Abstract
Aims/Introduction
A dietary supplementation product enriched with glutamine, dietary fiber and oligosaccharide (GFO) is widely applied for enteral nutrition support in Japan. The aim of the present study was to evaluate the effects of GFO ingestion on secretion of incretins, gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), and glucagon-like peptide-2 (GLP-2).
Materials and Methods
We carried out a cross-over study involving 20 healthy Japanese volunteers. The participants received GFO or 17 g of glucose, the equivalent carbohydrate in GFO as the control. Plasma glucose, serum insulin, and plasma total GIP, total GLP-1 and total GLP-2 levels during GFO or glucose loading were determined.
Results
GFO loading produced significantly higher plasma GLP-1 levels at 30 min and 60 min, area under the curve-GLP-1 value, and area under the curve-GLP-2 value after administration compared with those by glucose loading. In contrast, plasma GIP levels at both 30 and 60 min, and area under the curve-GIP value after glucose loading were significantly higher than those after GFO loading.
Conclusions
These results show that GFO ingestion stimulates GLP-1 and GLP-2 secretion, and reduces GIP secretion compared with glucose ingestion. Therefore, GFO could have an intestinotrophic effect as well as an ameliorating effect on metabolic disorders through modification of release of gut hormones.
Keywords: Glucagon-like peptide-2, Incretin, Oligosaccharide
Introduction
Various hormones secreted from diverse enteroendocrine cells in the gastrointestinal tract regulate nutrient absorption and metabolism in the intestinal environment1. Gastric inhibitory polypeptide (GIP) secreted from K-cells in the proximal small intestine and glucagon-like peptide-1 (GLP-1) secreted from L-cells in the distal small intestine and colon, respectively, are recognized as incretins, which amplify insulin secretion from pancreatic β-cells glucose-dependently. Incretins are responsible for approximately half of the postprandial insulin secretion2,3. It is well known that not only carbohydrate, but also fat and protein ingestion, stimulate incretin secretion4,5. Previous studies have shown that GIP secretion is higher after mixed meal (containing carbohydrate, fat, and protein) loading compared with that by oral glucose loading6,7. In contrast, the GLP-1 secretory response is not significantly different between oral glucose loading and mixed meal loading6,7. These findings suggest that GIP secretion is more susceptible than GLP-1 secretion to nutritional composition.
GLP-1 and glucagon-like peptide-2 (GLP-2) are proglucagon-derived peptides secreted simultaneously from intestinal L-cells in response to meal ingestion1. GLP-2 has no such insulinotropic effect, but has other various biological effects on the intestine, including stimulation of intestinal mucosal growth8,9, inhibition of gastric acid secretion10, enhancement of intestinal epithelial barrier function11, and upregulation of sodium-glucose cotransporter 1 (SGLT-1) transport activity12 and intestinal blood flow13–15. Thus, GLP-2 is known as an intestinotrophic hormone. Processing of proglucagon in the intestine generates equimolar amounts of GLP-1 and GLP-2. A sampling study of the mesenteric circulation draining the intestinal bed has confirmed that GLP-1 and GLP-2 are secreted at an equal rate (1:1)16.
A dietary supplementation product enriched with glutamine, dietary fiber and oligosaccharide (GFO®; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) is widely applied for enteral nutrition support in Japan. Glutamine, the most abundant component of GFO, is one of the major fuel sources for intestinal cells17–19. Amino acids, especially glutamine, have been shown to stimulate incretin secretion20–24. In fact, a previous report showed that glutamine treatment before meal ingestion augmented the GLP-1 response and reduced postprandial glycemia in type 2 diabetes patients, with effectiveness comparable with that of the DPP-4 inhibitor, sitagliptin25. The dietary fibers contained in GFO are polydextrose and hydrolyzed guar gum, which are water-soluble. The oligosaccharide contained in GFO is lactosucrose, a kind of galacto-oligosaccharide. Fiber and oligosaccharide are prebiotics that stimulate the growth and activity of gastrointestinal microflora. It is known that short-chain fatty acids (SCFA)26, which are produced by ingestion of dietary fiber and some kinds of oligosaccharide, promote GLP-1 secretion. In our recent report, we found that plasma GLP-1 levels were much higher in GFO-administered mice than in glucose-administered mice27. In the present report, we show that GFO alleviates experimental colitis in mice27. In addition, it is reported that GFO prevents gut bacterial translocation in an experimental intestinal infection model28. Thus, it is established that GFO has protective effects on the intestinal tract. We speculate that GFO stimulates release of GLP-2, an intestinotrophic hormone, along with GLP-1 secretion to attenuate development of mucosal damage.
To date, there is no assessment of the effects of GFO on GLP-2 secretion. Additionally, there is no report on the effect of dietary supplementation products, such as GFO, on the secretion of incretins. The aim of the present study was to evaluate incretin (GIP and GLP-1) and GLP-2 secretion in response to GFO administration, in comparison with the administration of glucose in an equivalent amount of carbohydrate to that in GFO.
Materials and Methods
Participants
A total of 20 healthy Japanese volunteers (10 men and 10 women) were recruited into the present study. The participants had no history of hypertension, hyperlipidemia or kidney and liver disease, and did not take any drugs during the 2 weeks before the study. The study was designed in compliance with the ethics regulations of the Helsinki Declaration and Kyoto University. Informed consent was obtained from all participants.
Study Design
We carried out a randomized cross-over design experiment with two single oral loadings: GFO loading and glucose loading. The participants' age, height and bodyweight were determined. Blood samples for measurement of liver and kidney function, glycated hemoglobin (HbA1c; National Glycohemoglobin Standardization Program [NGSP]), triglycerides (TG), total cholesterol and high-density lipoprotein (HDL) cholesterol levels were drawn after an overnight fast. After the participants fasted overnight for 10–16 h, they received three packs of GFO (1 pack = 36 kcal; protein 3.6 g, fat 0 g, carbohydrates 6.01 g, fiber 5.0 g, Na 0.2–12 mg, galactsyl-scurose 1.45 g, glutamine 3.0 g) dissolved in water or water-dissolved 17 g of glucose, which is an equivalent amount of the carbohydrate in GFO, as control (54 kcal). The carbohydrates contained in GFO, excluding lactosucrose and fiber, are composed mainly of dextrin and sucrose. Blood samples were collected at 0, 30, 60, 120 and 180 min after GFO or glucose loading, and were centrifuged at 1,880 g at 4°C for 10 min. After collecting supernatant of the samples, plasma and serum were stocked at −80°C. Blood was distributed into chilled tubes containing ethylenediaminetetra-acetic acid and aprotinin (500 kIU/mL blood, Trasylol; SRL Inc., Tokyo, Japan) for analyses of GIP, GLP-1 and GLP-2. Plasma glucose (PG), serum insulin (IRI), serum C-peptide (CPR), serum triglyceride (TG), plasma total incretin (GIP and GLP-1) and GLP-2 levels were measured at the indicated times.
Analytical Methods
The PG levels were measured by the glucose oxidase method. Serum IRI and CPR levels were measured by enzyme-linked immunosorbent assay. TG levels were measured by enzymatic method (free glycerol elimination method). HbA1c was measured using high-performance liquid chromatography (HPLC) and are expressed as a NGSP value calculated by the formula: HbA1c (NGSP value) (%) = 1.02 × HbA1c (Japan Diabetes Society (JDS) value) (%) + 0.2529. Homeostasis model assessment of insulin resistance (HOMA-IR), HOMA of β-function (HOMA-β), and insulinogenic index were evaluated as basal insulin resistance, basal insulin secretion and early phase insulin secretion, respectively30–32. Total GIP and total GLP-1 levels were measured using a human GIP ELISA kit (Merck Millipore Corp, Bilerica, MA, USA) and human GLP-1 ELISA kit (Meso Scale Discovery, Gaithersburg, MD, USA), respectively33. Total GLP-2 levels were measured using a human GLP-2 ELISA kit (Phoenix Pharmaceuticals, Burlingame, USA)34.
Calculations and Statistical Analysis
A repeated-measures anova was carried out with spss (version 17; SPSS Inc., Chicago, IL, USA). If a significant interaction of treatment and time was detected, values at single time-points were compared by one-way anova with Bonferroni's post-hoc test. The area under the curve of PG (AUC-PG), IRI (AUC-IRI), CPR (AUC-CPR), TG (AUC-TG), total GIP (AUC-GIP), total GLP-1 (AUC-GLP-1) and total GLP-2 (AUC-GLP-2) were calculated by the trapezoidal rule. Statistical differences in values of AUCs were analyzed using Student's t-test. P-values <0.05 were considered statistically significant. Data are presented as mean ± standard error.
Results
The profiles of the participants are shown in Table1. Mean age was 32.2 ± 2.0 years, and mean body mass index was 22.4 ± 0.8 kg/m2. HOMA-β and HOMA-IR were 111 ± 15 and 1.2 ± 0.8, respectively. No participants had liver or kidney dysfunction (data not shown). HbA1c, TG, total cholesterol and LDL cholesterol levels were within normal limits in the fasting state.
Table 1.
Characteristics of the participants
| n (male/female) | 20 (10/10) |
| Age (years) | 32.2 ± 2.0 |
| BMI (kg/m2) | 22.4 ± 0.8 |
| HbA1c (%) | 5.2 ± 0.1 |
| HOMA-β | 111 ± 15 |
| HOMA-IR | 1.2 ± 0.8 |
| Total cholesterol (mg/dL) | 190.5 ± 7.6 |
| Triglycerides (mg/dL) | 72.3 ± 8.6 |
| LDL cholesterol (mg/dL) | 109.3 ± 7.0 |
| HDL cholesterol (mg/dL) | 60.0 ± 3.4 |
Data presented as mean ± standard error. BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein.
The profiles of PG, IRI, and CPR in GFO loading and glucose loading are shown in Figure1. Fasting concentrations of PG (Figure1a), IRI (Figure1c), and CPR (Figure1e) did not differ between GFO loading and glucose loading. At 30 min after glucose loading, PG was significantly higher than that after GFO loading (GFO 112.7 ± 3.2 mg/dL vs glucose 129.8 ± 4.1 mg/dL, P < 0.01; Figure1a). AUC-PG in glucose loading was significantly higher than those in GFO loading (GFO 15,221 ± 255 mg/dL × min vs glucose 16,009 ± 375 mg/dL × min, P < 0.05; Figure1b). IRI and TG showed no significant differences at each time-point between either loading (Figure1c,g). At 30 min after glucose loading, CPR was significantly higher than after GFO loading (GFO 3.6 ± 0.3 mg/dL vs glucose 4.2 ± 0.3 mg/dL, P < 0.05; Figure1e), whereas AUC-IRI, AUC-CPR and AUC-TG were similar in both loading groups (Figure1d,f,h).
Figure 1.
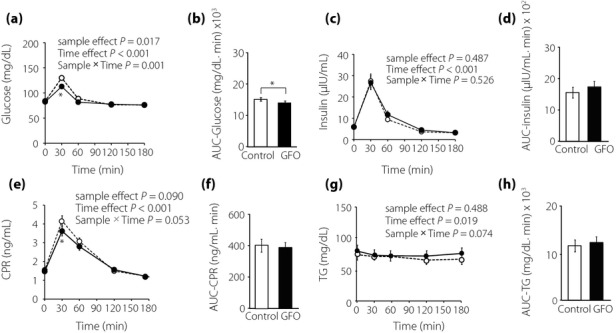
Glucose, insulin, serum C-peptide (CPR), and serum triglyceride (TG) levels during GFO and glucose tolerance. The (a) glucose, (c) insulin, (e) CPR, and (g) TG levels were measured after oligosaccharide (GFO) and glucose loading. Glucose loading and GFO loading groups are represented by white circles with dot line and black circles with solid line, respectively. *P < 0.05 versus glucose group. Total glucose and insulin secretion were evaluated by (b) area under the curve (AUC)-glucose, (d) AUC-insulin, (f) AUC-CPR, and (h) AUC-TG, respectively. Glucose loading and GFO loading groups are represented by white and black squares, respectively. *P < 0.05
Plasma GLP-1 levels were significantly higher in GFO loading than in glucose loading at 30 min (GFO 26.5 ± 2.4 pg/mL vs glucose 21.1 ± 1.9 pg/mL, P < 0.05) and 60 min (GFO 22.1 ± 1.8 pg/mL vs glucose 12.1 ± 1.4 pg/mL, P < 0.01; Figure2a). AUC-GLP-1 in GFO loading was significantly higher than that in glucose loading (GFO 3,371 ± 344 pg/mL × min vs glucose 2,445 ± 272 pg/mL × min, P < 0.01; Figure2b). Plasma GIP levels were significantly higher in glucose loading than in GFO loading at 30 min (GFO 89.7 ± 5.8 pg/mL vs glucose 126.7 ± 11.1 pg/mL, P < 0.01) and 60 min (GFO 66.0 ± 3.2 pg/mL vs glucose 84.3 ± 4.9 pg/mL, P < 0.01; Figure2c). AUC-GIP in glucose loading was significantly higher than in GFO loading (GFO 11,206 ± 450 pg/mL × min vs glucose 13,077 ± 671 pg/mL × min, P < 0.01; Figure2d). Plasma GLP-2 levels showed no significant difference at each time-point between glucose and GFO loading (Figure2e); however, the time-course of GLP-2 differed between the two groups (sample × time, P < 0.01). Additionally, AUC-GLP-2 in GFO loading was significantly higher than in glucose loading (GFO 756.4 ± 78.1 ng/mL × min vs glucose 618.6 ± 66.9 ng/mL × min, P < 0.01; Figure2f).
Figure 2.
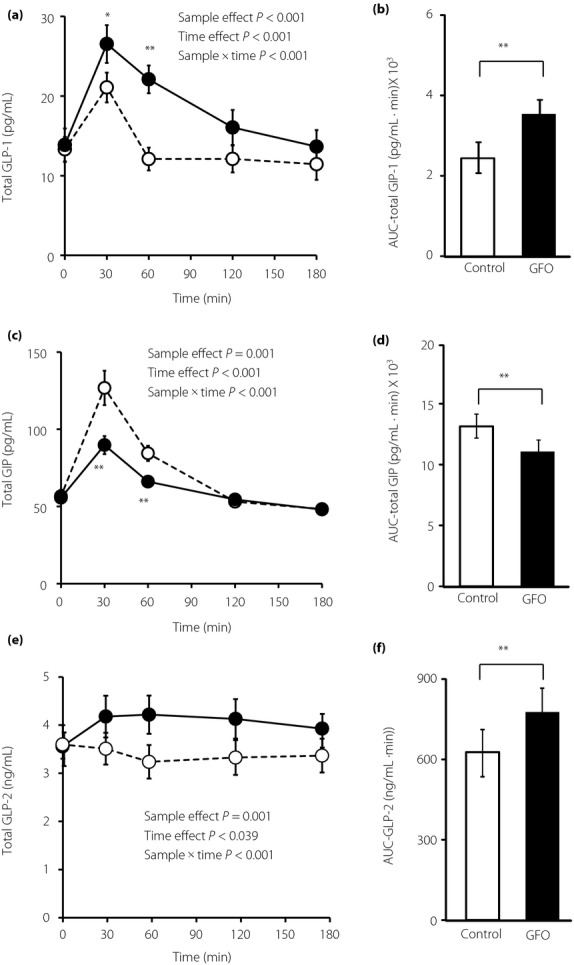
Incretin and glucagon-like peptide-2 (GLP-2) levels during oligosaccharide (GFO) and glucose tolerance. Total (a) glucagon-like peptide-1 (GLP-1), (c) total gastric inhibitory polypeptide (GIP), and (e) total GLP-2 levels were measured after GFO and glucose loading. Glucose loading and GFO loading groups are represented by white circles with dotted line and black circles with solid line. *P < 0.05, **P < 0.01 versus glucose group. GLP-1, GIP, and GLP-2 secretion were evaluated by (b) AUC-GLP-1, (d) AUC-GIP and (f) AUC-GLP-2, respectively. Glucose loading and GFO loading groups are represented by white and black squares, respectively. **P < 0.01
Discussion
In the present study, we evaluated incretins (GIP and GLP-1) and GLP-2 levels in response to single administration of GFO or glucose equivalent to the amount of carbohydrate in GFO. GFO loading produced significantly higher plasma GLP-1 levels at 30, 60 and 120 min after administration, as well as higher AUC values compared with those by glucose loading. In contrast, both plasma GIP levels at 30 and 60 min, and AUC values were significantly higher by glucose loading than those by GFO loading.
GFO is composed of glutamine, dietary fiber and oligosaccharide. Glutamine has been shown to stimulate GLP-1 secretion20–22. Our finding in the present study of higher plasma GLP-1 levels after GFO loading might well reflect the GLP-1 secretory effects of glutamine. Although glutamine also increases plasma GIP levels, it is less effective than glucose20. Our findings on plasma GIP levels are in accord with this finding.
The dietary fibers contained in GFO are polydextrose and hydrolyzed guar gum, which are water-soluble. Previous studies have shown that the glycemic response is markedly flattened by polydextrose or guar gum ingestion35,36. It was also reported that the plasma GIP concentration is significantly reduced after ingestion of guar gum bread compared with control bread containing an equal amount of carbohydrate35. GFO also contains dextrin, one of the polysaccharides, for which absorption is much delayed compared with glucose. These findings considered together suggest that more carbohydrate might pass the K-cells of the proximal small intestine and reach the L-cells in the lower intestine, resulting in less GIP response and greater GLP-1 response after administration of GFO compared with those after glucose administration.
The oligosaccharide contained in GFO is lactosucrose, a kind of galacto-oligosaccharide. As well as being a soluble fiber, it is known that lactosucrose is a prebiotic that produces SCFAs, such as acetate, propionate and butyrate, in the colon by anaerobic bacterial fermentation37. A previous study suggested that SCFAs stimulate GLP-1 secretion through GPR43 and GPR4126. However, unlike the case of long-term administration, a single oral administration of GFO was carried out in the present study, which should not affect anaerobic bacterial fermentation. GFO also contains sucralose, the artificial sweetener, but a previous study has found that sucralose ingestion does not stimulate either GLP-1 or GIP release38.
Processing of proglucagon in the intestine generates equimolar amounts of GLP-1 and GLP-2 concomitantly. A sampling study of the mesenteric circulation draining the intestinal bed confirmed that GLP-1 and GLP-2 are secreted at an equal rate (1:1)16. In the present study, compared with that by glucose loading, plasma GLP-2 levels were significantly higher after 30 min of GFO loading, which was accompanied by higher plasma levels of GLP-1, suggesting that GFO stimulates GLP-2 secretion by the same mechanism as that by GLP-1 secretion. The metabolic clearance rate of GLP-2 is slower than that of GLP-139. Thus, the basal blood level of GLP-2 could be relatively higher than that of GLP-1, masking the impact of GFO on GLP-2 levels. Our observations also accord with previous reports that the serum GLP-2 level is relatively higher than that of the GLP-1 level40,41. We previously found that plasma GLP-1 levels are higher in GFO-administered mice than those in glucose-administered mice, and that GFO alleviates experimental colitis in mice27.
A previous study showed that GLP-2 stimulates intestinal apoB48-containing lipoprotein secretion in mice, possibly through the increased lipid uptake42. In that study, blood TG levels also were increased along with apoB48 secretion by GLP-2 administration. However, in the present study, no significant differences were observed in TG levels between either loading (Figure1g). It is possible that this inconsistent result depends on the presence or absence of oil in the loading test. The previous study administered olive oil (200 μL/mice) to mice with GLP-2 injection42. In contrast, we did not include any oil in the loading test in the present study. We also carried out the loading test after participants fasted overnight for 10–16 h.
The present results clearly show that GFO ingestion stimulates GLP-1 and GLP-2 secretion, and represses GIP secretion compared with simple glucose loading. Previous reports have established the crucial role of GIP in linking overnutrition to obesity43,44. We recently reported that chronic reduction of GIP secretion ameliorates high-fat diet-induced obesity and insulin resistance45. In addition, treatment of type 2 diabetes with GLP-1 receptor agonists is associated with reductions of HbA1c, fasting plasma glucose and bodyweight46. We therefore expected that reducing the GIP effect and stimulating the GLP-1 effect by functional foods, such as GFO, could have desirable effects on glucose and/or lipid metabolic abnormality. However, in the present study, the contribution ratio of each component of GFO to the PG levels and the secretion patterns remains unclear; separate, single-nutrient administration studies are required. In addition, oil and GFO co-loading test is required to determine whether or not GFO loading increases lipid uptake through an increase of GLP-2 secretion.
In conclusion, we showed that a combination of glutamine, dietary fiber and GFO provides intestinal protection, and is also effective for metabolic disorders through modification of gut hormone release.
Acknowledgments
EJ, AM, AH, NH, SY, YK, KS, DN, KS, TH, KI, HT and KS have no conflict of interest to disclose. NI served as a medical advisor for Takeda, Taisho Pharmaceutical, GlaxoSmithKline and Mitsubishi Tanabe Pharma, he lectured for MSD, Sanofi, Novartis Pharma, Dainippon Sumitomo Pharma, Kyowa Kirin and Mitsubishi Tanabe Pharma, and received payment for his services. NI also received a clinical commissioned/joint research grant from MSD, Eli Lilly Japan, Shiratori Pharmaceutical, Roche Diagnostics and the Japan Diabetes Foundation, and also received a scholarship grant from MSD, Japan Tobacco Inc., Nippon Boehringer Ingelheim, Takeda, Dainippon Sumitomo Pharma, Astellas Pharma, Daiichi-Sankyo and Mitsubishi Tanabe Pharma.
References
- Moran-Ramos S, Tovar AR, Torres N. Diet: friend or foe of enteroendocrine cells–how it interacts with enteroendocrine cells. Adv Nutr. 2012;3:8–20. doi: 10.3945/an.111.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Invest. 2010;1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RM, Morgan LM, Tredger JA, et al. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- Karhunen LJ, Juvonen KR, Huotari A, et al. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008;149:70–78. doi: 10.1016/j.regpep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Yamane S, Harada N, Hamasaki A, et al. The effects of glucose and meal ingestion on incretin secretion in Japanese subjects with normal glucose tolerance. J Diabtes Invest. 2012;3:80–85. doi: 10.1111/j.2040-1124.2011.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- Litvak DA, Hellmich MR, Evers BM, et al. Glucagon-like peptide 2 is a potent growth factor for small intestine and colon. J Gastrointest Surg. 1998;2:146–150. doi: 10.1016/s1091-255x(98)80005-x. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Erlich P, Asa SL, et al. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wøjdemann M, Wettergren A, Hartmann B, et al. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab. 1999;84:2513–2517. doi: 10.1210/jcem.84.7.5840. [DOI] [PubMed] [Google Scholar]
- Benjamin MA, McKay DM, Yang PC, et al. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–119. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol. 1997;273:R1965–R1971. doi: 10.1152/ajpregu.1997.273.6.R1965. [DOI] [PubMed] [Google Scholar]
- Guan X, Stoll B, Lu X, et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–147. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- Taylor-Edwards CC, Burrin DG, Holst JJ, et al. Glucagon-like peptide-2 (GLP-2) increases small intestinal blood flow and mucosal growth in ruminating calves. J Dairy Sci. 2011;94:888–898. doi: 10.3168/jds.2010-3540. [DOI] [PubMed] [Google Scholar]
- Bremholm L, Hornum M, Andersen UB, et al. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept. 2011;168:32–38. doi: 10.1016/j.regpep.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Mojsov S, Heinrich G, Wilson IB, et al. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880–11889. [PubMed] [Google Scholar]
- Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr. 2001;131:2505S–2508S. doi: 10.1093/jn/131.9.2505S. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Burrin DG, Stoll B, et al. Intestinal glutamate metabolism. J Nutr. 2000;130:978S–982S. doi: 10.1093/jn/130.4.978S. [DOI] [PubMed] [Google Scholar]
- Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Farooqi IS, Keogh JM, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Williams L, da Silva Xavier G, et al. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Zheng Y, Parker HE, et al. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Stenberg M, Frid AH, et al. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–1253. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- Flatt PR, Kwasowski P, Howland RJ, et al. Gastric inhibitory polypeptide and insulin responses to orally administered amino acids in genetically obese hyperglycemic (ob/ob) mice. J Nutr. 1991;121:1123–1128. doi: 10.1093/jn/121.7.1123. [DOI] [PubMed] [Google Scholar]
- Samocha-Bonet D, Wong O, Synnott EL, et al. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr. 2011;141:1233–1238. doi: 10.3945/jn.111.139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–3671. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E, Yamane S, Hamasaki A, et al. Enteral supplement enriched with glutamine, fiber, and oligosaccharide attenuates experimental colitis in mice. Nutrition. 2013;29:549–555. doi: 10.1016/j.nut.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Azuma H, Mishima S, Oda J, et al. Enteral supplementation enriched with glutamine, fiber, and oligosaccharide prevents gut translocation in a bacterial overgrowth model. J Trauma. 2009;66:110–114. doi: 10.1097/TA.0b013e318193109b. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Kuzuya T, Hagura R, et al. Insulin response to oral glucose load is consistently decreased in established non-insulin-dependent diabetes mellitus: the usefulness of decreased early insulin response as a predictor of non-insulin-dependent diabetes mellitus. Diabet Med. 1996;13:S109–S119. [PubMed] [Google Scholar]
- Fukushima M, Taniguchi A, Sakai M, et al. Homeostasis model assessment as a clinical index of insulin resistance. Diabetes Care. 1999;22:1911–1912. doi: 10.2337/diacare.22.11.1911. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Harada N, Hamasaki A, Muraoka A, et al. Plasma GIP and GLP-1 levels after glucose loading are associated with different factors in Japanese subjects. J Diabetes Invest. 2011;2:193–199. doi: 10.1111/j.2040-1124.2010.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F, Linsalata M, Clemente C, et al. The effects of fluorouracil, epirubicin, and cyclophosphamide (FEC60) on the intestinal barrier function and gut peptides in breast cancer patients: an observational study. BMC Cancer. 2013;13:56. doi: 10.1186/1471-2407-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby SJ, Ellis PR, Morgan LM, et al. Effect of partially depolymerized guar gum on acute metabolic variables in patients with non-insulin-dependent diabetes. Diabet Med. 1996;13:358–364. doi: 10.1002/(SICI)1096-9136(199604)13:4<358::AID-DIA24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Jie Z, Bang-Yao L, Ming-Jie X, et al. Studies on the effects of polydextrose intake on physiologic functions in Chinese people. Am J Clin Nutr. 2000;72:1503–1509. doi: 10.1093/ajcn/72.6.1503. [DOI] [PubMed] [Google Scholar]
- Kihara M, Sakata T. Production of short-chain fatty acids and gas from various oligosaccharides by gut microbes of carp (Cyprinus carpio L.) in micro-scale batch culture. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:333–340. doi: 10.1016/s1095-6433(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–G739. doi: 10.1152/ajpgi.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. Glucagon and glucagon-like peptide 1 and 2. Results Probl Cell Differ. 2010;50:121–135. doi: 10.1007/400_2009_35. [DOI] [PubMed] [Google Scholar]
- de Hollanda A1, Jiménez A1, Corcelles R, et al. Gastrointestinal hormones and weight loss response after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2014 doi: 10.1016/j.soard.2014.01.022. doi: 10.1016/j.soard.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Wang JH1, Inoue T, Higashiyama M, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J1, Longuet C, Maida A, et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Harada N, Yamane S, et al. Transcriptional regulatory factor X6 (Rfx6) increases gastric inhibitory polypeptide (GIP) expression in enteroendocrine K-cells and is involved in GIP hypersecretion in high fat diet-induced obesity. J Biol Chem. 2013;288:1929–1938. doi: 10.1074/jbc.M112.423137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasteska D, Harada N, Suzuki K, et al. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high fat diet condition. Diabetes. 2014;63:2332–2343. doi: 10.2337/db13-1563. [DOI] [PubMed] [Google Scholar]
- Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247–1258. doi: 10.1016/j.clinthera.2012.04.013. e22. [DOI] [PubMed] [Google Scholar]


