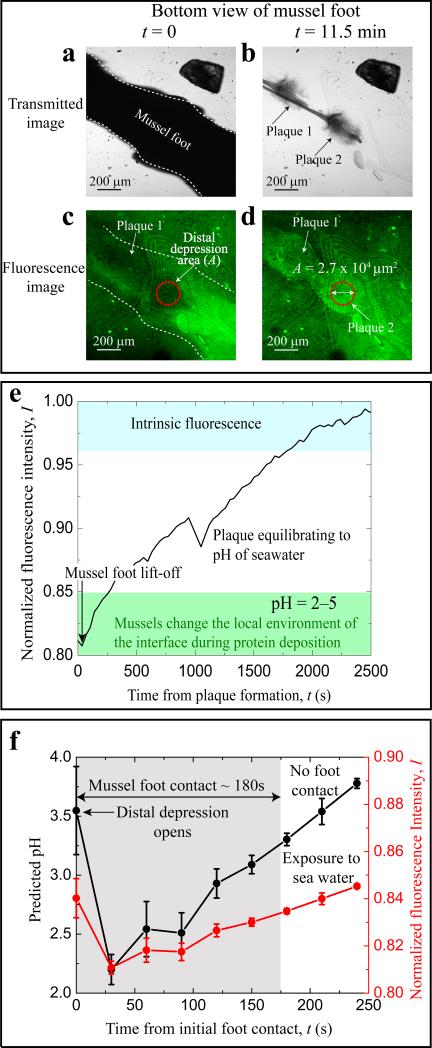
The fluorescent intensity of the contact area between the distal depression and the surface (red circle in Figure 1c and d, diameter ~209 μm) was then recorded over the next 15 min (Initial I ~ 0.84, s.d. ± 0.03, n = 3, during foot lift-off from the surface in Figure 2). Images were captured for 200 s starting from 30s after the initial foot contact (i.e. 30 s after the initial foot contact t = 0). Note that 200 s is a fraction of the 5 min required for plaque formation in adult mussels (Maheo 1970).
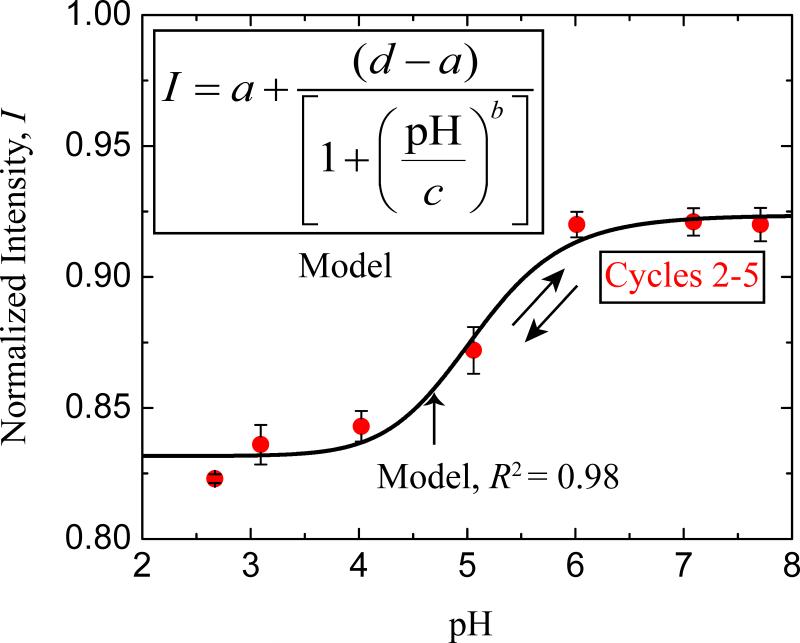
Figure 1.
The fluorochrome, Oregon Green 488, tethered to a bilayer adsorbed to mica shows a reversible response to pH change. The correlation of fluorescence intensity with pH was initiated by decreasing the ambient pH incrementally from pH 7.7 to 2.7 (cycle #1 not shown see Figure S2). The pH was then titrated back and forth between pH 2.7 and pH 7.7 for five cycles (Figure 1). Fluorescent yield underwent significant hysteresis between cycles 1 and 2, but followed a similar sigmoidal trajectory for cycles 2 to 5 (red solid circles). The error bars on the red solid circles indicate the SD in the intensities of the fluorescent dye (n=4). The black solid line in the plot of Normalized Intensity vs pH represents a ‘4 parameter logistic nonlinear regression’ model fit to the experimental data points (red solid circles). The equation used for modeling is denoted in the graph inset.
<<Editor: In Fig. 1 ‘’Normalized Intensity” should read ‘’Normalized intensity”. This will need a full single column layout>>
Figure 2.
Fluorescent images and intensities of the plaque substratum interface during plaque formation by juvenile mussels (length < 10mm). Transmitted light images taken at t = 0 (30 s after initial contact) and t = 11.5 min, respectively, of an Oregon Green DHPE/DMPC-labeled mica surface during foot contact (a) and following foot disengagement (b) from the new plaques. Corresponding fluorescence images are in (c) and (d). Distal depression of the foot is highlighted by a red circle (A = 2.7 × 104μm2, diameter ~209 μm). (e) Normalized fluorescence intensity (I) after disengagement of foot from plaque and direct equilibration with seawater. (f) Normalized fluorescent intensity (right axis) and pH (left axis) during actual mussel foot-surface contacts (shaded gray area) which typically lasted ≤180s in juvenile mussels. Equation 1 was used to convert the fluorescent intensity to pH.
<<Editor: In Fig 2f (r.h.side, in red) ‘’Intensity” should read ‘’intensity”. Fig. 2 will need a full double column layout>>
Atomic Force Microscopy (AFM)
The adsorption of Oregon Green® 488 DHPE and DMPC to mica was visualized by AFM. An MFP-3D-Bio Atomic Force Microscope (AFM, Asylum Research) was used to obtain images with an SNL probe (Bruker) under tapping mode at room temperature (22 °C). Oregon Green® 488 DHPE and DMPC was deposited on a mica surface (area ~ 1 cm2) by adsorbing 50 μl of the solution from a 1 mg ml−1 (Oregon Green® 488 DHPE, 1 mol% of the total lipid composition) concentration in 150 mM NaCl.
Adhesive plaque formation on Oregon Green DHPE/DMPC mica surfaces
Juvenile marine mussels (M. californianus), than <<Editor: more than or less than??>> 5 mm in length, were dorsally glued to a dry glass slide using a two-part epoxy. After the epoxy cured, the slide with mounted mussels was rinsed with 150 mM NaCl, followed by immersion in seawater at 4°C, and exposed to a wet mica surface coated with Oregon Green DHPE and DMPC lipids (Figures S1 and S3). In this configuration, the ventral side of each mussel was facing the mica surface. Seawater was maintained at 15°C for the duration of the experiment. Imaging was done from the underside of the glass slide using an inverted confocal microscope, Olympus Fluoview 1000 Spectral FLV0005 (Melville, New York). Marine mussels were allowed to attach to an Oregon Green® 488-tagged DHPE/DMPC bilayer coated mica surface. Once the mussel foot was attached and motionless on the substratum, imaging commenced using an inverted confocal microscope. Foot contact was monitored by confocal and transmitted light microscopy, and images were taken every 30 s. The area of the distal depression (part of the foot where the byssal plaque is formed) that was quantified is marked with a red circle (A= 2.7 × 104 μm2).
‘4 parameter logistic nonlinear regression’ model
| (1) |
where a, b, c, d are the parameters of the model pH corresponds to the explanatory variable and I to the response variable. a and d are parameters that respectively represent the lower and upper asymptotes, and b is the slope parameter (Draper et al. 1966, Neter et al. 1996). c is the abscissa of the mid-height point with ordinate at (a+b)/2. The values for a, b, c, d obtained from fitting (R2 = 0.983) the experimental data points (Figure 1) are 0.924, 12.220, 5.072 and 0.832 respectively with a P-value < 0.0001.
To predict the interfacial pH during mussel adhesive plaque formation from normalized fluorescence readings, a ‘4 parameter logistic nonlinear regression’ analysis (Draper et al. 1966, Neter, et al. 1996) was used to model the experimentally measured normalized fluorescent intensities, I of the modified surface with changing pH (Figure 1).
Results
The in situ pH conditions under the foot of juvenile M. californianus during the deposition of adhesive mfps was interrogated (1) by incorporating covalently tethered a pH-sensitive fluorochrome in a lipid-bilayer membrane on mica (Figure S1), followed by (2) fluorescence intensity measurements of the lipid-bilayer during foot contact and plaque formation by real-time confocal laser scanning microscopy (CLSM).
As observed in other tethered fluorochromes, an irreversible hysteresis in fluorescence yield occurred between the first and 2nd cycle probably indicating a dye redistribution often associated with clustering (O'Reilly et al. 2005). This hysteresis necessitated preconditioning Oregon Green 488-tethered surfaces with incremental pH equilibrations ranging from pH 7.6 to 2.6. After the cycling of buffers from low to high pH, the fluorescence intensity of the lipid bilayer vs pH became reproducibly sigmoidal (mid-point pH 4.7), and the surfaces were considered ready for mussel attachment (Figure 1). The low fluorescence under a foot upon initial contact with a surface indicated a local interfacial pH below the dye pKa ~ 4.7. That is to say, the foot appears to significantly acidify the interface during initial protein deposition. As the plaque ages over a course of time, the intrinsic fluorescence of the plaque is apparent (Figure S4) and is disregarded for the calculation of the interfacial pH since the initial pH after foot contact (< 200s) is the focus of this work. The role of acidification was earlier proposed to retard the oxidation of Dopa residues in the mfps for the formation of hydrogen bonds or metal-catechol coordination to secure the proteins/plaque to the substratum (Waite 1987).
The plaque intensity eventually increased to I = 0.96 (corresponding to fluorochrome-labeled bilayers on mica at seawater pH ~ 8.2), ~2000 s after the foot disengaged from the surface (Figure 2e). The most plausible explanation for the increase in fluorescent intensity is diffusion of hydroxide ions from seawater (~ pH 8.2) into the plaque-fluorochrome interface as would be expected for a non-living biomaterial equilibrating with the surrounding seawater. The fluorescence intensity of the plaque interface decreased when placed in seawater of reduced pH (Figures S1 and S3). This trend, however, is only consistent to pH 6, below which plaque fluorescence intensity increases. The latter trend is opposite to the predicted behavior of the fluorochrome, and likely due to the pH titration of a fluorescent intermediate formed during the chemical crosslinking the plaques (Smith &Haskell 2000; Rzepecki & Waite 1993). It should be noted that the intrinsic fluorescence of plaques took between 900 and 1200 s, on average, to develop, was independent of the interfacial fluorescence of Oregon Green 488 DHPE/DMPC (Figure S5) and continued to increase (no plateau at t = 2500 s) with the chemical maturation of the plaque in seawater (blue shaded region in Figure 1e).
The interfacial pH just prior to foot lift-off (t = 175 s) was estimated to be 3.5 (SD ± 1.4) based on the normalized intensities (eg I = 0.84, SD ± 0.03, n = 6). Indeed, the pH during initial foot contact and protein secretion (Figure 2f) could be as low as pH 2.1 although the model has lower predictive confidence in this range. These results indicate that mussels substantially acidify the local environment at the substratum-plaque interface during plaque formation (Figure 3).
Figure 3.
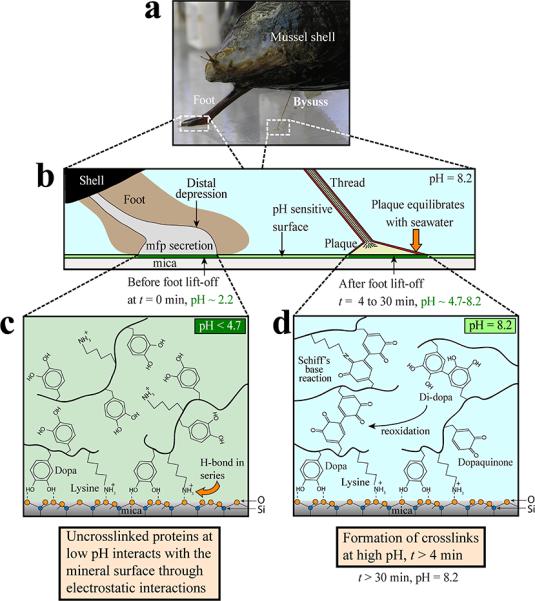
pH and mussel adhesive plaque formation on a pH-sensitive mica surface depicting chemistry under reducing (acidic pH) and oxidizing (neutral to slightly alkaline pH) conditions. (a) M. californianus with extended foot and a single completed plaque and thread. (b) Foot contact with a mica surface evicts seawater from the distal depression and lowers the pH to ~2.2 (c) The foot disengages from the surface and a plaque is deposited. The uncross-linked proteins at low pH interact with the mineral surface through bidentate catechol-mediated interactions. (d) The foot has disengaged from the plaque allowing its equilibration with the ambient seawater. The pH increase to pH 8 is linked directly and indirectly (via catecholoxidase) to formation of cross-links within the plaque.
<<Editor: Fig. 3 will require a full double column layout>>
Although the interfacial fluorescence (ie the fluorochrome in the lipid-bilayer) cannot be readily distinguished from plaque intrinsic fluorescence at t > 4 min, suffice it to say that total fluorescence continues to increase without saturating. As the interfacial fluorescence should saturate at ~pH 7, the steady increase in intrinsic fluorescence must be coming from the oxidation of Dopa residues (Rzepecki & Waite 1993, Smith & Haskell 2000) not adsorbed to the surface (Figure 3d) but instead are implicated in protein cross-linking (McDowell et al. 1999, Zhao et al. 2006). The cross linking of plaque mfps strengthens plaque cohesion against drag and lift forces in the wave-swept intertidal zone.
Discussion
This study provides in situ evidence that marine mussels impose an acidic pH (pH ~2) under the foot during plaque formation. Acid secretion by molluscan epithelia has been known for some time (Thompson 1969), but this is the first report linking the local pH and adhesion. Deposition of adhesive proteins at acidic pH has important implications for both mussel biology and mussel-inspired technology. For the mussel, the acidic pH: (1) allows delivery of mfps to surfaces as metastable complex fluids (Wei et al. 2014); (2) combined with antioxidants (Yu et al. 2011; Nicklisch, et al. 2013), low pH stabilizes the catecholic moiety of Dopa enabling formation of bidentate H-bonds and coordination complexes with surface oxides (Lee et al. 2011); (3) favors the formation of cationic functionalities eg Lys, Arg, His for long-range attraction to electronegative surfaces (Danner et al. 2012); and (4) in combination with seawater (pH 8.2), serves as a switch for initiating protein insolubility, quinone based cross-linking and catechol-mediated metal chelation (Lee et al. 2011). An additional though more speculative adaptive asset of low pH is that it may be used to kill surface microbes (Martinez Rodriguez 2014); Robert T Baier, personal communication). All of these advantages are equally favorable features in mussel-inspired synthetic polymers. This work provides an increased understanding of the ways marine mussels tailor the local environment of the distal depression during plaque formation to prevent the auto-oxidation of Dopa residues. The insights gained here should aid in the development of strategies for deploying Dopa-based or mussel-inspired wet adhesives while retaining the adhesive functionality of redox sensitive chemical groups.
Is acid-mediated secretion of adhesive molecules limited to mussels or widely practiced by other sessile organisms? If low pH is a precaution limited to Dopa-based protein adhesives, then it may be imposed during adhesion by sandcastle worms (Waite et al. 1992), cnidarian hydroids (Hwang et al. 2013), turbellarians (Swann et al. 1996) and tunicates (Dorsett et al. 1987), all of which are known to use Dopa-proteins. If more widely practiced, it may offer a significant potential control point against biofouling that has not previously been considered. On this note, Dopa-deficient cement proteins of barnacles rely on pH and ionic strength to undergo a triggered self-assembly reminiscent of amyloid formation (Nakano & Kamino 2014).
Supplementary Material
Acknowledgements
This research was supported by grants from NIH (R01 DE018468). The MRL Central Facilities (which include AFM and QCM) is supported by the MRSEC Program of the NSF under Award No. DMR 1121053; a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org). The fluorescent imaging was performed at the NRI-MCDB microscopy facility that is supported by NIH Grant Number: 1 S10 OD010610-01A1.
Footnotes
Author contributions
N.R.M.R, S.D, Y.K. and J.H.W designed the experiments; N.R.M.R, S.D and J.H.W. analyzed, the data and wrote the manuscript. J.H.W co-supervised the whole project with J.N.I., who helped analyze the results.
Supplemental material available: This document contains methods, model parameter, supporting figures and supporting video description. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- Ahn BK, Lee DW, Israelachvili JN, Waite JH. Surface-initiated self-healing of polymers in aqueous media. Nature materials. 2014;13:867–872. doi: 10.1038/nmat4037. [DOI] [PubMed] [Google Scholar]
- Carrington E, Waite JH, Sarà G, Sebens KP. Mussels as a model system for integrative ecomechanics. Marine Science. 2015;7 doi: 10.1146/annurev-marine-010213-135049. [DOI] [PubMed] [Google Scholar]
- Danner EW, Kan Y, Hammer MU, Israelachvili JN, Waite JH. Adhesion of mussel foot protein Mefp-5 to mica: an underwater superglue. Biochemistry. 2012;51:6511–6518. doi: 10.1021/bi3002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett LC, Hawkins CJ, Grice JA, Lavin MF, Merefield PM, Parry DL, Ross IL. Ferreascidin: a highly aromatic protein containing 3, 4-dihydroxyphenylalanine from the blood cells of a stolidobranch ascidian. Biochemistry. 1987;26:8078–8082. [Google Scholar]
- Draper NR, Smith H, Pownell E. Applied regression analysis. Wiley; New York: 1966. [Google Scholar]
- Fullenkamp DE, Rivera JG, Gong YK, Lau KH, He L, Varshney R, Messersmith PB. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials. 2012;33:3783–3791. doi: 10.1016/j.biomaterials.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DS, Masic A, Prajatelistia E, Iordachescu M, Waite JH. Marine hydroid perisarc: A chitin-and melanin-reinforced composite with DOPA–iron (III) complexes. Acta Biomater. 2013;9:8110–8117. doi: 10.1016/j.actbio.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Mussel-inspired adhesives and coatings. Annu Rev Mater Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lee BP, Messersmith PB. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448:338–U334. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- Longo GS, de la Cruz MO, Szleifer I. Molecular theory of weak polyelectrolyte thin films. Soft Matter. 2012;8:1344–1354. [Google Scholar]
- Maheo R. Study of position and secretion activity of byssus of Mytilus edulis L. Cah Biol Mar. 1970;11:475–&. [Google Scholar]
- Martinez Rodriguez NR. Wet adhesion: the advancement from mussel plaques to applications. University of California Santa Barbara; Santa Barbara: 2014. [Google Scholar]
- Martinez Rodriguez NR, Das S, Kaufman Y, Wei W, Israelachvili J, Waite JH. Mussel adhesive protein provides cohesive matrix for collagen type-1α. Biomaterials. 2015;51:51–57. doi: 10.1016/j.biomaterials.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell LM, Burzio LA, Waite JH, Schaefer J. Rotational echo double resonance detection of cross-links formed in mussel byssus under high-flow stress. J Biol Chem. 1999;274:20293–20295. doi: 10.1074/jbc.274.29.20293. [DOI] [PubMed] [Google Scholar]
- Nakano M, Kamino K. Amyloid-like conformation and interaction for the self-assembly in barnacle underwater cement. Biochemistry. 2014;54:826–835. doi: 10.1021/bi500965f. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. Irwin; Chicago: 1996. [Google Scholar]
- Nicklisch SC, Das S, Martinez Rodriguez NR, Waite JH, Israelachvili JN. Antioxidant efficacy and adhesion rescue by a recombinant mussel foot protein-6. Biotechnol Prog. 2013;29:1587–1593. doi: 10.1002/btpr.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JP, Butts CP, I'Anso IA, Shaw AM. Interfacial pH at an isolated silica-water surface. J Am Chem Soc. 2005;127:1632–1633. doi: 10.1021/ja0443326. [DOI] [PubMed] [Google Scholar]
- Rzepecki L, Waite J. The byssus of the zebra mussel, Dreissena polymorpha. I: Morphology and in situ protein processing during maturation. Mol Mar Biol Biotechnol. 1993;2:255–266. [PubMed] [Google Scholar]
- Smith GJ, Haskell TG. The fluorescent oxidation products of dihydroxyphenylalanine and its esters. J Photochem Photobiol, B. 2000;55:103–108. doi: 10.1016/s1011-1344(00)00020-8. [DOI] [PubMed] [Google Scholar]
- Swann C, Waite J, Huggins L. Cross-linking of DOPA-proteins in the eggshell of Bdelloura candida, a marine worm: a PIXE study. Nucl Instrum Methods Phys Res, Sect B. 1996;109:301–304. [Google Scholar]
- Tamarin A, Lewis P, Askey J. Structure and formation of byssus attachment plaque in Mytilus. J Morphol. 1976;149:199–221. doi: 10.1002/jmor.1051490205. [DOI] [PubMed] [Google Scholar]
- Thompson T. Acid secretion in the Pacific Ocean gastropods. Aust J Zool. 1969;17:755–764. [Google Scholar]
- Waite JH. Adhesion in byssally attached bivalves. Biol Rev. 1983;58:209–231. [Google Scholar]
- Waite JH. Nature's underwater adhesive specialist. Int J Adhes Adhes. 1987;7:9–14. [Google Scholar]
- Waite JH, Andersen NH, Jewhurst S, Sun C. Mussel adhesion: finding the tricks worth mimicking. Journal of adhesion. 2005;81:297–317. [Google Scholar]
- Waite JH, Jensen RA, Morse DE. Cement precursor proteins of the reef-building polychaete Phragmatopoma californica (Fewkes). Biochemistry. 1992;31:5733–5738. doi: 10.1021/bi00140a007. [DOI] [PubMed] [Google Scholar]
- Wei W, Tan Y, Martinez Rodriguez NR, Yu J, Israelachvili JN, Waite JH. A mussel-derived one component adhesive coacervate. Acta Biomater. 2014;10:1663–1670. doi: 10.1016/j.actbio.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonge C. On the primitive significance of the byssus in the Bivalvia and its effects in evolution. J Mar Biol Assoc U K. 1962;42:113–125. [Google Scholar]
- Yonge CM, Thompson TE. Living marine molluscs. 1976. <<Editor: Details??>>.
- Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat Chem Biol. 2011;7:588–590. doi: 10.1038/nchembio.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Robertson NB, Jewhurst SA, Waite JH. Probing the adhesive footprints of Mytilus californianus byssus. J Biol Chem. 2006;281:11090–11096. doi: 10.1074/jbc.M510792200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.