Abstract
Aims/Introduction
Variants in cell cycle regulation genes, CDKAL1 and CDKN2A/2B, have been suggested to be associated with type 2 diabetes, and also play a role in insulin procession in non-diabetic European individuals. Rs7754580 in CDKAL1 and rs7020996 in CDKN2A/2B were found to be associated with gestational diabetes in Chinese individuals. In order to understand the metabolism mechanism of greatly upregulated maternal insulin signaling during pregnancy and the pathogenesis of gestational diabetes, we investigated the impact of rs7754580 and rs7020996 on gestational insulin regulation and procession.
Materials and Methods
We recruited 1,146 unrelated, non-diabetic, pregnant Han Chinese women (age 28.5 ± 4.1 years, body mass index 21.4 ± 2.6 kg/m2), and gave them oral glucose tolerance tests. The indices of insulin sensitivity, insulin disposition, insulin release and proinsulin to insulin conversion were calculated. Rs7754580 in the CDKAL1 gene and rs7020996 in the CDKN2A/2B gene were genotyped. Under an additive model, we analyzed the associations between the variants and gestational insulin indices using logistic regression.
Results
By adjusting for maternal age, body mass index and the related interactions, CDKAL1 rs7754580 risk allele C was detected to be associated with increased insulin sensitivity (P = 0.011), decreased insulin disposition (P = 0.0002) and 2-h proinsulin conversion (P = 0.017). CDKN2A/2B rs7020996 risk allele T was found to be related to decreased insulin sensitivity (P = 0.002) and increased insulin disposition (P = 0.0001).
Conclusions
The study showed that cell cycle regulating genes might have a distinctive effect on gestational insulin sensitivity, β-cell function and proinsulin conversion in pregnant Han Chinese women.
Keywords: Genetic association, Gestational metabolisms, Insulin index
Introduction
The genes encoding cyclin-dependent kinases-inhibitor-2A/B (CDKN2A/B) and cyclin-dependent kinases 5 regulatory subunit-associated protein-like 1 (CDKAL1) have been widely reported to be related to type 2 diabetes and gestational diabetes1–6. The cyclin-dependent kinases are important in cell cycle control, and cell cycle dysregulation is suggested to be a common pathogenetic mechanism in diabetes7–9. Recent studies showed that cell cycle genes have been implicated in the restricted prolific capacity of β-cells during aging, and influence glucose-dependent transcriptional networks in islets10,11. Furthermore, variants in genes CDKAL1 and CDKN2A/2B were found to affect insulin procession and secretion without detectable change in fasting glucose in non-diabetic European individuals12.
Phenomenal alterations in metabolism occur during pregnancy. In order to compensate the increased metabolic demands, maternal pancreatic islets increase in both mass and secretion13,14. However, the genetic basis of the upregulated insulin signaling during pregnancy remains to be investigated. To ascertain the impact of cell cycle genes on gestational insulin signaling, and understand the mechanism of the changed gestational metabolism and the pathogenesis of gestational diabetes, we examined whether variants in genes CDKAL1 and CDKN2A/2B are correlated with gestational insulin procession including insulin sensitivity, insulin disposition, insulin release or proinsulin to insulin conversion in pregnant Han Chinese women.
Materials and Methods
Study Population
A total of 1,146 pregnant participants with normal glucose tolerance (NGT) were randomly recruited from April 2010 to April 2012 in Sichuan Provincial People's Hospital, located in Chengdu city of Southwest China. There were 4,707 women in total who attended the hospital for antenatal care during these 2 years. Pregnant women residing in the city choose the local hospital personally. All these women were advised to undergo a two-step approach based on the previous American Diabetes Association (ADA) diabetes diagnosis criteria for diagnosis of gestational diabetes in their second trimester of 24–28 gestational weeks15. Approximately 4,388 women (93.2% of total 4,707) signed an agreement to participate in the study project. Mainly based on 50-g, 1-h glucose challenge test (GCT) results and the need for rescreening in these women, more than 1,895 pregnant women were advised to take a 100-g, 3-h oral glucose tolerance test (OGTT). Approximately 1,765 women completed the examination, among which 1,146 women showed normal results for the 100-g, 3-h OGTT according to the previous ADA diagnosis criteria. All participants were of Han Chinese ancestry and had no previous diagnosis of glucose intolerance. Their NGT status was also confirmed by examining their medical records after delivery. The recruitment process of the pregnant participants, and inclusion and exclusion criteria are shown in Figure1.
Figure 1.
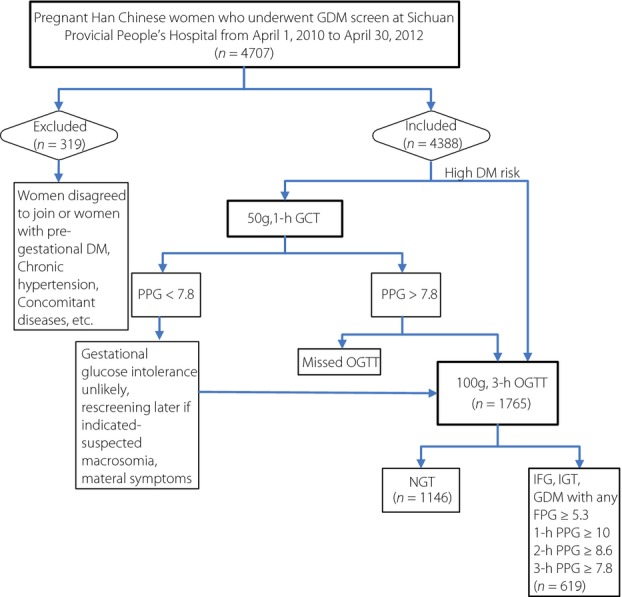
The recruitment process of the pregnant participants. DM, diabetes mellitus; GDM, gestational diabetes; OGTT, oral glucose tolerance test; PPG, postprandial glucose.
The blood glucose level of OGTT was measured with a glucose oxidase method by the Glucose Analyzer (SECOMAM, Domont, France), and the insulin, proinsulin and C-peptide levels were measured with a chemiluminescent immunoassay by the ARCHITECT i2000SR System (Abbott, Chicago, IL, USA) in the Central Clinical Isotopic Laboratory of the Sichuan Provincial People's Hospital. The glucose, insulin and C-peptide levels were measured to the nearest 0.1 mmol/L, 0.1 μU/mL and 0.1 ng/mL, respectively. The height and bodyweight of these pregnant women were measured to the nearest 0.1 cm and 0.1 kg. Table1 showed the clinical characteristics of the studied pregnant Han Chinese women at their 24–28 gestational weeks.
Table 1.
Characteristics of the studied pregnant Chinese women at 24–28 gestational weeks
| Maternal age | Height | Weight | BMI | SBP | DBP | FPG | 30-min PPG | 2-h PPG | 3-h PPG | FPI | PPI | 2-h PPI | 3-h PPI | FPPI | 2-h PPPI | FPC | 2-h PPC | Matsuda ISI | HOMA-B | HOMA-IR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | (m) | (kg) | (kg/m2) | (mmHg) | (mmHg) | (mmol/L) | (mmol/L) | (mmol/L) | (mmol/L) | (μU/mL) | (μU/mL) | (μU/mL) | (μU/mL) | (pmol/L) | (pmol/lL) | (ng/mL) | (ng/mL) | |||
| 28.6 ± 4.0 | 159.5 ± 6.5 | 57.7 ± 8.5 | 21.4 ± 2.7 | 111.2 ± 12.1 | 69.8 ± 8.0 | 4.8 ± 0.7 | 7.9 ± 1.7 | 6.9 ± 1.7 | 5.0 ± 1.4 | 8.0 ± 7.2 | 58.1 ± 30.5 | 53.5 ± 37.3 | 23.0 ± 21.1 | 5.6 ± 0.3 | 26.3 ± 1.4 | 1.6 ± 0.7 | 7.8 ± 3.1 | 6.4 ± 2.8 | 121.8 ± 39.7 | 0.89 ± 0.6 |
Data are presented as mean ± standard deviation. SBP, systolic blood pressure; DBP, diastolic blood pressure; FPC, fasting plasma C-peptide level; FPG, fasting plasma glucose level; FPI, fasting plasma insulin level; FPPI, fasting plasma proinsulin level; HOMA-B, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance; Matsuda ISI, Matsuda index of insulin sensitivity; PPC, postprandial plasma C-peptide level; PPG, postprandial plasma glucose level; PPI, postprandial plasma insulin level; PPPI, postprandial plasma proinsulin level.
Ethical Statement
Written informed consent was obtained from every participant. The study protocol was approved by the Review Board of Clinical Research and the Ethics Committee of the Sichuan Academy of Medical Science, Sichuan Provincial People's Hospital.
Deoxyribonucleic Acid Extraction
Blood samples were collected as dried blood spots in the 24–28 gestational week of pregnancy. Total deoxyribonucleic acid was isolated from purified peripheral blood lymphocytes. The deoxyribonucleic acid was extracted by using the methods previously described16. Deoxyribonucleic acid samples with an OD260/OD280 ratio between 1.8–2.0 and concentration more than 40 ng/mL were used for genotyping.
Genetic Variants and Genotyping
Rs7754580 in CDKAL1 and rs7020996 in CDKN2A/2B were genotyped in the present study, because both variants were confirmed to be significantly linked to type 2 diabetes and gestational diabetes, and specifically reported in the Han Chinese population5,6. The variants were genotyped with the Sequenom system at the Huada Gene Laboratory (Shengzhen, China). The genotyping call success rate was 100 and 99.91%, respectively; among the total 1,146 samples, 60 were run in duplicates with both 100% concordance rates.
Calculations
The OGTT-derived measures including insulin sensitivity index Matsuda index of insulin sensitivity (Matsuda ISI)17, homeostasis model assessment of insulin resistance index (HOMA-IR)18, insulin disposition index, early-phase insulin release index and proinsulin conversion index were set as physiological indices with regard to insulin procession in the study. Based on the 100-g OGTT results, the area under the curve (AUC) for glucose, insulin and proinsulin was calculated according to the trapezoidal method. Insulin disposition index was defined as Matsuda ISI × insulinogenic index ([Ins30−Ins0]/[Glu30−Glu0]); here, Ins30 and Ins0 represent postprandial 30 min and fasting insulin levels, respectively, whereas Glu30 and Glu0 represent postprandial 30 min and fasting glucose levels. Early-phase insulin release was determined as InsAUC0–30/GluAUC0–30 where InsAUC0–30 is the area under the curve of total insulin and GluAUC0–30 is the area under the curve of total glucose during the first 30 min of the OGTT. The ratio of proinsulin0/insulin0 in the fasting state and 2-hproinsulinAUC0–120/insulinAUC0–120 from 0 to 120 min were calculated as two indices of proinsulin conversion.
The association between each variant and the quantitative traits, such as fasting or postprandial levels of insulin, glucose and C peptides, were also tested.
Statistical Analysis
The quantitative variable of clinical characteristics was reported using mean ± standard deviation according to a normal distribution. Hardy–Weinberg equilibrium (HWE) for the distribution of a variant in population was tested by Pearson's χ2-test. The allelic frequencies of CDKAL1 rs7754580 and CDKN2A/2B rs7020996 followed HWE (P = 0.22 and 0.93, respectively), and could be used for the following analysis. To model the insulin indices, age, body mass index (BMI) and their interactions in these pregnant Han Chinese women, linear regression was used to fit all the insulin indices with genotypes, and the insulin indices were logarithmically transformed to normal distribution in the analysis (Table2). To explore the association between each variant and insulin indices, a logistic regression was carried out using the insulin index as response variable and genotypes as predictors, with adjustment for age and BMI under the genetic additive model. The best-fitting logistic model with multiple variables was chosen by likelihood ratio test (Table3). No variables were transformed in the logistic regression.
Table 2.
Interaction analysis between the insulin indices and age, between the insulin indices and body mass index, and between age and body mass index
| Maternal age | BMI | Matsuda ISI | HOMA-IR | Disposition index | Early-phase insulin release | Initial proinsulin conversion | 2-h proinsulin conversion | |
|---|---|---|---|---|---|---|---|---|
| Maternal age | – | <0.0001 | <0.0001 | 0.32 | <0.0001 | 0.71 | 0.056 | 0.066 |
| BMI | <0.0001 | – | <0.0001 | <0.0001 | 0.069 | <0.0001 | 0.182 | 0.001 |
P-values were calculated by linear regression. All the insulin indices were logarithmically transformed. BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; Matsuda ISI, Matsuda index of insulin sensitivity.
Table 3.
Association of rs7754840 and rs7020996 with insulin sensitivity, disposition index, early-phase insulin release and proinsulin conversion in pregnant Han Chinese women
| Gene | CDKAL1 | CDKN2A/B | |
|---|---|---|---|
| Variant | rs7754840 | rs7020996 | |
| Allele MAF (%) | G/C(37.7) | C/T(42.0) | |
| Matsuda ISI: with adjustment for age, BMI, age × BMI, age × Matsuda ISI, BMI × Matsuda ISI, age × BMI × Matsuda ISI | P-values and coefficient (95% CI) | 0.011 and −9.28 (−16.42 to −2.13) | 0.002 and 5.05 (1.79–8.31) |
| Z-value (SE) | −2.55 (3.64) | 3.04 (1.66) | |
| HOMA IR: with adjustment for age, BMI, age × BMI, BMI × HOMA-IR | P-values and coefficient (95% CI) | 0.194 and −2.07 (−5.18 to −1.05) | 0.168 and −1.73 (−4.19 to 0.73) |
| Z-value (SE) | −1.30 (1.59) | −1.38 (1.26) | |
| Disposition index: Matsuda ISI × ([Ins30−Ins0]/[Glu30−Glu0]): with adjustment for age, | P-values and coefficient (95% CI) | 0.0002 and 1.82 (0.87–2.77) | 0.0001 and −0.99 (−1.50 to −0.48) |
| BMI, age × BMI, age × disposition index | Z-value (SE) | 3.75 (0.49) | −3.82 (0.26) |
| Early-phase insulin release: InsAUC0–30/GluAUC0–30: with adjustment for age, BMI, | P-values and coefficient (95% CI) | 0.351 and 5.41 (−5.96 to 16.79) | 0.962 and 0.17 (−6.95 to 7. 29) |
| age × BMI, BMI × early-phase insulin release | Z-value (SE) | 0.93 (5.80) | 0.05 (3.63) |
| Initial proinsulin conversion: Proins0/Ins0: with adjustment for age, BMI, age × BMI | P-values and coefficient (95% CI) | 0.079 and 2.97 (−0.34 to 6.27) | 0.119 and −1.89 (−4.26 to −0.48) |
| Z-value (SE) | 1.76 (1.69) | −1.56 (1.21) | |
| 2-h proinsulin conversion: ProinsAUC0–120/InsAUC0–120: with adjustment for age, | P-values and coefficient (95% CI) | 0.017 and 48.99 (8.65–89.35) | 0.254 and 16.24 (−11.65 to 44.13) |
| BMI, age × BMI, BMI × 2-h proinsulin conversion | Z-value (SE) | 2.38 (20.58) | 1.14 (14.23) |
Assuming an additive genetic model, P-values and coefficiency (95% confidence interval [CI]) were calculated by logistic regression with adjustment. No transformation was applied to normalize the data. P-value <0.05 for single test significant level and P-value <0.002 for multiple testing significant level, the significant P-value appears in bold. The minor allele in minor allele frequency (MAF) is also highlighted in bold. The risk alleles are shadowed. AUC, area under the curve; BMI, body mass index; CI, confidence interval; Glu0, fasting glucose levels; Glu30, postprandial 30 min glucose levels; HOMA-IR, homeostasis model assessment of insulin resistance; Ins0, fasting insulin levels; Ins30, postprandial 30 min insulin levels; SE, standard error.
The statistical power of the current sample was estimated by software package G*power 3.1.7 (http://www.softpedia.com/get/Science-CAD/G-Power.shtml). With the 1,146 participants, we had a power of 81% with a type I error rate of 0.05 to detect associations (Z-value ≤−1.96 or Z-value ≥1.96) between each variant and the outcomes of insulin indices under normal distribution with mean = 0 and variance = 1.
The STATA 11.0 (StataCorp, College Station, TX, USA) was used to carry out statistical analyses. P < 0.004 calculated using the Bonferroni correction was set for multicomparisons significance level, given 12 independent tests were carried out for the two variants.
Results
Analysis of Interactions
In these 1,146 NGT pregnant women, age was found to be positively associated with Matsuda ISI and insulin disposition index (both P < 0.05). Age and BMI were also significantly correlated (P < 0.0001). Furthermore, BMI was positively associated with Matsuda ISI, HOMA-IR, index of early-phase insulin release and 2-h proinsulin conversion (all P < 0.05). The fasting proinsulin conversion was not associated with age or BMI (Table2). Table2 lists all the interactions between the insulin indices and age, between the insulin indices and BMI, and between age and BMI. Therefore, to examine age- and BMI-independent effects of the variant on the insulin procession, we adjusted the main effect between the variant and each insulin index for age, BMI, and all the interactions. The detailed adjustment for each index is shown in Table3.
No significant association was found between each variant and the quantitative traits, such as fasting or postprandial levels of insulin, glucose and C peptides.
Associations of Insulin Sensitivity/Resistance
As shown in Table3, both CDKAL1 rs7754580 and CDKN2A/2B rs7020996 were detected to be associated with Matsuda ISI after adjusting for age, BMI and the related interactions (P = 0.011 and P = 0.002, respectively). However, the non-risk allele of rs7754580 was negatively associated with Matsuda ISI; on the contrary, the non-risk allele of rs7020996 was positively associated with Matsuda ISI.
Both variants were not found to be connected with HOMA-IR in these NGT pregnant Chinese women.
Associations of Insulin Procession
Table3 also shows that both rs7754580 in CDKAL1 and rs7020996 in CDKN2A/2B were strongly associated with insulin disposition index at a level of P = 0.0002 and P = 0.0001 after the adjustment. The non-risk allele of CDKAL1 rs7754580 was positively related to insulin disposition, whereas that of CDKN2A/2B rs7020996 was negatively associated with insulin disposition.
Except that CDKAL1 rs7754580 might impact the 2-h proinsulin conversion (P = 0.017), there were no significant connections between each variant and early-phase insulin release, or between each variant and initial proinsulin conversion.
Discussion
Both CDKAL1 rs7754580 and CDKN2A/B rs7020996 have been reported to be related to gestational diabetes. In our current study, the major allele of rs7754580 was negatively associated with Matsuda ISI, showing that CDKAL1 could play a role in reducing gestational insulin sensitivity; whereas the major allele of rs7020996 was positively associated with Matsuda ISI, suggesting CDKN2A/2B could promote gestational insulin sensitivity. Also, the disposition index provides a useful measure of β-cell function, the major allele of CDKAL1 rs7754580 was positively related to insulin disposition index, whereas that of CDKN2A/2B rs7020996 was negatively associated with insulin disposition index, indicating that the two genes might contribute to the gestational β-cell function. In addition, both rs7754580 and rs7020996 were associated with Matsuda ISI, which reflects whole-body insulin sensitivity, and they were not associated with HOMA-IR, which primarily reflects hepatic insulin sensitivity. We also noted the effects of CDKAL1 rs7754580 and CDKN2A/2B rs7020996 on insulin sensitivity and insulin disposition, the additive effect of CDKN2A/2B rs7020996 on Matusda ISI was 4.15 (P = 0.042), whereas CDKAL1 rs7754580 had a very weak additive effect; conversely, rs7020996 had no additive impact on gestational insulin disposition, whereas rs7754580 had a large additive effect (8.04, P = 0.005). Currently, the function of these cell cycle regulation genes has not been fully elucidated. Rs7754580 locates in the intron of CDKAL1, the CDKAL1 protein shares considerable domain and amino acid homology with inhibitor of cyclin-dependent kinase 5 (CDK5). Recently, CDKAL1 has been shown to be a tail-anchored protein in the endoplasmic reticulum of insulinoma cells, regulating glucose-stimulated insulin secretion19; and CDKAL1 has also been shown to be a methylthiotransferase that modifies transfer ribonucleic acid lysine to enhance translational fidelity of transcripts, including the one encoding proinsulin20. Rs7020996 locates within the upstream of CDKN2A/2B gene, comprehensive chromatin modifications and hypermethylation of CDKN2A/2B gene promoter were proposed to be critical in oncogene-induced islet-like cell21 and β-cell senescence22,23, and in tumorigenesis of pancreatic carcinogenesis24. Here, it is interesting that variants in the non-coding region (intron and upstream) of CDKAL1 and CDKN2A/2B could connect with gestational insulin disposition and insulin sensitivity when maternal pancreatic β-cell mass and insulin secretion increased during pregnancy. However, how these variants played a role is unclear. It will be interesting to explore the potential impact of gene regulatory regions in gestational metabolism, and further investigate the function of these cell cycle regulatory genes in the adaptively changes during pregnancy.
In our current study, rs7754580 in CDKAL1 and rs7020996 in CDKN2A/2B were detected to be associated with gestational insulin sensitivity and insulin disposition, showing the distinctive role of cell cycle regulatory genes on insulin procession during pregnancy. In non-diabetic non-pregnant individuals, risk alleles of variants in CDKAL1 and CDKN2A/2B genes were connected with reduced insulin secretion in the presence of increased proinsulin12; significant associations of CDKAL1 and CDKN2A/2B genetic variants with insulin disposition were also reported in non-diabetic Finnish men25; but there was little systematic evidence for effects of the cell cycle genes on insulin sensitivity in non-pregnant populations12. As increased islet mass and secretion are adaptive mechanisms that occur during pregnancy, it seems reasonable that both maternal β-cell function and insulin sensitivity improved, and thus the association with gestational insulin disposition was also enhanced.
There was a limitation to the present study. The OGTT result could be measured only once in these NGT women, although the non-diabetic status was confirmed by examining the medical records of the women after delivery. Also, the NGT status of the pregnant women could lead to a truncation effect on the association with insulin sensitivity, as the non-diabetic individual could have a predisposition that favors both low insulin resistance and high insulin secretion. Furthermore, the contribution of the variants in different ethnic groups has not yet been investigated. Hence, further large-scale studies are required to validate our findings.
Our current study showed the distinctive impact of CDKAL1 rs7754580 and CDKN2A/2B rs7020996 on insulin sensitivity and β-cell function during pregnancy in pregnant Han Chinese women.
Acknowledgments
This study was supported by research grants (to Shunyao Liao, Shaoping Deng and Meijie) from the Key project of Sichuan Provincial Department of Science and Technology (2011sz0029, 2009sz0223), Sichuan Provincial Health Department (100450, 120074), Department of Science and Technology in Chengdu (11PPYB050SF-289) and National Natural Science Foundation of China (81471430). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank Zhenlin Yang and Yi Shi for suggestions of protocol design; Hongji Yang and Xiaolun Huang for coordination between clinical research departments; He Lin for genotyping of the samples; and the clinical teams for participation in collection and phenotypic characterization of the samples. Zhenlin Yang, Yi Shi and He Lin are currently working in the Sichuan Provincial Key Laboratory for Human Disease Gene Study, and the Institute of Laboratory Medicine, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital; Hongji Yang and Xiaolun Huang are currently working in the Institute of Transplantation, Sichuan Academy of Medical Science, Sichuan Provincial People's Hospital. The authors declare no conflict of interest.
References
- Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram M, Radha V, Mohan V. Replication of recently described type 2 diabetes gene variants in a South Indian population. Metabolism. 2010;59:1760–1766. doi: 10.1016/j.metabol.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Wen J, Rönn T, Olsson A, et al. Investigation of type 2 diabetes risk alleles support CDKN2A/B, CDKAL1, and TCF7L2 as susceptibility genes in a Han Chinese cohort. PLoS One. 2010;5:e9153. doi: 10.1371/journal.pone.0009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bao W, Rong Y, et al. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum Reprod Update. 2013;19:376–390. doi: 10.1093/humupd/dmt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Li Q, Gao S. Meta-analysis of the relationship between common type 2 diabetes risk gene variants with gestational diabetes mellitus. PLoS One. 2012;7:e45882. doi: 10.1371/journal.pone.0045882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nie M, Li W, et al. Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PLoS One. 2011;6:e26953. doi: 10.1371/journal.pone.0026953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Ridderstråle M, Groop L. Genetic dissection of type 2 diabetes. Mol Cell Endocrinol. 2009;297:10–17. doi: 10.1016/j.mce.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Salpeter SJ, Khalaileh A, Weinberg-Corem N, et al. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes. 2013;62:2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Asso A, Castaño C, Grilli A, et al. Glucose regulation of a cell cycle gene module is selectively lost in mouse pancreatic islets during ageing. Diabetologia. 2013;56:1761–1772. doi: 10.1007/s00125-013-2930-0. [DOI] [PubMed] [Google Scholar]
- Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123:990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimas AS, Lagou V, Barker A, et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63:2158–2171. doi: 10.2337/db13-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol. 1978;5:818–820. doi: 10.1111/j.1471-0528.1978.tb15835.x. [DOI] [PubMed] [Google Scholar]
- Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;1:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S103–S105. doi: 10.2337/diacare.26.2007.s103. [DOI] [PubMed] [Google Scholar]
- Liao S, Liu Y, Tan Y, et al. Association of genetic variants of melatonin receptor 1B with gestational plasma glucose level and risk of glucose intolerance in pregnant Chinese women. PLoS One. 2012;7:e40113. doi: 10.1371/journal.pone.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index [letter] Diabetes Care. 2010;33:e93. doi: 10.2337/dc10-0646. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Brambillasca S, Altkrueger A, Colombo SF, et al. CDK5 regulatory subunit-associated protein 1-like 1 (CDKAL1) is a tail-anchored protein in the endoplasmic reticulum (ER) of insulinoma cells. J Biol Chem. 2012;287:41808–41819. doi: 10.1074/jbc.M112.376558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei FY, Suzuki T, Watanabe S, et al. Deficit of tRNALys modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121:3598–3608. doi: 10.1172/JCI58056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DQ, Wang Q, Burkhardt BR, et al. In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation. Am J Stem Cells. 2012;1:114–127. [PMC free article] [PubMed] [Google Scholar]
- Rane SG, Cosenza SC, Mettus RV, et al. Germ line transmission of the Cdk4 (R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Mol Cell Biol. 2002;22:644–656. doi: 10.1128/MCB.22.2.644-656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Ellenrieder V. Senescence in pancreatic carcinogenesis: from signalling to chromatin remodelling and epigenetics. Gut. 2013;62:1364–1372. doi: 10.1136/gutjnl-2012-302793. [DOI] [PubMed] [Google Scholar]
- Stancáková A, Kuulasmaa T, Paananen J, et al. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. 2009;58:2129–2136. doi: 10.2337/db09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]


