Abstract
Aims/Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in developed countries, and it was required to monitor patients with prediabetes. However, there have been few reports establishing the risk for diabetes mellitus (DM) among patients with prediabetes. The purpose of the present study was to evaluate the effect of NAFLD on the progression of DM among insurance beneficiaries with prediabetes, using data from specific health check-ups and the fatty liver index (FLI).
Materials and Methods
We used a retrospective cohort study that enrolled 967 insurance beneficiaries with prediabetes who had rarely drunk or could not drink alcohol, or whose alcohol consumption was <19 g/day from two health insurance societies. We divided insurance beneficiaries into FLI <30, intermediates FLIs and FLI ≥60, and compared the incidence rate of DM among the groups after 3 years' follow up, using multiple logistic regression models.
Results
During 3 years' follow up, progression of diabetes was seen in 65 men (11.5%) and 24 women (6.0%). Logistic regression analyses showed that those with NAFLD had significantly higher risks of developing DM; this was the case in both men (odds ratio 2.68, 95% confidential interval 1.29–5.56) and women (odds ratio 10.35, 95% confidential interval 3.22–33.31).
Conclusions
Among insurance beneficiaries with prediabetes, those with NAFLD had a significantly higher risk of DM than those without NAFLD. The FLI might be useful for detecting individuals who have an especially higher risk for DM, and developing more effective guidance for delivering healthcare services in Japan.
Keywords: Diabetes mellitus, Non-alcoholic fatty liver, Prediabetes
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in developed countries1. Furthermore, a recent study showed that the prevalence of NAFLD was 68.5% in obese, 15.2% in non-obese subjects and 24.6% in total among Japanese2. NAFLD is strongly associated with insulin resistance3,4. The rate of newly diagnosed prediabetes was 75% in patients with NAFLD, and 25% in those without NAFLD5. Furthermore, a recent study reported that NAFLD is a strong and independent risk factor for prediabetes6, and that liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyltransferase (GGT), were strongly associated with diabetes mellitus (DM) risk7,8. It has also been reported that fatty liver is a risk factor for impaired fasting glucose (IFG) and type 2 DM in Japanese people9, and NAFLD is a risk factor for type 2 DM in middle-aged men10.
The diagnosis of NAFLD is regarded as clinically problematic because of the invasive character of the gold standard method of liver biopsy. Therefore, previous studies clarified NAFLD by using ultrasound1,2,6,9,10, magnetic resonance spectroscopy5, indices of fatty liver11–13, or two of these4,14. Bedogni et al.11 introduced the fatty liver index (FLI), which was estimated using multivariate models including several biomarkers. These included body mass index (BMI), waist circumference (WC), triglycerides (TGs) and GGT, measured in specific health check-ups in Japan. Specific health check-ups and guidance were implemented to reduce the number of persons with lifestyle-related diseases including DM, in fiscal year (FY) 200815. There are few studies to report that FLI is valuable in identifying type 2 diabetes among Asian populations16.
Furthermore, the FLI was required to monitor patients with prediabetes for identification, quantification and characterization of the population of high-risk individuals targeted for ongoing DM primary prevention efforts17. Also, it was reported that the prevalence of prediabetes increased significantly in both men and women from the 1980s to the 2000s in a general Japanese population18. The National Health and Nutrition Survey reported that approximately 9.5 million people were strongly suspected as having DM, and a further 11.0 million people were possible candidates for having DM19. However, a previous study in Asian populations did not classify prediabetes among subjects without DM16. Previous studies in Japan did not include subjects with impaired glucose tolerance (IGT) and IFG9,10; but nevertheless, there were 20.5 million people at high risk of having diabetes. Additionally, a recent study reported that NAFLD was a stronger predictor for prediabetes than metabolic syndromes6.
However, there have been few reports establishing the risk for DM among patients with prediabetes among Asian populations, in which the prevalence of DM has rapidly increased in recent decades with economic development including food supply and dietary patterns, technology transfer, and cultural admixture20. Therefore, we carried out the present study to evaluate the effect of NAFLD on the progression of DM among these patients.
Methods
Participants
The inclusion and exclusion flowchart is shown in Figure1. We identified 8,982 insurance beneficiaries aged 40 years or older as of 31 March 2009 who worked for health insurance societies located in Fukuoka and Shizuoka Prefectures (Japan), and who attended specific health check-ups at FY2008. For the present study, we excluded 413 insurance beneficiaries who had not attended specific health check-ups at FY2011. From those, we identified 4,830 insurance beneficiaries who were not drinkers or whose alcohol consumption was less than 19 g per day. After converting hemoglobin A1c (HbA1c), which was in the Japan Diabetes Society (JDS) units to the National Glycohemoglobin Standardization Program (NGSP) units21,22, we excluded 292 insurance beneficiaries whose HbA1c was higher than 6.4% or those taking treatments for DM based on a self-administered questionnaire (insurance beneficiaries with exiting DM), 2,387 insurance beneficiaries without any treatments for DM whose HbA1c was less than 5.7% (insurance beneficiaries with normal glucose tolerance) and 844 of those who did not have their HbA1c values measured or who did not answer questionnaires about treatments for DM (insurance beneficiaries with insufficient data about DM). Thus, we identified 1,307 insurance beneficiaries without treatments for DM whose HbA1c levels ranged 5.7–6.4%. We excluded 141 insurance beneficiaries whose HbA1c, or responses to questionnaires about both drinking behaviors and alcohol consumptions or dietary habits, were not available at FY2011. Furthermore, we excluded 193 insurance beneficiaries who had been diagnosed with stroke, coronary heart disease, chronic kidney disease or anemia based on a self-administered questionnaire. We also excluded six insurance beneficiaries who had been treated for chronic viral hepatitis (International Classification of Diseases 10th revision code: B18) at FY2011, using claims data. Finally, we arrived at 967 insurance beneficiaries as study participants.
Figure 1.
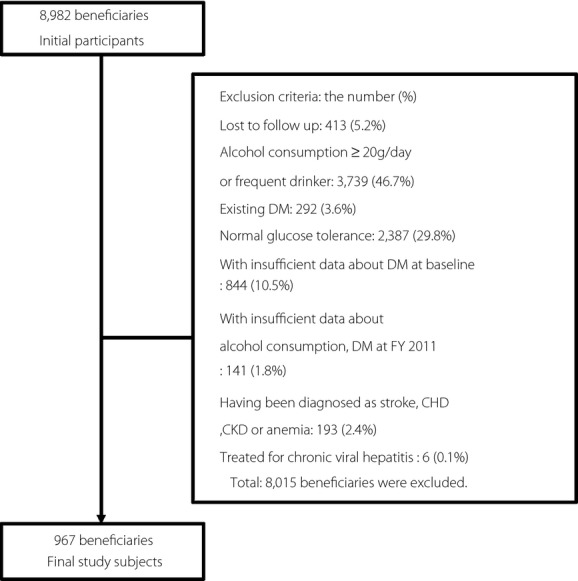
Inclusion and exclusion flowchart. CHD, coronary heart disease; CKD, chronic kidney failure; DM, diabetes mellitus; FY, fiscal year.
The present study was approved by the Kyushu University Institutional Review Board for Clinical Research.
Definition of Variables
The FLI score was used as a surrogate measure for fatty liver. This measure was calculated using the following equation:
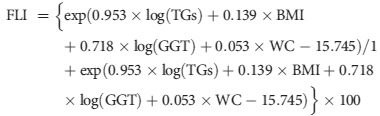 |
The FLI varies between 0 and 100. According to a previous study12, FLI <30 can be used to rule out (sensitivity = 87%; negative likelihood ratio = 0.2) and FLI ≥60 to rule in hepatic steatosis (specificity = 86%; positive likelihood ratio = 4.3). Thus, we defined participants with FLI scores of 60 or higher as having NAFLD, and those with FLI scores of 30 or lower as not having NAFLD. The rest of them were defined as having intermediate FLIs.
Participants with HbA1c values higher than 6.4% or those taking treatments for DM based on self-administered questionnaire at FY2011 were defined as newly diagnosed DM. Ages were categorized into three groups: 40–49, 50–59 and 60 years or older. HbA1c values at baseline were categorized into four groups according to quartiles. Participants whose systolic blood pressure (SBP) was higher than 140 mmHg or whose diastolic blood pressure (DBP) was higher than 90 mmHg, or who used antihypertensive drugs based on self-administered questionnaire were defined as having hypertension. Participants whose low-density cholesterol (LDL-C) was higher than 3.6 mmol/L, or who used cholesterol-lowering drugs based on self-administered questionnaire, were defined as having hypercholesterolemia. Lifestyle habits were respectively categorized based on a self-administered questionnaire. Those who had smoked over the past month and had smoked a total of over 100 cigarettes, or who had smoked over a period of 6 months, were defined as smokers. Those who had habitually exercised for over 30 min twice a week for at least 1 year, or who habitually walked for over 1 h a day, were defined as involved in physical activity. Eating before sleeping, eating fast and prefecture were used as explanatory variables.
Statistical Analysis
Participant characteristics were constructed using frequencies and proportions for categorical variables, and using median and interquartile ranges for a continuous variable. Categorical variables were compared between the three groups using Pearson's χ2-test, and the continuous variable was compared between the three groups using the Kruskal–Wallis test.
Multiple logistic regression analyses were used to estimate adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) for the incidence of DM. After stratification by sex, we set the incidence of DM as the dependent variable, and age, FLI categories, smoking habits, physical activity, eating before sleeping, eating fast, the presence of hypertension, the presence of hypercholesterolemia and prefecture as independent variables. Statistical analyses were carried out using pasw version 18.0 (SPSS Inc., Chicago, IL, USA). P-values <0.05 were regarded as statistically significant.
Results
Demographic and physical characteristics of the participants are shown in Table1. The number of those with NAFLD, intermediate FLIs, and no NAFLD were 499 (51.6%), 281 (29.1%) and 187 (19.3%), respectively. The median age between the groups was significantly different in men (P < 0.001), but not significantly different in women (P = 0.588). Biochemical characteristics are shown in Table2. The median HbA1c was significantly different in both sexes (P < 0.001). Lifestyle habits and comorbidity are shown in Table3. The prevalence of hypertension and hypercholesterolemia was significantly different in both sexes (P < 0.001, P = 0.001 in men; P < 0.001, P < 0.001 in women). The proportion of participants who ate fast was significantly different in men (P = 0.017), and the proportion of participants who ate before sleeping was significantly different in women (P = 0.010).
Table 1.
Demographic and physical characteristics of participants according to fatty liver index, by sex
| Total | FLI | P-value | |||||
|---|---|---|---|---|---|---|---|
| <30 | Intermediate | ≥60 | |||||
| Men | (n = 565) | (n = 209) | (n = 200) | (n = 156) | |||
| Median age (years) | [IQR] | 49.0 [8.0] | 50.0 [8.0] | 50.0 [8.0] | 47.0 [8.0] | <0.001† | |
| Age (years) | 40–49 | 271 (48.0%) | 93 (18.6%) | 86 (43.0%) | 92 (59.0%) | 0.008 | |
| 50–59 | 258 (45.7%) | 97 (19.4%) | 102 (51.0%) | 59 (37.8%) | |||
| ≥60 | 36 (6.4%) | 19 (3.8%) | 12 (6.0%) | 5 (3.2%) | |||
| Fukuoka Prefecture | 119 (21.1%) | 36 (17.2%) | 48 (24.0%) | 35 (22.4%) | 0.216 | ||
| Median BMI (kg/m2) | [IQR] | 24.3 [4.2] | 21.6 [2.5] | 24.8 [2.5] | 27.7 [3.6] | <0.001† | |
| Median WC (cm) | [IQR] | 87.2 [10.7] | 80.0 [7.0] | 88.0 [6.5] | 95.0 [10.2] | <0.001† | |
| Women | (n = 402) | (n = 290) | (n = 81) | (n = 31) | |||
| Median age (years) | [IQR] | 52.0 [7.0] | 52.0 [7.0] | 52.0 [9.0] | 51.0 [8.0] | 0.588† | |
| Age (years) | 40–49 | 117 (29.1%) | 84 (29.0%) | 22 (27.2%) | 11 (35.5%) | 0.788 | |
| 50–59 | 247 (61.4%) | 180 (62.1%) | 49 (60.5%) | 18 (58.1%) | |||
| ≥60 | 38 (9.5%) | 26 (9.0%) | 10 (12.3%) | 2 (6.5%) | |||
| Fukuoka Prefecture | 105 (26.1%) | 75 (25.9%) | 23 (28.4%) | 7 (22.6%) | 0.807 | ||
| Median BMI (kg/m2) | [IQR] | 22.7 [4.9] | 21.6 [3.4] | 26.0 [3.4] | 29.8 [5.5] | <0.001† | |
| Median WC (cm) | [IQR] | 82.0 [11.7] | 79.3 [9.4] | 89.5 [6.5] | 98.2 [8.7] | <0.001† | |
| Total | (n = 967) | (n = 499) | (n = 281) | (n = 187) | |||
| Median age (years) | [IQR] | 51.0 [8.0] | 51.0 [8.0] | 51.0 [7.0] | 48.0 [9.0] | <0.001† | |
| Age (years) | 40–49 | 388 (40.1%) | 177 (35.5%) | 108 (38.4%) | 103 (55.1%) | <0.001 | |
| 50–59 | 505 (52.2%) | 277 (55.5%) | 151 (53.7%) | 77 (41.2%) | |||
| ≥60 | 74 (7.7%) | 45 (9.0%) | 22 (7.8%) | 7 (3.7%) | |||
| Sex | Male | 565 (58.4%) | 209 (41.9%) | 200 (71.2%) | 156 (83.4%) | <0.001 | |
| Female | 402 (41.6%) | 290 (58.1%) | 81 (28.8%) | 31 (16.6%) | |||
| Fukuoka Prefecture | 224 (23.2%) | 111 (22.2%) | 71 (25.3%) | 42 (22.5%) | 0.610 | ||
| Median BMI (kg/m2) | [IQR] | 23.6 [4.6] | 21.8 [3.0] | 25.0 [2.8] | 27.9 [4.1] | <0.001† | |
| Median WC (cm) | [IQR] | 85.0 [12.0] | 79.8 [8.2] | 88.5 [6.4] | 95.5 [10.5] | <0.001† | |
Compared using the Kruskal–Wallis test. Other comparisons made using the χ2 test.
BMI, body mass index; FLI, fatty liver index; IQR, interquartile range; WC, waist circumference.
Table 2.
Biochemical characteristics of participants according to fatty liver index by sex
| Total | FLI | P-value | |||||
|---|---|---|---|---|---|---|---|
| <30 | Intermediate | ≥60 | |||||
| Men | (n = 565) | (n = 209) | (n = 200) | (n = 156) | |||
| Median HbA1c at baseline, % | [IQR] | 5.9 [0.3] | 5.8 [0.3] | 5.9 [0.3] | 5.9 [0.2] | 0.274† | |
| Q1 | 5.7 | 154 (27.3%) | 61 (29.2%) | 56 (28.0%) | 37 (23.7%) | 0.629 | |
| Q2 | 5.8 | 128 (22.7%) | 53 (25.4%) | 40 (20.0%) | 35 (22.4%) | ||
| Q3 | 5.9 | 110 (19.5%) | 38 (18.2%) | 42 (21.0%) | 30 (19.2%) | ||
| Q4 | ≥6.0 | 173 (30.6%) | 57 (27.3%) | 62 (31.0%) | 54 (34.6%) | ||
| Median TGs (mmol/L) | [IQR] | 1.5 [1.2] | 1.0 [0.5] | 1.6 [0.9] | 2.4 [1.4] | <0.001† | |
| Median GGT (U/L) | [IQR] | 35.0 [28.0] | 24.0 [12.0] | 38.0 [25.0] | 56.5 [42.0] | <0.001† | |
| Women | (n = 402) | (n = 290) | (n = 81) | (n = 31) | |||
| Median HbA1c at baseline (%) | [IQR] | 5.8 [0.3] | 5.8 [0.2] | 5.9 [0.2] | 6.0 [0.4] | <0.001† | |
| Q1 | 5.7 | 120 (29.9%) | 97.0 (33.4%) | 17.0 (21.0%) | 6.0 (19.4%) | 0.003 | |
| Q2 | 5.8 | 88 (21.9%) | 67.0 (23.1%) | 19.0 (23.5%) | 2.0 (6.5%) | ||
| Q3 | 5.9 | 77 (19.2%) | 56.0 (19.3%) | 15.0 (18.5%) | 6.0 (19.4%) | ||
| Q4 | ≥6.0 | 117 (29.1%) | 70.0 (24.1%) | 30.0 (37.0%) | 17.0 (54.8%) | ||
| Median TGs (mmol/L) | [IQR] | 1.0 [0.6] | 0.9 [0.5] | 1.3 [0.8] | 1.5 [1.1] | <0.001† | |
| Median GGT (U/L) | [IQR] | 19.0 [15.0] | 17.0 [8.0] | 30.0 [23.0] | 37.0 [44.0] | <0.001† | |
| Total | (n = 967) | (n = 499) | (n = 281) | (n = 187) | |||
| Median HbA1c at baseline, % | [IQR] | 5.8 [0.3] | 5.8 [0.3] | 5.9 [0.3] | 5.9 [0.2] | <0.001† | |
| Q1 | 5.7 | 274 (28.3%) | 158 (31.7%) | 73 (26.0%) | 43 (23.0%) | 0.028 | |
| Q2 | 5.8 | 216 (22.3%) | 120 (24.0%) | 59 (21.0%) | 37 (19.8%) | ||
| Q3 | 5.9 | 187 (19.3%) | 94 (18.8%) | 57 (20.3%) | 36 (19.3%) | ||
| Q4 | ≥6.0 | 290 (30.0%) | 127 (25.5%) | 92 (32.7%) | 71 (38.0%) | ||
| Median TGs (mmol/L) | [IQR] | 1.2 [1.0] | 0.9 [0.5] | 2 [0.9] | 2.3 [1.4] | <0.001† | |
| Median GGT (U/L) | [IQR] | 28.0 [27.0] | 20.0 [13.0] | 35.0 [25.0] | 53.0 [44.0] | <0.001† | |
Compared using the Kruskal–Wallis test. Other comparisons made using the χ2 test.
SI conversion factor: To convert triglycerides to millimoles per liter, multiply by 0.0113.
FLI, fatty liver index; GGT, gamma-glutamyltransferase; IQR, interquartile range; TGs, triglycerides.
Table 3.
Lifestyle habits and comorbidity of participants according to fatty liver index, by sex
| Total | FLI | P-value | |||||
|---|---|---|---|---|---|---|---|
| <30 | Intermediate | ≥60 | |||||
| Men | (n = 565) | (n = 209) | (n = 200) | (n = 156) | |||
| Hypertension | 160 (28.3%) | 38 (18.2%) | 68 (34.0%) | 54 (34.6%) | <0.001 | ||
| Median SBP (mmHg) | [IQR] | 122 [24] | 116 [24] | 126 [24] | 128 [22] | <0.001† | |
| Median DBP (mmHg) | [IQR] | 78 [16] | 72 [15] | 80 [18] | 80 [14] | <0.001† | |
| Use of antihypertensive drugs | 53 (9.4%) | 13 (6.2%) | 25 (12.5%) | 15 (9.6%) | 0.093 | ||
| Hypercholesterolemia | 274 (48.5%) | 80 (38.3%) | 108 (54.0%) | 86 (55.1%) | 0.001 | ||
| Median LDL-C (mmol/L) | [IQR] | 3.5 [1.0] | 3.3 [0.9] | 3.6 [0.9] | 3.6 [1.0] | 0.002† | |
| Use of cholesterol lowering drugs | 39 (6.9%) | 6 (2.9%) | 20 (10.0%) | 13 (8.3%) | 0.012 | ||
| Alcohol consumption <19 g/day | 506 (89.6%) | 190 (90.9%) | 178 (89.0%) | 138 (88.5%) | 0.714 | ||
| No drinkers | 320 (56.6%) | 122 (58.4%) | 104 (52.0%) | 94 (60.3%) | 0.242 | ||
| Smoking | 251 (44.4%) | 98 (46.9%) | 80 (40.0%) | 73 (46.8%) | 0.293 | ||
| Physical activities | 174 (30.8%) | 63 (30.1%) | 65 (32.5%) | 46 (29.5%) | 0.803 | ||
| Eating fast | 230 (40.7%) | 71 (34.0%) | 83 (41.5%) | 76 (48.7%) | 0.017 | ||
| Eating before sleeping | 213 (37.7%) | 80 (38.3%) | 69 (34.5%) | 64 (41.0%) | 0.441 | ||
| Women | (n = 402) | (n = 290) | (n = 81) | (n = 31) | |||
| Hypertension | 85 (21%) | 49 (16.9%) | 21 (25.9%) | 15 (48.4%) | <0.001 | ||
| Median SBP (mmHg) | [IQR] | 120 [25] | 117 [24] | 125 [22] | 130 [26] | <0.001† | |
| Median DBP (mmHg) | [IQR] | 74 [12] | 71 [16] | 79 [14] | 80 [18] | <0.001† | |
| Use of antihypertensive drugs | 43 (10.7%) | 21 (7.2%) | 13 (16.0%) | 9 (29.0%) | <0.001 | ||
| Hypercholesterolemia | 200 (49.8%) | 123 (42.4%) | 54 (66.7%) | 23 (74.2%) | <0.001 | ||
| Median LDL-C (mmol/L) | [IQR] | 3.5 [1.1] | 3.4 [1.1] | 3.8 [1.1] | 3.7 [1.3] | 0.001† | |
| Use of cholesterol lowering drugs | 41 (10.2%) | 24 (8.3%) | 11 (13.6%) | 6 (19.4%) | 0.081 | ||
| Alcohol consumption <19 g /day | 363 (90.3%) | 269 (92.8%) | 68 (84.0%) | 26 (83.9%) | 0.027 | ||
| No drinkers | 294 (73.1%) | 204 (70.3%) | 64 (79.0%) | 26 (83.9%) | 0.111 | ||
| Smoking | 24 (6.0%) | 18 (6.2%) | 5 (6.2%) | 1 (3.2%) | 0.798 | ||
| Physical activities | 106 (26.4%) | 78 (26.9%) | 21 (25.9%) | 7 (22.6%) | 0.870 | ||
| Eating fast | 136 (33.8%) | 91 (31.4%) | 33 (40.7%) | 12 (38.7%) | 0.242 | ||
| Eating before sleeping | 114 (28.4%) | 70 (24.1%) | 31 (38.3%) | 13 (41.9%) | 0.010 | ||
| Total | (n = 967) | (n = 499) | (n = 281) | (n = 187) | |||
| Hypertension | 245 (25.3%) | 87 (17.4%) | 89 (31.7%) | 69 (36.9%) | <0.001 | ||
| Median SBP (mmHg) | [IQR] | 122 [24] | 117 [22] | 126 [24] | 128 [20] | <0.001† | |
| Median DBP (mmHg) | [IQR] | 76 [16] | 72 [14] | 79 [16] | 80 [14] | <0.001† | |
| Use of antihypertensive drugs | 96 (9.9%) | 34 (6.8%) | 38 (13.5%) | 24 (12.8%) | 0.004 | ||
| Hypercholesterolemia | 474 (49.0%) | 203 (40.7%) | 162 (57.7%) | 109 (58.3%) | <0.001 | ||
| Median LDL-C (mmol/L) | [IQR] | 3.5 [1.0] | 3.3 [1.0] | 3.6 [0.9] | 3.6 [1.0] | <0.001† | |
| Use of cholesterol lowering drugs | 80 (8.3%) | 30 (6.0%) | 31 (11.0%) | 19 (10.2%) | 0.029 | ||
| Alcohol consumption <19 g/day | 869 (89.9%) | 459 (92.0%) | 246 (87.5%) | 164 (87.7%) | 0.079 | ||
| No drinkers | 614 (63.5%) | 326 (65.3%) | 168 (59.8%) | 120 (64.2%) | 0.297 | ||
| Smoking | 275 (28.4%) | 116 (23.2%) | 85 (30.2%) | 74 (39.6%) | <0.001 | ||
| Physical activities | 280 (29.0%) | 141 (28.3%) | 86 (30.6%) | 53 (28.3%) | 0.769 | ||
| Eating fast | 366 (37.8%) | 162 (32.5%) | 116 (41.3%) | 88 (47.1%) | 0.001 | ||
| Eating before sleeping | 327 (33.8%) | 150 (30.1%) | 100 (35.6%) | 77 (41.2%) | 0.018 | ||
Compared using the Kruskal–Wallis test. Other comparisons made using the χ2 test.
SI conversion factor: To convert cholesterol to millimoles per liter, multiply by 0.0259.
FLI, fatty liver index; DBP, diastolic blood pressure; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol SBP, systolic blood pressure.
Table4 compares the proportions, unadjusted odds ratios (ORs) and 95% CIs for diabetes, and the results of multiple logistic regression analyses according to FLI by sex. During the study period, progression of diabetes was seen in 65 men (11.5%) and 24 women (6.0%). The incidence of DM was significantly different in both sexes (P = 0.008, P < 0.001). Participants with intermediate FLIs and those with NAFLD had significantly higher risks of DM, in both men (OR 2.35, 2.88; 95% CI 1.18–4.70, 1.42–5.83; P for trend 0.003) and women (OR 2.95, 10.86; 95% CI 1.07–8.19, 3.83–30.82; P for trend <0.001). Logistic regression analyses showed that those with intermediate FLIs and those with NAFLD had significantly higher risks of developing DM; this was the case in both men (OR 2.28, 2.68; 95% CI 1.12–4.63, 1.29–5.56; P for trend 0.023) and women (OR 3.01, 10.35; 95% CI 1.03–8.78, 3.22–33.31; P for trend <0.001).
Table 4.
Comparison of odds ratios and 95% confidence intervals for diabetes, and results of multiple logistic regression analyses, according to fatty liver index by sex
| FLI | P-value | |||
|---|---|---|---|---|
| <30 | Intermediate | ≥60 | ||
| Men | ||||
| No. DM cases | 13 (6.2%) | 27 (13.5%) | 25 (16.0%) | 0.008† |
| No. patients with HbA1c > 6.4 | 13 (6.2%) | 26 (13.0%) | 21 (13.5%) | |
| No. patients taking treatments for DM | 0 (0.0%) | 4 (2.0%) | 8 (5.1%) | |
| Unadjusted odds ratio | Reference | 2.35 [1.18–4.70] | 2.88 [1.42–5.83] | 0.003 |
| Adjusted odds ratio | Reference | 2.28 [1.12–4.63] | 2.68 [1.29–5.56] | 0.023 |
| Women | ||||
| No. DM patients | 9 (0.3%) | 7 (8.6%) | 8 (25.8%) | <0.001† |
| No. patients with HbA1c >6.4 | 6 (0.2%) | 7 (8.6%) | 7 (22.6%) | |
| No. patients taking treatments for DM | 3 (0.1%) | 1 (1.2%) | 1 (3.2%) | |
| Unadjusted odds ratio | Reference | 2.95 [1.07–8.19] | 10.86 [3.83–30.82] | <0.001 |
| Adjusted odds ratio | Reference | 3.01 [1.03–8.78] | 10.35 [3.22–33.31] | <0.001 |
Compared using the χ2-test. Other comparisons made using the trend test.
Hosmer–Lemeshow goodness of fit: P = 0.714 in men, and P = 0.651 in women, respectively.
Adjusted by age, smoking habits, physical activities, eating habits before sleeping,eating fast, hypertension, hypercholesterolemia and prefecture.
DM, diabetes mellitu; FLI, fatty liver index.
Discussion
The present study showed that NAFLD assessed by FLI and questionnaires is an independent risk factor for DM among insurance beneficiaries with prediabetes undergoing specific health check-ups. The results are consistent with previous studies, which reported that NAFLD is a risk factor for prediabetes or type 2 DM among subjects without DM or prediabetes6,10,14. Above all, the present results are similar to those of a previous study that used the FLI, in terms of risk for DM. In the previous study, risk was especially high among women with NAFLD, although adjusted ORs were estimated at remarkably high values: OR 4.71, 95% CI 1.68–7.28 in men and OR 22.77, 95% CI 6.78–76.44 in women14.
NAFLD could progress to non-alcoholic steatohepatitis (NASH) and liver cirrhosis. Therefore, the current study's results also suggest that it would be advantageous for insurers to carefully monitor for early detection of NAFLD in insurance beneficiaries with prediabetes, and to intervene earlier in both diseases. For example, weight loss is an effective intervention to prevent DM among patients with prediabetes23–26, whereas a recent randomized controlled trial reported that community-based lifestyle modifications targeting BMI 23 kg/m2 were effective in reducing and normalizing liver fat in NAFLD patients27. Furthermore, although thiazolidinediones were only used for type 2 DM in Japan, they reduced insulin resistance to prevent DM among patients with IGT or IFG28,29, and decreased AST, ALT and hepatic fat content among non-alcoholic steatohepatitis patients with IGT or type 2 DM30,31. In addition to issues around application to prediabetes, a recent meta-analysis investigating ethnic differences in insulin sensitivity and response reported that insulin sensitivity of East Asians with impaired glucose regulation was significantly higher than that of Africans32. These interventions would be effective if aimed at insurance beneficiaries with NAFLD and prediabetes.
There were several limitations to the present study. First, we used FLI as a surrogate marker for NAFLD, because specific health check-ups did not include scanning tests, such as ultrasounds or magnetic resonance spectroscopy. Second, as each component of the FLI is a risk factor for diabetes by itself, it would be controversial if NAFLD was an independent predictor for DM and if cut-off points of the FLI were appropriate. Third, we used a self-administered questionnaire to define DM. Therefore, information bias could exist. Furthermore, it is also unclear whether the findings could be applied to other populations as well as Japanese. Finally, as the present study did not obtain fasting plasma glucose and a 2-h oral glucose tolerance test, the prevalence of DM would be underestimated.
However, the FLI has also validated in Korean populations33, while it was developed and its validity has been examined in European populations11,34. It was required to monitor those with prediabetes for identification and quantification17; nevertheless, just nine patients with prediabetes have received specific health guidance at FY2008.Therefore, it is expected that insurers would develop a disease management program for insurance beneficiaries who had an especially higher risk for DM. Because of the increasing prevalence of NAFLD, a simpler surrogate measure would be important to prevent NAFLD, and its adverse hepatic and extrahepatic consequences. As diagnosing NAFLD by using scanning tests requires radiological equipment and experts, the utilization of simpler and more cost-effective screening methods for NAFLD is necessary to identify people who might have NAFLD at annual health check-ups. Also, because it could be calculated by measures commonly used during specific health check-ups, the FLI and questionnaires could be used as surrogate measures for NAFLD during these check-ups.
In conclusion, among insurance beneficiaries with prediabetes, those with NAFLD had a significantly higher risk of DM than those without NAFLD. The FLI might be useful for detecting those who had an especially higher risk for DM, and for developing more effective guidance for delivering healthcare services in Japan.
Acknowledgments
This study was supported in part by a Grant-In-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant no. 25293160). The authors declare no conflict of interest.
References
- Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- Nishioji K, Sumida Y, Kamaguchi M, et al. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011–2012. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0948-9. doi: 10.1007/s00535-014-0948-9. [DOI] [PubMed] [Google Scholar]
- Lattuada G, Ragogna F, Perseghin G. Why does NAFLD predict type 2 diabetes? Curr Diab Rep. 2011;11:167–172. doi: 10.1007/s11892-011-0190-2. [DOI] [PubMed] [Google Scholar]
- Gastaldelli A, Kozakova M, Højlund K, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–1544. doi: 10.1002/hep.22845. [DOI] [PubMed] [Google Scholar]
- Ortiz-Lopez C, Lomonaco R, Orsak B, et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD) Diabetes Care. 2012;35:873–878. doi: 10.2337/dc11-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelber-Sagi S, Lotan R, Shibolet O, et al. Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int. 2013;33:1406–1412. doi: 10.1111/liv.12200. [DOI] [PubMed] [Google Scholar]
- Schneider AL, Lazo M, Ndumele CE, et al. Liver enzymes, race, gender and diabetes risk: the Atherosclerosis Risk in Communities (ARIC) Study. Diabet Med. 2013;30:926–933. doi: 10.1111/dme.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol. 2013;15(178):159–171. doi: 10.1093/aje/kws469. [DOI] [PubMed] [Google Scholar]
- Yamada T, Fukatsu M, Suzuki S, et al. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol. 2010;25:352–356. doi: 10.1111/j.1440-1746.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kihara Y, Taguchi M, et al. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940–2944. doi: 10.2337/dc07-0792. [DOI] [PubMed] [Google Scholar]
- Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert IM, Heier M, Rathmann W, et al. Association between markers of fatty liver disease and impaired glucose regulation in men and women from the general population: the KORA-F4-study. PLoS ONE. 2011;6:e22932. doi: 10.1371/journal.pone.0022932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt L, Göbl CS, Tura A, et al. Fatty liver index predicts further metabolic deteriorations in women with previous gestational diabetes. PLoS ONE. 2012;7:e32710. doi: 10.1371/journal.pone.0032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkau B, Lange C, Vol S, et al. Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol. 2010;7(10):56. doi: 10.1186/1471-230X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babazono A, Kuwabara K, Hagiihara A, et al. Do interventions to prevent lifestyle-related diseases reduce healthcare expenditures? A randomized controlled clinical trial. J Epidemiol. 2011;21:75–80. doi: 10.2188/jea.JE20100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Lee WJ, Hwang JY, et al. Assessment of the fatty liver index as an indicator of hepatic steatosis for predicting incident diabetes independently of insulin resistance in a Korean population. Diabet Med. 2013;30:428–435. doi: 10.1111/dme.12104. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999-2010. Diabetes Care. 2013;36:2286–2293. doi: 10.2337/dc12-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai N, Doi Y, Ninomiya T, et al. Trends in the prevalence of type 2 diabetes and prediabetes in community-dwelling Japanese subjects: The Hisayama Study. J Diabetes Invest. 2014;5:162–169. doi: 10.1111/jdi.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare. 2013. Outline for the Results of the National Health and Nutrition Survey in Japan. Available from: http://www.mhlw.go.jp/stf/houdou/0000032074.html Accessed February 27, 2014. (in Japanese)
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Watanabe M, Nishida J, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med. 2011;171:1352–1360. doi: 10.1001/archinternmed.2011.275. [DOI] [PubMed] [Google Scholar]
- Sakane N, Sato J, Tsushita K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health. 2011;11:40. doi: 10.1186/1471-2458-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- Kodama K, Tojjar D, Yamada S, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kwon SY, Lee SW, et al. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 2011;31:1600–1601. doi: 10.1111/j.1478-3231.2011.02580.x. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S, Webb M, Assy N, et al. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J Gastroenterol. 2013;19:57–64. doi: 10.3748/wjg.v19.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]


