Abstract
Background
Stress ulcers and related upper gastrointestinal bleeding are well-known complications in intensive care unit (ICU) patients. Proton pump inhibitor (PPI)-based stress ulcer prophylaxis (SUP) has been widely prescribed in noncritically ill patients who are at low risk for clinically significant bleeding, which is then injudiciously continued after hospital discharge. This study aimed to evaluate the incidence of inappropriate prescribing of PPI-based preventative therapy in ICU versus non-ICU patients that subsequently continued postdischarge, and to estimate the costs incurred by the unwarranted outpatient continuation of PPI therapy.
Methods
A retrospective review of patient data at a major teaching hospital in Korea was performed. During the 4-year study period, adult patients who were newly initiated on PPI-based SUP during hospital admission and subsequently discharged on a PPI without a medical indication for such therapy were captured for data analysis. The incidence rates of inappropriate prescribing of PPIs were compared between ICU and non-ICU patients, and the costs associated with such therapy were also examined.
Results
A total of 4,410 patients, more than half of the inpatient-initiated PPI users, were deemed to have been inadvertently prescribed a PPI at discharge in the absence of a medical need for acid suppression. The incidence of inappropriate outpatient continuation of the prophylaxis was higher among ICU patients compared with non-ICU patients (57.7% versus 52.2%, respectively; P=0.001). The total expenditure accrued through the continuation of nonindicated PPI therapy was approximately US$40,175.
Conclusion
This study confirmed that excess usage of PPIs for SUP has spread to low-risk, non-ICU patients. The overuse of unwarranted PPI therapy can incur large health care expenditure, as well as clinical complications with minimal therapeutic benefits. Educating clinicians regarding SUP guidelines and the adverse effects of long-term use of acid suppression can improve the cost effectiveness of PPI therapy.
Keywords: proton pump inhibitor, stress ulcer, prophylaxis, gastrointestinal bleeding, intensive care unit, critical care
Introduction
Stress ulceration in the upper gastrointestinal (GI) tract is an acute condition that can be detected endoscopically in the majority of critically ill patients within 24 hours of admission to an intensive care unit (ICU).1 The stress-induced upper GI hemorrhage is a well-known complication in critically ill patients, but the incidence of clinically significant GI bleeding is highly variable across studies depending on the definition used, the severity of the illness, and the outcome parameters evaluated in clinical settings.2–4 Nevertheless, most sources report its incidence as approximately 0.1%–4% at most.2,5–9 The incidence rate has improved substantially over the past two to three decades, which is largely attributable to the advances in the therapeutic monitoring and management of critical care patients.10,11 Despite the low risk of clinically relevant sequelae, published guidelines recommend a routine administration of stress ulcer prophylaxis (SUP) with acid suppressive therapy for high-risk patients.12–14 The rationale for this recommendation is to prevent clinically important GI bleeding due to its strong association with patient mortality and a prolonged ICU stay of roughly 4–8 days.15 Therefore, clinicians widely recognize the prophylaxis of stress ulcers and related GI bleeding as a crucial component of pharmacotherapy in ICU patients. However, several studies have raised concerns that prophylactic therapy against stress ulcers is frequently prescribed to low-risk patients, such as those admitted to general medical floors, without supporting evidence.2,16,17 This practice is problematic as the overuse of acid suppressants in the absence of an indication for SUP or other acid–peptic related disorders can incur large health care expenditure, as well as adverse clinical outcomes with minimal therapeutic benefits.
The necessity of SUP is largely determined by the presence of relevant risk factors for clinically significant GI hemorrhage. Cook et al2 in 1994 identified two primary risk factors associated with the highest risk of clinically important GI bleeding in intensive care patients: coagulopathy (odds ratio [OR]: 4.3); and respiratory failure requiring prolonged mechanical ventilation (OR: 15.6). Several other risk factors were also specified in the first practice guidelines published in 1999 by the American Society of Health-System Pharmacists (ASHP): major trauma; severe head injury; multiple organ failure; burns covering more than 25%–30% of the body; and major surgical procedures.12 An updated guideline published in 2006 also suggests that acid suppression is warranted in patients with at least one of those independent risk factors: coagulopathy; mechanical ventilation for >48 hours; or a history of GI bleeding or ulceration within the past year.14 According to current practice guidelines, risk factors other than the aforementioned three do not independently predispose a patient to stress ulcer bleeding; therefore, SUP should be withheld in a majority of hospitalized patients unless they have multiple risk factors.18 Nevertheless, several studies demonstrate that noncritically ill patients who are lacking an indication warranting SUP are abundantly initiated on acid suppressive therapy upon hospital admission.16,19–21 Moreover, the SUP agents are often inadvertently continued as a discharge medication (up to 68.8%), and hence the unwarranted therapy persists beyond hospital stay.16,22–25 The resultant prolonged use of acid suppressants can lead to adverse clinical complications, as well as to the economic waste of resources.16
Of the several antiulcer agents, recent guidelines from the Surviving Sepsis Campaign suggest that proton pump inhibitors (PPIs), the new class of antisecretory drugs, be considered over histamine 2 receptor antagonists (H2RAs) for the provision of SUP.13 Also, several studies showed a lower incidence of GI hemorrhage with PPIs than with their progenitor agents, H2 blockers.3,4,26 These findings have potentially encouraged the shift toward PPIs, which have recently eclipsed H2RAs as the agents of first choice for SUP in the United States (76% versus 23%, respectively).27 However, the excessive utilization of PPIs is concerning given the possible association of chronic PPI therapy with an increased risk for adverse complications, such as Clostridium difficile colitis,28,29 pneumonia,30–32 and diminished bone marrow density.33,34
In light of the clinical and economic concerns, as well as lack of guidelines regarding prescribing SUP in general medicine patients, this study was designed to assess the incidence of inappropriate PPI use for SUP in ICU versus non-ICU settings that continued postdischarge from a major academic medical center in Korea, and to estimate the expenditure that originated from the unindicated outpatient continuation of PPI therapy.
Methods
After obtaining approval from the Ajou Institutional Review Board in June 2014, a retrospective review of electronic medical records of patients admitted to the Ajou University Hospital (Yeongtong-gu, Suwon-si, Gyeonggi-do, South Korea) between January 1, 2010 and December 31, 2013 was performed. This hospital is a 1,108-bed university-affiliated, tertiary care teaching hospital with 138 medical and surgical ICU beds in Korea. Informed consent was not obtained from the study patients, as patient records were anonymized and deidentified prior to analysis. Adult patients, aged 18 years and older, were initially selected if they had received as an inpatient at least one dose of PPI therapy (rabeprazole, lansoprazole, esomeprazole, omeprazole, or pantoprazole), which was identified by the World Health Organization Anatomical Therapeutic Chemical classification codes (http://www.whocc.no/atc_ddd_index/). To ensure that new starters on PPIs, whose therapy was inadvertently continued posthospital discharge, were captured for analysis, the following data extraction logic was designed. Those patients who had a PPI on their preadmission medication list (or home medication list) were assumed to have an appropriate indication prior to admission and hence were excluded from further analysis. The rationale for this criterion was to account for a potentially proper utilization and to avoid overrating of inadequate prescribing at discharge. Any patient lacking an appropriate International Classification of Diseases, Tenth Revision (ICD-10) diagnosis that warrants PPI therapy (Table 1) prior to or during hospital admission was considered for receiving the acid suppressant for the purpose of SUP. In contrast, those patients with an appropriate diagnosis requiring PPI therapy were eliminated from analysis; this elimination process had to be employed to capture PPI therapy that was apparently attributed to SUP, as this off-label indication is not assigned a specific ICD-10 code. Of the remaining patients on PPI prophylaxis, those who were injudiciously prescribed a PPI at hospital discharge without an appropriate indication were identified by reviewing their discharge medication list. For the collected cases, the estimated total costs linked to inadequate continuation of a PPI postdischarge were calculated based on the specific regimen, as well as on the individual price of the medication prescribed. For example, if a patient is discharged on oral pantoprazole 40 mg daily for 30 days, the total cost associated with the regimen is obtained by multiplying the unit price of 771 won and a therapy duration of 30 days. To convert currencies, the US dollar–South Korean won exchange rate of 1,098.9 was utilized. If a patient was admitted multiple times during the course of the study period, each admission was separately counted.
Table 1.
Appropriate diagnoses for acid suppressive therapy
| Diagnosis | ICD-10 codes | |
|---|---|---|
| Diseases of the esophagus, stomach, and duodenum (K20–K31) | Esophagitis | K20 |
| Gastroesophageal reflux disease | K21.x | |
| Other diseases of the esophagus | K22.x | |
| Gastric ulcer | K25.xx | |
| Duodenal ulcer | K26.xx | |
| Peptic ulcer | K27.xx | |
| Gastrojejunal ulcer | K28.xx | |
| Gastritis and duodenitis | K29.x | |
| Dyspepsia | K30.x | |
| Other diseases of the stomach and duodenum | K31.x | |
| Other indications | Esophageal varices | I85.x |
| Congenital stenosis or stricture of the esophagus | Q39.3 | |
| Heartburn | R12 | |
| Gastrointestinal hemorrhage | K92.0–K92.2 | |
| Zollinger–Ellison syndrome | E16.4 | |
| Helicobacter pylori infection | B98.0 | |
| External causes of morbidity and mortality | NSAID | Y45.3 |
| Anticoagulants | Y44.2 |
Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision; NSAID, nonsteroidal anti-inflammatory drug.
For statistical analysis, nominal data were analyzed using Pearson’s chi-square test and presented in the form of frequency distributions. Statistical analyses were performed using SPSS version 18.0 (IBM Corporation, Armonk, NY, USA) with P<0.05 considered to be statistically significant.
Results
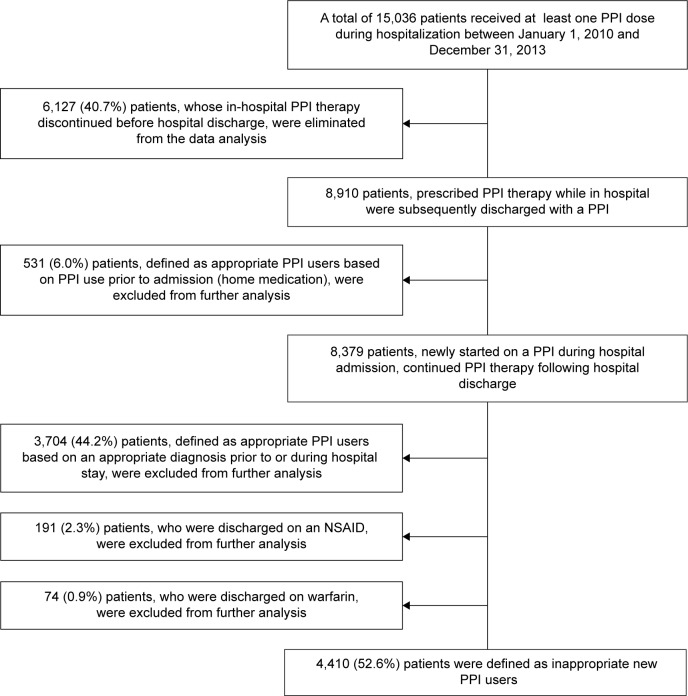
A total of 15,036 patients were dispensed at least one PPI dose while in hospital during the 4-year study period (Figure 1). Of these, 6,127 (40.7%) patients, whose inpatient PPI therapy discontinued before hospital discharge, were excluded from further analysis for estimating the costs associated with inappropriate outpatient continuation of the antisecretory agent. Therefore, the total number of patients who were prescribed PPI therapy as an inpatient and who were subsequently discharged on a PPI was 8,910. Of these continuous PPI users, 531 (6.0%) were further excluded from this analysis, as they had been using a PPI prior to admission as a home medication (assumed to have an appropriate indication for acid suppression). Of the resultant 8,379 hospital-initiated PPI users, 622 (7.4%) were admitted to ICUs during their hospital stay while the remaining 7,757 (92.6%) were non-ICU patients. Approximately 44.2% of patients (3,704/8,379) with an appropriate diagnosis (as noted in Table 1) that warrants antiulcer therapy prior to or during their hospital admission were also eliminated from further data analysis. Additionally, 191 (2.3%) and 74 (0.9%) patients were discharged with a nonsteroidal anti-inflammatory drug and warfarin, respectively, and to avoid overestimation of inappropriate PPI utilization following hospital discharge, those patients were also eliminated from the economic evaluation; the concomitant use of a PPI with the ulcerogenic or anticoagulant drug is categorized as appropriate because of the risk of GI bleeding. The resultant 4,410 (52.6%) patients, more than half of the hospital-initiated PPI users, received the acid suppressant presumably for prophylactic purposes, and these patients were then deemed inadequately prescribed a PPI at hospital discharge.
Figure 1.
Flow diagram of the process of identifying and including study patients.
Abbreviations: PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug.
Table 2 presents the yearly trends in the incidence of inappropriate PPI use posthospital discharge and the costs associated with it. The incidence rates of inappropriate outpatient continuation of the prophylactic treatment were generally higher among ICU patients when compared with those admitted to general medicine floors, and the difference were statistically significant (57.7% versus 52.2%, respectively; P=0.001, based on Pearson’s chi-square test). During the 4-year study period, unnecessary prescribing of PPI therapy at discharge became more prevalent, and the incidence rates increased substantially in 2013 both in ICUs and in non-ICUs (71.3% versus 62.1%, respectively; P<0.001). The total expenditure incurred due to the continuation of nonindicated PPI therapy was approximately US$40,175. This amount includes costs paid by both insurance and patients. All Korean citizens have health coverage under the National Health Insurance Program, and typically a universal copayment rate is applied for pharmaceutical expenditure. The copayment rate for outpatient medicine is 30%; therefore, patients were required to pay roughly USD $12,053 as copay for unwarranted therapy during the study period.
Table 2.
Incidence rates and costs of inadvertent outpatient continuation of PPI-based SUP
| Year | ICU stay, n (%) | No ICU stay, n (%) | Total inappropriate use, n (%) | Total cost, US$ | Patient cost, US$ |
|---|---|---|---|---|---|
| 2010 | 68/141 (48.2%) | 676/1,745 (38.7%) | 744/1,886 (39.4%) | $6,610 | $1,983 |
| 2011 | 63/135 (46.7%) | 728/1,593 (45.7%) | 791/1,728 (45.8%) | $7,029 | $2,109 |
| 2012 | 57/106 (53.8%) | 805/1,451 (55.5%) | 862/1,557 (55.4%) | $7,422 | $2,226 |
| 2013 | 171/240 (71.3%) | 1,842/2,968 (62.1%) | 2,013/3,208 (62.7%) | $19,115 | $5,734 |
| Total | 359/622 (57.7%) | 4,051/7,757 (52.2%) | 4,410/8,379 (52.6%) | $40,175 | $12,053 |
Abbreviations: PPI, proton pump inhibitor; SUP, stress ulcer prophylaxis; ICU, intensive care unit.
The top 20 ICD-10 diagnostic codes assigned to the 4,410 study patients are listed in Table 3. Of these patients, hypertensive diseases (I10–I15), malignant neoplasms (C76–C80), and diabetes mellitus (E10–E14) were the top three diagnostic categories (51.6%, 35.2%, and 28.9%, respectively).
Table 3.
Top 20 diagnostic categories associated with inappropriate PPI utilization
| Diagnosis categories (ICD-10 codes) | Patient count (%) |
|---|---|
| Hypertensive diseases – includes essential hypertension, hypertensive heart and renal disease, and secondary hypertension (I10–I15) | 2,274 (51.6%) |
| Malignant neoplasms of ill-defined, secondary, and unspecified sites (C76–C80) | 1,553 (35.2%) |
| Diabetes mellitus – includes type 1 diabetes mellitus, type 2 diabetes mellitus, and malnutrition-related diabetes mellitus (E10–E14) | 1,273 (28.9%) |
| Malignant neoplasms of digestive organs (C15–C26) | 1,186 (26.9%) |
| Ischemic heart diseases – includes angina pectoris, acute myocardial infarction, subsequent myocardial infarction, acute ischemic heart disease, and chronic ischemic heart disease (I20–I25) | 669 (15.2%) |
| Benign neoplasms (D10–D36) | 514 (11.7%) |
| Other diseases of the upper respiratory tract – includes rhinitis, nasopharyngitis, pharyngitis, sinusitis, laryngitis, and laryngotracheitis (J30–J39) | 506 (11.5%) |
| Cerebrovascular diseases – includes subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infarction, stroke, and occlusion and stenosis of the cerebral arteries (I60–I69) | 463 (10.5%) |
| Diseases of the liver – includes alcoholic liver disease, toxic liver disease, hepatic failure, chronic hepatitis, and fibrosis and cirrhosis of the liver (K70–K77) | 414 (9.4%) |
| Renal failure – includes acute renal failure and chronic kidney disease (N17–N19) | 392 (8.9%) |
| Influenza and pneumonia (J09–J18) | 302 (6.8%) |
| Other forms of heart disease – includes pericarditis, endocarditis, nonrheumatic valve disorders, myocarditis, cardiomyopathy, conduction disorders, cardiac arrest, arrhythmias, and heart failure (I30–I52) | 298 (6.8%) |
| Malignant neoplasms of respiratory and intrathoracic organs (C30–C39) | 292 (6.6%) |
| Chronic lower respiratory diseases – includes bronchitis, emphysema, chronic obstructive pulmonary disease, asthma, and bronchiectasis (J40–J47) | 277 (6.3%) |
| Arthrosis (M15–M19) | 269 (6.1%) |
| Metabolic disorders – includes disorders of amino acid metabolism, carbohydrate metabolism, lipoprotein metabolism, volume depletion and disorders of fluid, electrolyte, and acid–base balance (E70–E90) | 263 (6.0%) |
| Spondylopathies (M45–M49) | 234 (5.3%) |
| Disorders of the gallbladder, biliary tract, and pancreas (K80–K87) | 227 (5.1%) |
| Other soft tissue disorders – includes bursopathies, fibroblastic disorders, shoulder lesions, and enthesopathies (M70–M79) | 191 (4.3%) |
| Malignant neoplasms, stated or resumed to be primary, of lymphoid, hematopoietic, and related tissue (C81–C96) | 191 (4.3%) |
Note: Total patient number =4,410.
Abbreviations: PPI, proton pump inhibitor; ICD-10, International Classification of Diseases, Tenth Revision.
Discussion
Prescribing a PPI for the prophylaxis of stress ulcers and related bleeding is considered off-label or unlabeled use, which means that the agent is utilized in a manner not indicated in the US Food and Drug Administration (FDA)-approved package insert.27 This practice enables clinicians to legally prescribe PPI therapy for prophylactic purposes based on their individual evidence-based clinical judgment, regardless of the US FDA approval for that particular indication.27 Many health care practitioners widely accept off-label prescribing for SUP as standard of care in critically ill patients.2 However, only scant clinical evidence exists in the medical literature that assists clinicians in evaluating the therapeutic benefits, as well as the safety concerns, related to prescribing PPIs for SUP. In addition, there is insufficient guidance as to when it is appropriate to initiate, discontinue, or withhold the prophylaxis. The paucity of reliable clinical data is attributed to the fact that antisecretory agents are not required by the US FDA to undergo extensive testing or a rigorous review process to achieve that off-label indication in their official product labeling.27 Additionally, critically ill patients are mostly excluded from or under-represented in clinical trials, as they present a particular set of challenges that make it difficult to design studies involving those patient population.35,36 As a consequence, the optimal strategy to assess the appropriateness of the prophylactic treatment remains uncertain. Nevertheless, off-label prescribing has boosted the widespread use of prophylactic PPI among hospitalized patients. A previous study also demonstrates that the most predominant class of medications used off-label in ICUs is PPIs (55%), and SUP is the most predominant off-label indication.27 At the Ajou University Hospital, PPIs presumably intended for SUP are often dispensed without being linked to a particular medical diagnosis, possibly due to the fact that there is no specific ICD-10 code assigned for SUP.
Another factor that influenced the substantial increase in PPI use in hospital includes the recommendations from practice guidelines developed by professional organizations. Despite the paucity and the low quality of evidence on therapeutic benefits of SUP, professional organizations universally endorse pharmacologic interventions to prevent stress ulcers and related bleeding in high-risk patients.12–14 The first evidence-based guidelines from ASHP (published in 1999) base its recommendations on the results from a systematic review published almost two decades ago. The study demonstrates that prophylaxis with H2RAs lowers the occurrence of overt GI bleeding (OR: 0.58; 95% confidence interval [CI]: 0.42–0.79) and clinically significant bleeding (OR: 0.44; 95% CI: 0.22–0.88).8 However, PPIs were not categorized as the agent of choice in the ASHP guidelines, as there were not sufficient data concerning their therapeutic efficacy and safety at the time of writing. In 2008, the Eastern Association for the Surgery of Trauma released therapeutic guidelines on SUP, which specified that PPIs are equivalent to H2RAs in preventing overt bleeding from stress ulcers (level 1 recommendation).37 More recently, the Surviving Sepsis Campaign guidelines13 suggest that PPIs are superior to H2RAs in reducing the rate of stress ulcer bleeding (grade 2C recommendation). Currently, new practice guidelines are under development by the ASHP and also by the Society of Critical Care Medicine, and both are expected to be released in 2015.18
Recent guidelines’ suggestion of considering PPIs over H2RAs for SUP is largely based on the results from several meta-analyses that compared the efficacy and safety of the two classes of antisecretory agents.13 PPIs provide profound acid suppression, but whether their high potency in increasing gastric pH translates into improved clinically important endpoints remains uncertain.18 Thus far, four meta-analyses have reported that PPIs are associated with lower rates of GI bleeding than H2RAs, although the overall quality of the included studies is questionable.3,4,26,38 The first meta-analysis was performed by Pongprasobchai et al38 in 2009, which included three trials enrolling a total of 569 critically ill patients. The overall incidence of clinically significant bleeding was lower with PPI therapy (3.5%) when compared with H2RA (8%) (OR: 0.42; 95% CI: 0.20–0.91).38 In 2010, Lin et al4 analyzed the results from seven trials with a total of 936 critical care patients. They reported no difference in clinically significant GI bleeding between treatment with PPIs and with H2RAs (overall pooled risk difference: −0.04; 95% CI: −0.09 to 0.01; P=0.08).4 Barkun et al26 included 13 trials with a total of 1,587 critically ill patients and found that PPIs were associated with less clinically important bleeding than H2RAs (OR: 0.30; 95% CI: 0.17–0.54). The most recent meta-analysis by Alhazzani et al3 included 1,720 critically ill patients from 14 trials and revealed greater efficacy with PPI therapy at decreasing clinically important GI bleeding (relative risk [RR]: 0.36; 95% CI: 0.19–0.68; P=0.002) and overt upper GI bleeding (RR: 0.35; 95% CI: 0.21–0.59; P<0.0001). Overall, pooled data suggest that patients with relevant risk factors may benefit more from receiving prophylactic treatment with PPIs than with H2RAs.3,4,26,38 However, due to the poor methodological quality and significant heterogeneity of the included studies, the results from the aforementioned meta-analyses should be interpreted cautiously. Other advantages of PPIs include their longer duration of action and no tachyphylaxis in contrast to H2RAs. In addition, acquisition costs for PPIs have decreased substantially with patent expiration and generic availability, although PPIs are generally more expensive than H2 blockers.27
PPIs are typically considered safe and well tolerated, but overuse of the agents is raising safety concerns due to their possible adverse effects associated with long-term therapy. Several recent clinical trials have associated the use of PPIs with an increased risk of severe complications, such as C. difficile-associated diarrhea (CDAD),28,29 pneumonia,30–32 osteoporosis,33,34 and interstitial nephritis.39–41 Also, higher mortality rates were noted in elderly patients discharged on PPI therapy from acute care hospitals.42 Another safety concern related to the excess usage of PPIs includes potential drug interactions, especially with clopidogrel. The concern is that PPIs inhibit an enzyme called CYP2C19, which is crucial in converting clopidogrel to an active metabolite.43 The US FDA issued a warning with regard to the concomitant use of clopidogrel and omeprazole/esomeprazole, and experts now recommend an individualized, risk–benefit approach when prescribing the acid reducers for those taking clopidogrel.43
This study found that, despite the fact that prophylactic treatment for preventing stress ulcer bleeding is recommended only for selected critically ill patients, overutilization of PPI therapy for SUP has spread to general medicine patients at the evaluated hospital in Korea. As many as 52.2% of non-ICU patients were prescribed PPI-based SUP and inappropriately continued the prophylaxis postdischarge over the study period. This is much higher than the findings of previous reports where the continuation of SUP at the time of discharge ranged from 18% to 34%.22–24 More importantly, inappropriate PPI use rates increased over the 4-year study period, and the majority of such cases were found among low-risk, non-ICU patients. The study results indicate that the overutilization of unwarranted PPI therapy could place tremendous pressure on the health care system and increase the costs of medication therapy and the management of adverse drug events.
Several studies suggest that the implementation of an interprofessional quality improvement initiative can reduce the inappropriate utilization of SUP during hospital admission, as well as postdischarge.16,44 In a recent study, Tasaka et al44 compared prescribing practices before and after the quality improvement initiative composed of an institution SUP guideline, pharmacist-led intervention, and an education/awareness campaign. The incidence of the inappropriate use of SUP was significantly lower in the intervention group when compared with the control group (nine and 19 per 100 patient-days, respectively; P=0.03).44 According to findings from a survey conducted to assess the factors influencing physicians’ prescribing behavior regarding SUP, fear of legal repercussions of not prescribing SUP and ignorance of the adverse effects of acid suppressive therapy showed a clinically relevant association with inappropriate prescribing of SUP.45 Raising awareness about the adverse effects of prolonged acid suppression and about the existing practice guidelines among prescribing physicians can reduce the unnecessary utilization of PPIs. Individual institutions may consider developing protocols regarding the provision of SUP, which can help reduce the overuse of PPIs and, at the same time, educate health care professionals about the proper indications for the prophylaxis. Also, pharmacists should take a leadership role in managing medication reconciliation at patient discharge to identify unnecessary therapy, such as SUP.24
Limitations
This study has several limitations. Because of the retrospective and single institution study design, our results may not be applicable to other institutions. Due to the absence of a specific ICD-10 code assigned to SUP, an assumption had to be made that patients lacking a proper diagnosis for acid-reducing therapy were administered a PPI for SUP. Diagnostic codes entered incorrectly or not entered by physicians could have potentially affected the rates of improper PPI use analyzed in this study. Inappropriate outpatient continuation of PPI therapy may have been exaggerated because of the elimination process utilized in this study to capture apparent hospital-initiated PPI users. Reviewing the preadmission medication list may have failed to identify actual PPI use prior to admission. Postdischarge utilization of over-the-counter PPIs was not captured by the data extraction logic implemented in this analysis, which could have potentially resulted in an underestimation of the improper continuation of the acid-reducing agents. All prescribed PPIs at discharge were assumed to be dispensed and taken by patients, although it does not necessarily mean that patients filled the prescriptions and completed the whole course of therapy.
Conclusion
This study confirmed that PPIs presumably intended for SUP were widely prescribed in non-ICU patients. More than half of both ICU and non-ICU patients with hospital-initiated PPI therapy for SUP were discharged with a prescription for PPIs, despite no medical cause to continue such therapy. Over the 4-year study period, the total cost attributed to inappropriate outpatient continuation of PPI therapy was estimated to be USD $40,175. Educating health care practitioners regarding existing guidelines on the provision of SUP and the adverse effects associated with the long-term use of acid-reducing therapy can improve the cost effectiveness of, as well as patient outcomes from, PPI therapy.
Acknowledgments
This study was supported by the new faculty research fund of Ajou University and the Bio and Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science, Information and Communications Technology, and Future Planning, Republic of Korea (number 2013M3A9B5075838).
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Grube RR, May DB. Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64(13):1396–1400. doi: 10.2146/ajhp060393. [DOI] [PubMed] [Google Scholar]
- 2.Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330(6):377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 3.Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41(3):693–705. doi: 10.1097/CCM.0b013e3182758734. [DOI] [PubMed] [Google Scholar]
- 4.Lin PC, Chang CH, Hsu PI, Tseng PL, Huang YB. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010;38(4):1197–1205. doi: 10.1097/CCM.0b013e3181d69ccf. [DOI] [PubMed] [Google Scholar]
- 5.D’Ancona G, Baillot R, Poirier B, et al. Determinants of gastrointestinal complications in cardiac surgery. Tex Heart Inst J. 2003;30(4):280–285. [PMC free article] [PubMed] [Google Scholar]
- 6.Faisy C, Guerot E, Diehl JL, Iftimovici E, Fagon JY. Clinically significant gastrointestinal bleeding in critically ill patients with and without stress-ulcer prophylaxis. Intensive Care Med. 2003;29(8):1306–1313. doi: 10.1007/s00134-003-1863-3. [DOI] [PubMed] [Google Scholar]
- 7.Shuman RB, Schuster DP, Zuckerman GR. Prophylactic therapy for stress ulcer bleeding: a reappraisal. Ann Intern Med. 1987;106(4):562–567. doi: 10.7326/0003-4819-106-4-562. [DOI] [PubMed] [Google Scholar]
- 8.Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA. 1996;275(4):308–314. [PubMed] [Google Scholar]
- 9.Qadeer MA, Richter JE, Brotman DJ. Hospital-acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings. J Hosp Med. 2006;1(1):13–20. doi: 10.1002/jhm.10. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med. 2010;38(11):2222–2228. doi: 10.1097/CCM.0b013e3181f17adf. [DOI] [PubMed] [Google Scholar]
- 11.Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med. 1994;20(5):335–340. doi: 10.1007/BF01720905. [DOI] [PubMed] [Google Scholar]
- 12.ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56(4):347–379. doi: 10.1093/ajhp/56.4.347. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spirt MJ, Stanley S. Update on stress ulcer prophylaxis in critically ill patients. Crit Care Nurse. 2006;26(1):18–20. 22–28. quiz 29. [PubMed] [Google Scholar]
- 15.Cook DJ, Griffith LE, Walter SD, et al. Canadian Critical Care Trials Group The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. 2001;5(6):368–375. doi: 10.1186/cc1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas L, Culley EJ, Gladowski P, Goff V, Fong J, Marche SM. Longitudinal analysis of the costs associated with inpatient initiation and subsequent outpatient continuation of proton pump inhibitor therapy for stress ulcer prophylaxis in a large managed care organization. J Manag Care Pharm. 2010;16(2):122–129. doi: 10.18553/jmcp.2010.16.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997. doi: 10.1001/archinternmed.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barletta JF, Sclar DA. Use of proton pump inhibitors for the provision of stress ulcer prophylaxis: clinical and economic consequences. Pharmacoeconomics. 2014;32(1):5–13. doi: 10.1007/s40273-013-0119-5. [DOI] [PubMed] [Google Scholar]
- 19.Gardner TB, Robertson DJ. Stress ulcer prophylaxis in non-critically ill patients: less may be more. Am J Gastroenterol. 2006;101(10):2206–2208. doi: 10.1111/j.1572-0241.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 20.Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol. 2006;101(10):2200–2205. doi: 10.1111/j.1572-0241.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 21.Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122. doi: 10.1111/j.1572-0241.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy CE, Stevens AM, Ferrentino N, et al. Frequency of inappropriate continuation of acid suppressive therapy after discharge in patients who began therapy in the surgical intensive care unit. Pharmacotherapy. 2008;28(8):968–976. doi: 10.1592/phco.28.8.968. [DOI] [PubMed] [Google Scholar]
- 23.Wohlt PD, Hansen LA, Fish JT. Inappropriate continuation of stress ulcer prophylactic therapy after discharge. Ann Pharmacother. 2007;41(10):1611–1616. doi: 10.1345/aph.1K227. [DOI] [PubMed] [Google Scholar]
- 24.Zeigler AJ, McAllen KJ, Slot MG, Barletta JF. Medication reconciliation effect on prolonged inpatient stress ulcer prophylaxis. Ann Pharmacother. 2008;42(7):940–946. doi: 10.1345/aph.1L123. [DOI] [PubMed] [Google Scholar]
- 25.Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565–1571. doi: 10.1345/aph.1P167. [DOI] [PubMed] [Google Scholar]
- 26.Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors vs histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol. 2012;107(4):507–20. doi: 10.1038/ajg.2011.474. quiz 521. [DOI] [PubMed] [Google Scholar]
- 27.Barletta JF, Lat I, Micek ST, Cohen H, Olsen KM, Haas CE, Critical Care Pharmacotherapy Trials Network Off-label use of gastrointestinal medications in the intensive care unit. J Intensive Care Med. 2013 Dec 20; doi: 10.1177/0885066613516574. Epub. [DOI] [PubMed] [Google Scholar]
- 28.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 29.Dial S, Delaney JA, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175(7):745–748. doi: 10.1503/cmaj.060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook DJ. Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis. Scand J Gastroenterol Suppl. 1995;210:48–52. doi: 10.3109/00365529509090271. [DOI] [PubMed] [Google Scholar]
- 31.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301(20):2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 32.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 33.Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(Suppl 2):S21–S26. doi: 10.1038/ajg.2009.48. [DOI] [PubMed] [Google Scholar]
- 34.Yang YX. Proton pump inhibitor therapy and osteoporosis. Curr Drug Saf. 2008;3(3):204–209. doi: 10.2174/157488608785699414. [DOI] [PubMed] [Google Scholar]
- 35.Flanagan BM, Philpott S, Strosberg MA. Protecting participants of clinical trials conducted in the intensive care unit. J Intensive Care Med. 2011;26(4):237–249. doi: 10.1177/0885066610390867. [DOI] [PubMed] [Google Scholar]
- 36.Vincent JL. We should abandon randomized controlled trials in the intensive care unit. Crit Care Med. 2010;38(10 Suppl):S534–S538. doi: 10.1097/CCM.0b013e3181f208ac. [DOI] [PubMed] [Google Scholar]
- 37.Guillamondegui OD, Gunter OL, Jr, Bonadies JA, et al. EAST Practice Management Guidelines Committee [webpage on the Internet] Stress ulcer prophylaxis. Chicago, IL: Eastern Association for the Surgery of Trauma; 2008. [Accessed January 19, 2015]. Available from: http://www.east.org/resources/treatment-guidelines/stress-ulcer-prophylaxis. [Google Scholar]
- 38.Pongprasobchai S, Kridkratoke S, Nopmaneejumruslers C. Proton pump inhibitors for the prevention of stress-related mucosal disease in critically-ill patients: a meta-analysis. J Med Assoc Thai. 2009;92(5):632–637. [PubMed] [Google Scholar]
- 39.Geevasinga N, Coleman PL, Webster AC, Roger SD. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol. 2006;4(5):597–604. doi: 10.1016/j.cgh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy M, Snyder R, Bachurina M. Long-term use of proton pump inhibitors: are they really safe? A case of delayed acute interstitial nephritis. J Am Geriatr Soc. 2009;57(8):1513–1514. doi: 10.1111/j.1532-5415.2009.02409.x. [DOI] [PubMed] [Google Scholar]
- 41.Simpson IJ, Marshall MR, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology (Carlton) 2006;11(5):381–385. doi: 10.1111/j.1440-1797.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 42.Maggio M, Corsonello A, Ceda GP, et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518–523. doi: 10.1001/jamainternmed.2013.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drepper MD, Spahr L, Frossard JL. Clopidogrel and proton pump inhibitors – where do we stand in 2012? World J Gastroenterol. 2012;18(18):2161–2171. doi: 10.3748/wjg.v18.i18.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462–469. doi: 10.1177/1060028013517088. [DOI] [PubMed] [Google Scholar]
- 45.Hussain S, Stefan M, Visintainer P, Rothberg M. Why do physicians prescribe stress ulcer prophylaxis to general medicine patients? South Med J. 2010;103(11):1103–1110. doi: 10.1097/SMJ.0b013e3181f6539d. [DOI] [PubMed] [Google Scholar]