Abstract
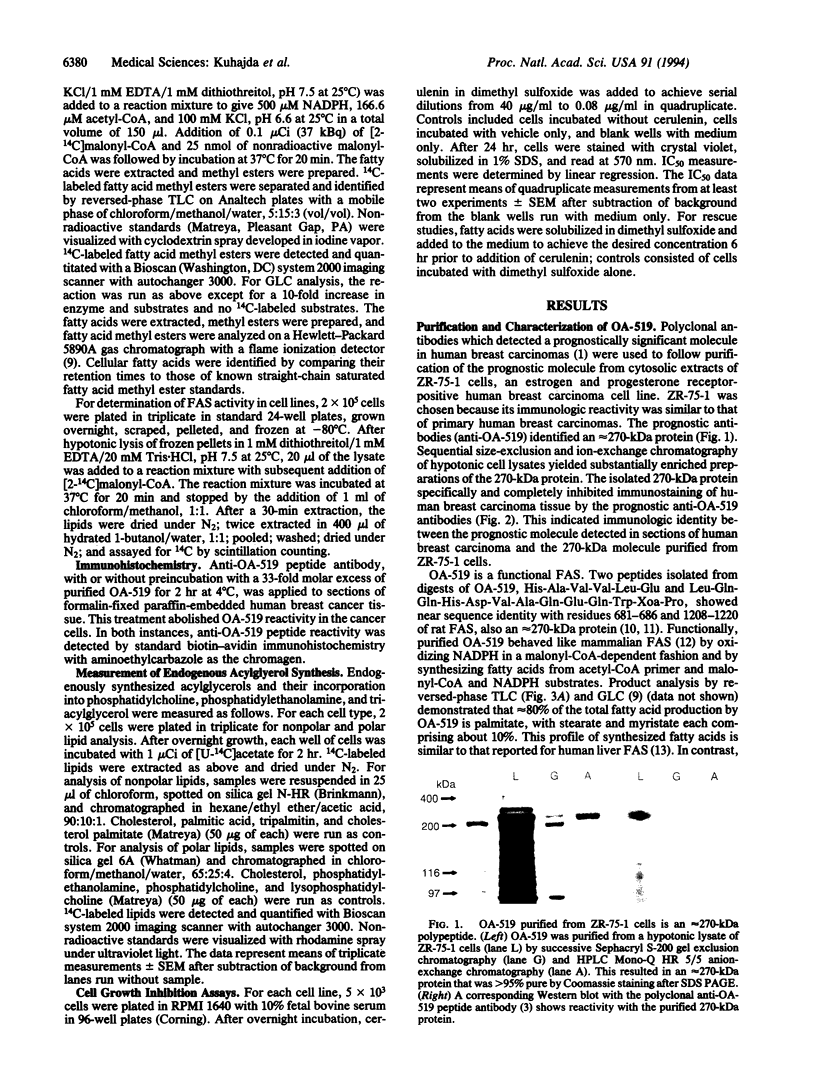
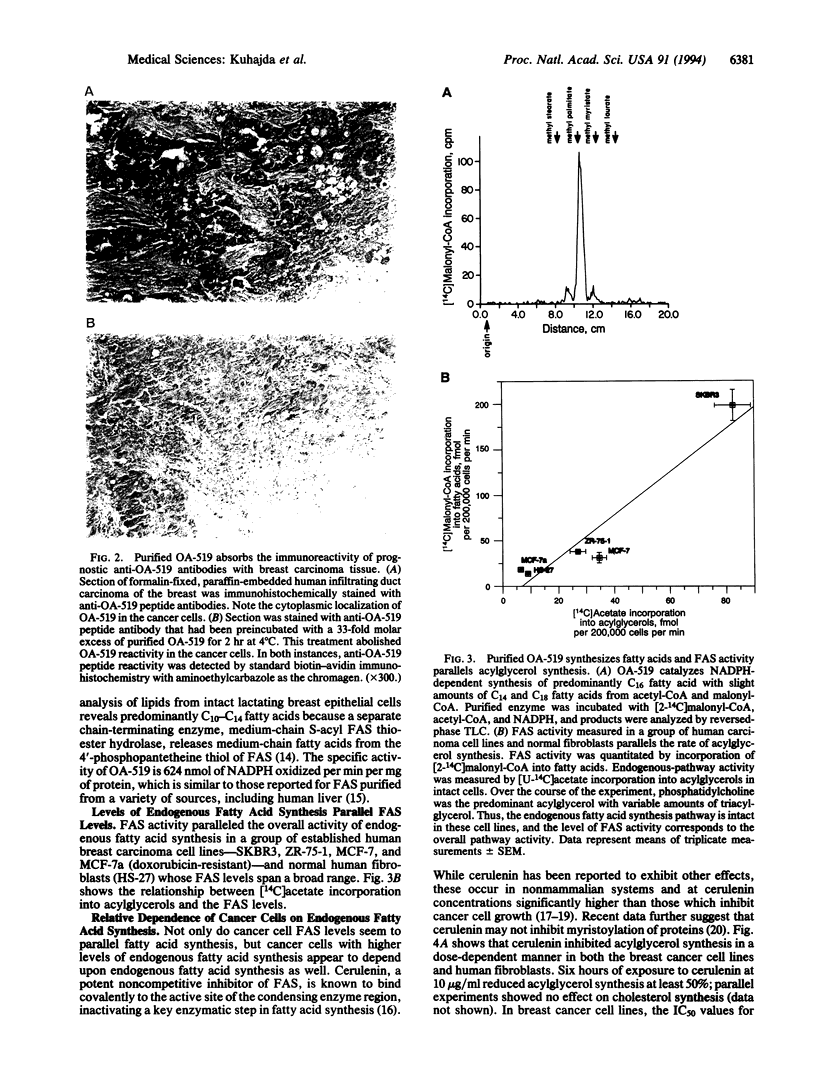
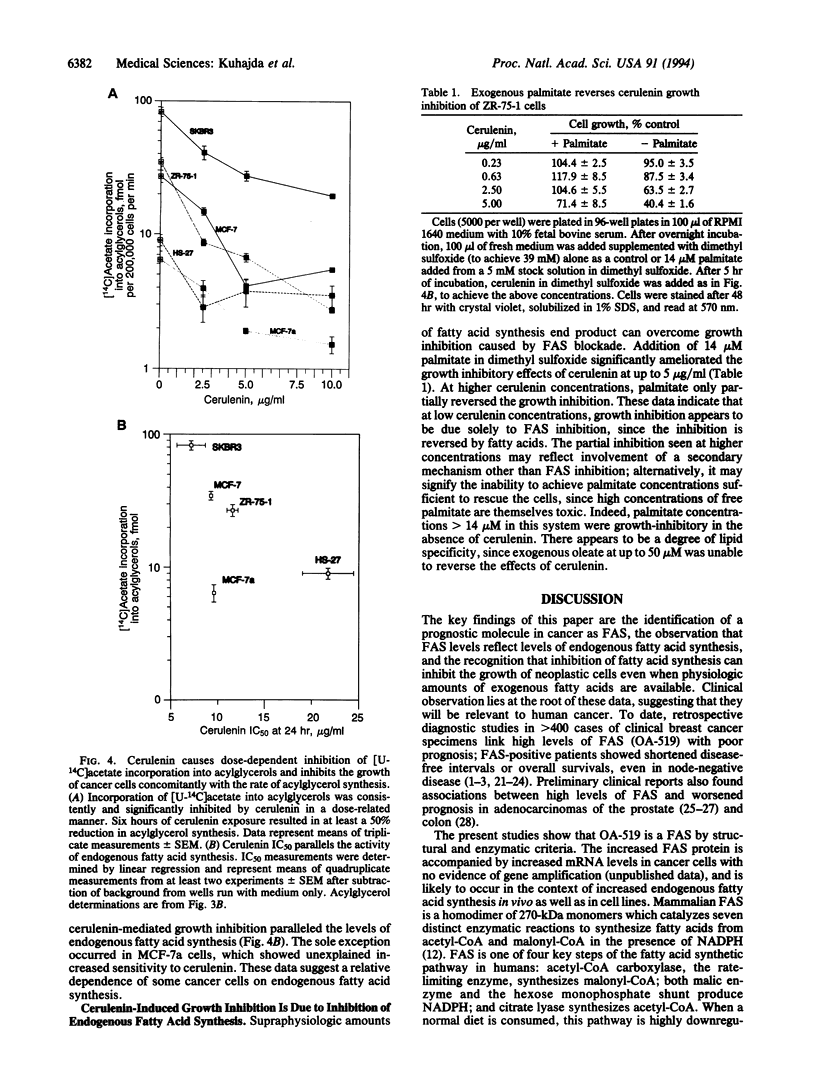
OA-519 is a prognostic molecule found in tumor cells from breast cancer patients with markedly worsened prognosis. We purified OA-519 from human breast carcinoma cells, obtained its peptide sequence, and unambiguously identified it as fatty acid synthase through sequence homology and enzymology. Tumor fatty acid synthase is an approximately 270-kDa polypeptide which specifically abolished immunostaining of human breast cancers by anti-OA-519 antibodies. Tumor fatty acid synthase oxidized NADPH in a malonyl-CoA-dependent fashion and synthesized fatty acids composed of 80% palmitate, 10% myristate, and 10% stearate from acetyl-CoA, malonyl-CoA, and NADPH with a specific activity of 624 nmol of NADPH oxidized per min per mg. Tumor cell lines with elevated fatty acid synthase showed commensurate increases in incorporation of [U-14C]acetate into acylglycerols demonstrating that fatty acid synthase increases occur in the context of overall increases in endogenous fatty acid synthesis. Cerulenin inhibited acylglycerol synthesis in tumor cells and fibroblast controls in a dose-dependent fashion and also caused a growth inhibition which generally paralleled the level of endogenous fatty acid synthesis. Supraphysiologic levels of palmitate, 14 microM in dimethyl sulfoxide, significantly reversed the growth inhibition caused by cerulenin at concentrations of up to 5 micrograms/ml, indicating that cerulenin-mediated growth inhibition was due to fatty acid synthase inhibition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy C. M., Witkowski A., Naggert J., Williams B., Randhawa Z., Smith S. Molecular cloning and sequencing of cDNAs encoding the entire rat fatty acid synthase. Proc Natl Acad Sci U S A. 1989 May;86(9):3114–3118. doi: 10.1073/pnas.86.9.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay G. K., Imagawa W., Wallace D., Nandi S. Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem. 1987 Feb 25;262(6):2750–2756. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen L. A., Thompson D. O., Maeura Y., Choi K., Blank M. E., Rose D. P. Dietary fat and mammary cancer. I. Promoting effects of different dietary fats on N-nitrosomethylurea-induced rat mammary tumorigenesis. J Natl Cancer Inst. 1986 Jul;77(1):33–42. [PubMed] [Google Scholar]
- Funabashi H., Kawaguchi A., Tomoda H., Omura S., Okuda S., Iwasaki S. Binding site of cerulenin in fatty acid synthetase. J Biochem. 1989 May;105(5):751–755. doi: 10.1093/oxfordjournals.jbchem.a122739. [DOI] [PubMed] [Google Scholar]
- Kuhajda F. P., Katumuluwa A. I., Pasternack G. R. Expression of haptoglobin-related protein and its potential role as a tumor antigen. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1188–1192. doi: 10.1073/pnas.86.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhajda F. P., Piantadosi S., Pasternack G. R. Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med. 1989 Sep 7;321(10):636–641. doi: 10.1056/NEJM198909073211003. [DOI] [PubMed] [Google Scholar]
- Moelling K., Schulze T., Knoop M. T., Kay J., Jupp R., Nicolaou G., Pearl L. H. In vitro inhibition of HIV-1 proteinase by cerulenin. FEBS Lett. 1990 Feb 26;261(2):373–377. doi: 10.1016/0014-5793(90)80595-a. [DOI] [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout G. J., Shull V. H., Dick J. D. Identification of clinical isolates of gram-negative nonfermentative bacteria by an automated cellular fatty acid identification system. J Clin Microbiol. 1991 Sep;29(9):1822–1830. doi: 10.1128/jcm.29.9.1822-1830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncari D. A. Fatty acid synthase from human liver. Methods Enzymol. 1981;71(Pt 100):73–79. doi: 10.1016/0076-6879(81)71011-5. [DOI] [PubMed] [Google Scholar]
- Roncari D. A. Mammalian fatty acid synthetase. I. Purification and properties of human liver complex. Can J Biochem. 1974 Mar;52(3):221–230. doi: 10.1139/o74-035. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Takabayashi K., Laux T., Beck K. F., Schreglmann R. Rat mammary gland fatty acid synthase: localization of the constituent domains and two functional polyadenylation/termination signals in the cDNA. Nucleic Acids Res. 1989 Jan 25;17(2):567–586. doi: 10.1093/nar/17.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurbaji M. S., Kuhajda F. P., Pasternack G. R., Thurmond T. S. Expression of oncogenic antigen 519 (OA-519) in prostate cancer is a potential prognostic indicator. Am J Clin Pathol. 1992 May;97(5):686–691. doi: 10.1093/ajcp/97.5.686. [DOI] [PubMed] [Google Scholar]
- Shurbaji M. S., Pasternack G. R., Kuhajda F. P. Expression of haptoglobin-related protein in primary and metastatic breast cancers. A longitudinal study of 48 fatal tumors. Am J Clin Pathol. 1991 Aug;96(2):238–242. doi: 10.1093/ajcp/96.2.238. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Aderem A. Myristoylation of proteins in the yeast secretory pathway. J Biol Chem. 1992 Feb 25;267(6):3922–3931. [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. J., Smith S. Biosynthesis of fatty acids by lactating human breast epithelial cells: an evaluation of the contribution to the overall composition of human milk fat. Pediatr Res. 1985 Jan;19(1):139–143. doi: 10.1203/00006450-198501000-00036. [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Stern A., Smith S. Purification and properties of fatty acid synthetase from a human breast cell line. Biochim Biophys Acta. 1981 Nov 13;662(1):125–130. doi: 10.1016/0005-2744(81)90232-1. [DOI] [PubMed] [Google Scholar]
- Tomáska L., Resnick R. J. Suppression of platelet-derived growth factor receptor tyrosine kinase activity by unsaturated fatty acids. J Biol Chem. 1993 Mar 5;268(7):5317–5322. [PubMed] [Google Scholar]
- Wakil S. J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989 May 30;28(11):4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- Weiss L., Hoffmann G. E., Schreiber R., Andres H., Fuchs E., Körber E., Kolb H. J. Fatty-acid biosynthesis in man, a pathway of minor importance. Purification, optimal assay conditions, and organ distribution of fatty-acid synthase. Biol Chem Hoppe Seyler. 1986 Sep;367(9):905–912. doi: 10.1515/bchm3.1986.367.2.905. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Ziegler L. D., Buzdar A. U. Current status of adjuvant therapy of early breast cancer. Am J Clin Oncol. 1991 Apr;14(2):101–110. doi: 10.1097/00000421-199104000-00002. [DOI] [PubMed] [Google Scholar]





