More than 380 million people are currently suffering from diabetes. The International Diabetes Federation estimated that this would rise to 592 million within a generation. Despite this relentless increase in the prevalence of type 2 diabetes worldwide, with the advent of potent statins, there has been a gradual decline in mortality from macrovascular complications, such as stroke and coronary heart disease. However, this is not the situation in diabetic nephropathy, which remains a leading cause of end-stage renal disease (ESRD) globally, with its incidence being still on a rising trend. Notably, once nephropathy develops, approximately 20–40% of patients inevitably progress to ESRD. Therefore, we need biomarkers that would enable early risk stratification in diabetic nephropathy (Figure1), so that those at higher risk of progression to ESRD are identified at early stages of nephropathy development. Long-term follow up of interventional studies, such as STENO-2, have clearly shown that, even at the stage of microalbuminuria, intensive multifactorial management targeting blood pressure (particularly with blockade of the renin–angiotensin system), glucose, cholesterol and lifestyle issues could reduce the progression to ESRD as well as the associated cardiovascular mortality. The availability of sensitive and well-validated biomarkers for nephropathy progression would allow targeted intensive management to be provided to those who would derive the most cost-effective benefits from such intervention.
Figure 1.
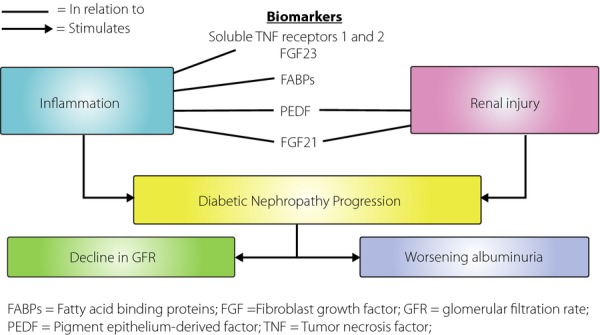
Emerging biomarkers of diabetic nephropathy in relation to disease pathogenesis. FABPs, fatty acid binding proteins; FGF, fibroblast growth factor; GFR, glomerular filtration rate; PADF, pigment epithelium-derived factor; TNF, tumor necrosis factor.
In the past, persistent microalbuminuria was the most studied biomarker in diabetic nephropathy. Both the presence and the incremental changes in microalbuminuria had been shown to correlate with the development and progression of chronic kidney disease in type 1 and type 2 diabetes. Furthermore, microalbuminuria was also shown to be a risk factor for the development of macrovascular complications in diabetic patients. These were all good reasons for incorporating the measurement of urinary albumin excretion into our clinical practice until the recent decade, when microalbuminuria was challenged for its high variability, low sensitivity and specificity in predicting kidney disease progression in diabetic nephropathy. This change in paradigm was supported by the observation that spontaneous remission of microalbuminuria could occur in more than half of diabetic patients. Furthermore, 20% of type 2 diabetic patients had their glomerular filtration rate (GFR) decline to ≤60 mL/min/1.73 m2 before or even without passing the stage of microalbuminuria. Therefore, the progression of diabetic nephropathy, if defined as a deterioration in chronic kidney disease (CKD) staging or a decline in GFR, is not necessarily in parallel with the progression in urinary albumin excretion. Hence, in a proportion of diabetic patients, microalbuminuria might not be sufficient as a marker to identify or stratify those at risk of kidney disease progression or ESRD, especially if we aim at intervening at an early stage of diabetic nephropathy.
This has led to active research on new prognostic biomarkers for progressive diabetic nephropathy in recent years. Of the emerging candidate biomarkers, serum cystatin C, fibroblast growth factor 23 (FGF23) and soluble tumor necrosis factor (TNF) receptors have provided the most promising data. Serum cystatin C is produced by all nucleated cells, freely filtered by the glomeruli and completely metabolized by the proximal renal tubules. An observational study by Krolewski et al.1 showed that the measurement of serum cystatin C improved the risk prediction of nephropathy progression to ESRD in type 2 diabetic patients. They recruited more than 1,000 diabetic patients from three Caucasian cohorts, with CKD stages 1–3 at baseline, and followed them up for 8–10 years. These subjects had baseline estimated GFR (eGFR) measured by both serum creatinine and serum cystatin C. Subjects given a higher baseline CKD stage by cystatin C-based eGFR than by creatinine-based eGFR had a significantly higher risk of progression to ESRD compared with those with concordant staging, suggesting a superior risk prediction by cystatin C-based eGFR. Markers of inflammation are also potential candidates for the prediction of progressive kidney disease in diabetes, as inflammation is clearly one of the key players in the pathogenesis of diabetic nephropathy (Figure1). Both FGF23, an endocrine hormone related to phosphate homeostasis and vitamin D activation, and circulating TNF receptors (1 and 2), had been shown to predict renal outcome in type 2 diabetes. Niewczas et al.2 showed that raised baseline levels of TNF receptors 1 and 2 predicted the progression to ESRD in more than 400 type 2 diabetic patients after 12 years of follow up, even after adjustment for urinary albumin excretion. Furthermore, it was shown in another study that the predictive effect of FGF23 on the risk of progression in diabetic nephropathy was dependent on the TNF receptor 1, likely because the downregulation of Klotho by TNF-α leads to FGF23 resistance and hence a compensatory increase in FGF23 expression. These suggested the superiority of using serum TNF receptor 1 over conventional biomarkers, such as microalbuminuria, as it could predict the progression to ESRD, a hard renal end-point, irrespective of the presence or absence of albuminuria. This is certainly relevant to the subset of diabetic patients discussed above, who suffered from GFR decline without passing through the stage of microalbuminuria. Recently, fatty acid binding proteins, a group of diverse proteins involved in lipid homeostasis, have also been reported as potential biomarkers for diabetic nephropathy. Elevated urinary levels of liver-fatty acid binding protein (L-FABP) were detected in diabetic patients even before the onset of microalbuminuria. As diabetic nephropathy is characterized by the renal accumulation of activated macrophages, it is perhaps not surprising that serum levels of adipocyte-fatty acid binding protein (A-FABP), which is highly expressed in macrophages, are independently associated with nephropathy staging in type 2 diabetes3. Data presented at the 2014 Diabetes Congress of the International Diabetes Federation – West Pacific Region suggest that serum A-FABP might also independently predict early eGFR decline in type 2 diabetic patients.
Another two potential novel biomarkers of progression in diabetic nephropathy have recently been identified: pigment epithelium-derived factor (PEDF) and FGF21 (Figure1)4,5. PEDF is a secreted circulating glycoprotein with anti-oxidative, anti-inflammatory and anti-angiogenic properties, whereas FGF21 is a hormone predominantly secreted from the liver and possess multiple metabolic regulatory properties. In a cohort involving more than 1,000 Chinese type 2 diabetic patients with baseline eGFR ≥60 mL/min/1.73 m2, both baseline circulating PEDF and FGF21 levels were shown to be independent predictors of the decline in renal function. In addition, in subgroups of diabetic patients with relatively well-preserved kidney function, with an eGFR ≥60 mL/min/1.73 m2 and normoalbuminuria, baseline serum PEDF and FGF21 levels were independently associated with the progression to micro- or albuminuria and eGFR decline respectively, even after adjusted for baseline eGFR levels. Further evaluation in other cohorts is certainly warranted to confirm these exciting findings. It is interesting to note that PEDF is expressed in the kidney, and renoprotective as well as anti-inflammatory effects of PEDF have been shown in diabetic rats with microalbuminuria. In contrast, impaired FGF21 signaling in the kidney has been found in db/db mice, a rodent model of type 2 diabetes. In the adipose tissue of obese mice, such impaired signaling or FGF21 resistance has been attributed to local inflammation and TNF-α induced downregulation of β-Klotho, the obligatory coreceptor protein for FGF21 signaling. Increased local FGF21 expression has also been found in response to drug-induced renal and liver injury, and exogenous FGF21 results in decreased renal apoptosis, suppressed diabetes-induced renal inflammation, oxidative stress and fibrosis in animal models. Taken together, the elevation in both serum PEDF and FGF21 levels might simply reflect compensatory responses, and reflect on the severity of the underlying renal inflammation and injury in type 2 diabetes, which would contribute to the development and progression of diabetic nephropathy. (Figure1) In addition, not only are serum PEDF and FGF21 potential biomarkers for identifying diabetic patients at risk of nephropathy progression, especially at early stages of diabetic nephropathy, FGF21 could also serve as a novel therapeutic target in the treatment of diabetic nephropathy. Beneficial effects of an FGF21 analog on metabolic parameters including hyperlipidemia, bodyweight and hyperinsulinemia have recently been shown in type 2 diabetic patients, despite the presence of FGF21 resistance.
To conclude, there is clearly a need for novel biomarkers with high sensitivity and specificity for predicting the progression of diabetic nephropathy. The identification of good biomarkers, in addition to providing useful tools for early renal risk stratification, could also bring new insights into the pathogenesis of diabetic nephropathy, and open up new therapeutic options for preventing nephropathy progression. With advances in proteomics, genomics and translational research, it is anticipated that more biomarkers will continue to be discovered. It will certainly be a long road from the discovery of a new biomarker to its clinical application as a routine assay or as a therapeutic agent for patient management. Universal validation and standardization of each and every biomarker involve significant time and cost. Even more demanding efforts and costs are entailed in drug development and clinical trials. Nevertheless, all scientists working in this field of biomarker research, whether in the past, present or future, deserve to be applauded for their unswerving efforts to prevent the development and progression of diabetic nephropathy, and bring new hope to people with diabetes.
Acknowledgments
The authors declare no conflict of interest.
References
- Krolewski AS, Warram JH, Forsblom C, et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care. 2012;35:2311–2316. doi: 10.2337/dc11-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung DC, Xu A, Tso AW, et al. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care. 2009;32:132–134. doi: 10.2337/dc08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Yeung CY, Lee PC, et al. Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E2169–E2177. doi: 10.1210/jc.2014-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Hui E, Woo YC, et al. Circulating fibroblast growth factor 21 levels predict progressive kidney disease in subjects with type 2 diabetes and normoalbuminuria. J Clin Endocrinol Metab. 2015 doi: 10.1210/jc.2014-3465. doi: 10.1210/jc.2014-3465. [DOI] [PubMed] [Google Scholar]


