Abstract
Aims/Introduction
Ezetimibe lowers serum lipid levels by inhibiting intestinal absorption of dietary and biliary cholesterol. However, the effect of ezetimibe on insulin resistance remains unclear. The aim of the present study was to examine this issue in patients with metabolic syndrome in local-dwelling Japanese, who were not being treated with lipid-lowering drugs.
Materials and Methods
In 2009, 1,943 participants received a health examination in the Tanushimaru Study, a Japanese cohort of the Seven Countries Study, of whom 490 participants had metabolic syndrome. Among them, 61 participants (41 men and 20 women) were examined in the present study. They were treated with 10 mg of ezetimibe once a day for 24 weeks, combined with standard diet and exercise therapy.
Results
Bodyweight (P < 0.001), body mass index (P < 0.001), systolic blood pressure (P = 0.003), diastolic blood pressure (P < 0.001), triglycerides (P = 0.002), non-high-density lipoprotein cholesterol (P = 0.001), low-density lipoprotein cholesterol (P < 0.001) and homeostasis model assessment of insulin resistance (P = 0.011) significantly decreased after the observational period. There were no statistically significant differences in the effects of ezetimibe between men and women. Univariate analysis showed that the reduction of homeostasis model assessment of insulin resistance was not associated with the improvement of other metabolic components.
Conclusions
Ezetimibe combined with standard diet and exercise therapy improves not only bodyweight and atherogenic lipid profiles, but also insulin resistance, blood pressure and anthropometric factors in metabolic syndrome in local-dwelling Japanese. Interestingly, the improvement of insulin resistance had no correlation with other metabolic components.
Keywords: Ezetimibe, Insulin resistance, Metabolic syndrome
Introduction
Ezetimibe lowers serum lipid levels by inhibiting intestinal absorption of dietary and biliary cholesterol. Its lipid-lowering effects on low-density lipoprotein cholesterol (LDL-c) are generally consistent in all subgroups ever analyzed, independent of baseline lipid profile, the presence of hypertension or diabetes mellitus and body mass index (BMI)1. Several clinical studies have reported that combination therapy with ezetimibe and statin strongly reduces LDL-c, remnant-like particle cholesterol (RLP-c) and triglycerides, and increases high-density lipoprotein cholesterol (HDL-c) in patients with metabolic syndrome or diabetes2–10. In particular, in patients with metabolic syndrome, combination therapy with ezetimibe and mild statin was significantly more effective than strong statin alone in reducing LDL-c11. It has also been reported that ezetimibe has some pleiotropic effects, such as improvement of inflammatory markers, insulin sensitivity, liver dysfunction, endothelial function and metabolic disorders12–15. However, all of these previous studies have been carried out in healthy volunteers or patients with dyslipidemia treated by statin, whereas no attention has been focused on patients with metabolic syndrome, who have not been treated with lipid-lowering drugs2–9,11,12. Therefore, we carried out an epidemiological study to elucidate the clinical effects of ezetimibe on insulin resistance and atherosclerotic markers in local-dwelling Japanese.
Materials and Methods
Participants and Study Design
In 2009, we carried out physical examinations on the inhabitants of Tanushimaru in Fukuoka (a cohort of the Seven Countries Study)16. Informed consent was obtained from all participants in accordance with the ethics committee guidelines of Kurume University. As previously reported, the demographic backgrounds of the participants in this area are similar to those of the Japanese general population17–20. We examined 1,943 people over the age of 40 years (774 men and 1,169 women), and identified 490 participants with metabolic syndrome. After excluding participants aged over 76 years (n = 125), we further excluded patients treated with lipid-lowering drugs (n = 99) from the present study. We invited those patients with metabolic syndrome (n = 266) who were not being treated with lipid-lowering drugs to participate in the study. Among them, 141 participants visited us for a detailed explanation of the protocol of this study. A total of 76 people declined our proposal, and in the end, 65 participants (43 men and 22 women) were enrolled in the present study. Four participants later dropped out, leaving a total of 61 participants (41 men and 20 women) available for analysis in the present study (Figure1).
Figure 1.
Flow chart of enrolled participants.
This was a single-arm interventional study without a control group. The enrolled participants were treated with 10 mg ezetimibe once a day for 24 weeks. Standard diet and exercise therapy for dyslipidemia were also recommended during the study period. Height and weight were measured, and BMI was calculated as weight (kilograms) divided by the square of height (square meters) as an index of obesity. Waist circumference was measured at the level of the umbilicus in the standing position. Blood pressure (BP) was measured in the supine position twice at 3-min intervals using an upright standard sphygmomanometer. Vigorous physical activity and smoking were avoided for at least 30 min before BP measurement. The second BP with the fifth-phase diastolic pressure was used for analysis. Carotid intima-media thickness (c-IMT) of the common carotid artery was determined by using duplex ultrasonography (Sonosite”TITAN”, ALOKA, Tokyo, Japan) with a 10-MHz transducer in the supine position. A single well-trained sonographer, who was blinded to the participants' background, recorded longitudinal B-mode images at the diastolic phase of the cardiac cycle. The images were magnified and printed with a high-resolution line recorder (LSR-100A; Toshiba, Tochigi, Japan). The c-IMT defined by Pignoli et al.21,22 was measured as the distance from the leading edge of the first echogenic line to the leading edge of the second echogenic line. The first line represented the lumen–intimal interface; the collagen-containing upper layer of the tunica adventitia formed the second line. At each longitudinal projection, the site of the greatest thickness, including plaque, was sought along the arterial walls nearest the skin and farthest from the skin from the common carotid artery to the internal carotid artery. Three determinations of c-IMT were carried out at the site of the greatest thickness and at two other points, 1 cm upstream and 1 cm downstream from this site. These three determinations were averaged. The greatest value among the six averaged IMTs (3 from the left and 3 from the right) was used as the representative value for each individual. Measurements of c-IMT were made twice in pre- and post-examinations. BMI, waist and BP were measured once a month.
Blood was drawn from the antecubital vein in the morning after a 12-h fast for determinations of lipids profiles (LDL-c, triglycerides, HDL-c, non-HDL-c and RLP-c), free fatty acid (FFA), fasting plasma glucose (FPG), fasting immune-reactive insulin (IRI), hemoglobin A1c (HbA1c [National Glycohemoglobin Standardization Program]), blood urea nitrogen (BUN), creatinine and uric acid. Fasting blood samples were centrifuged within 1 h after collection. Serum RLP-c was measured by immune-separation technique (using an immune-affinity gel containing monoclonal antibodies to human apolipoprotein [apo] B-100 and apo A-1)23. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as FPG (mg/dL) × fasting IRI (μU/mL)/405 and used as a marker of insulin resistance24. High-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) were measured as inflammatory markers. Asparatate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (γ-GTP) were measured as markers of liver dysfunction, and creatine phosphokinase (CPK) was measured as a marker of ezetimibe side-effects. All of the blood tests were measured in a commercially available laboratory (The Kyodo Igaku Laboratory, Fukuoka, Japan).
According to the new definition by the Japanese Committee for the Diagnostic Criteria of metabolic syndrome in April 2005, we defined metabolic syndrome as the presence of two or more abnormalities in addition to waist circumference (≥85 cm in men and ≥90 cm in women)25,26. Other abnormalities examined were dyslipidemia, hypertension and glucose intolerance/diabetes mellitus. Dyslipidemia in metabolic syndrome was defined as plasma triglycerides ≥150 mg/dL, or HDL <40 mg/dL in men or 50 mg/dL in women. Glucose intolerance/diabetes mellitus was diagnosed by the use of antidiabetic drugs and/or fasting glucose ≥110 mg/dL. Hypertension was diagnosed by the use of antihypertensive drugs and/or systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg.
Statistical Analysis
Results are presented as the mean ± standard deviation. Variables that were not normally distributed and/or showed homogeneity of variances were analyzed by the Mann–Whitney test for independent samples. Normally distributed variables were analyzed using a paired t-test. To determine factors influencing anthropometric and laboratory findings before and after ezetimibe treatments, paired t-test was carried out in all participants, and then the association was analyzed separately in men and women. The association between the reduction of HOMA-IR and metabolic components was tested using multiple regression analysis. P-values <0.05 were considered statistically significant. All statistical analyses were carried out using sas version 9.3 (SAS Inc., Cary, NC, USA).
Results
The 61 participants consisted of 41 men and 20 women with a mean age of 63.7 ± 8.1 years in men and 63.3 ± 9.6 years in women (Table1). The mean BMI was 27.2 ± 2.7 kg/m2 in men and 29.0 ± 4.4 kg/m2 in women. The mean waist circumference was 95.0 ± 6.4 cm in men and 101.6 ± 10.5 cm in women. The mean LDL-c was 128.1 ± 36.2 mg/dL in men and 136.8 ± 35.1 mg/dL in women, respectively.
Table 1.
Characteristics of study participants
| Men (n = 41) | Women (n = 20) | |
|---|---|---|
| Age (years) | 63.7 ± 8.1 | 63.3 ± 9.6 |
| BMI (kg/m2) | 27.2 ± 2.7 | 29.0 ± 4.4 |
| Waist circumference (cm) | 95.0 ± 6.4 | 101.6 ± 10.5 |
| Systolic blood pressure (mmHg) | 138.1 ± 12.9 | 133.3 ± 14.9 |
| Diastolic blood pressure (mmHg) | 87.3 ± 10.4 | 80.9 ± 8.6 |
| Triglycerides (mg/dL) | 191.4 ± 96.1 | 169.4 ± 87.3 |
| HDL-cholesterol (mg/dL) | 46.5 ± 10.5 | 57.4 ± 18.6 |
| Non-HDL-cholesterol (mg/dL) | 185.5 ± 95.6 | 208.8 ± 153.7 |
| LDL-cholesterol (mg/dL) | 128.1 ± 36.2 | 136.8 ± 35.1 |
| Fasting plasma glucose (mg/dL) | 108.5 ± 28.5 | 100.4 ± 14.7 |
| Hemoglobin Alc (%) | 5.7 ± 1.1 | 5.7 ± 0.4 |
| Insulin (uU/mL)* | 15.6 (2.7-119.2) | 11.8 (2.6–49.9) |
| HOMA-IR* | 6.0 (0.68–40.2) | 3.0 (0.7–10.2) |
| Medication for hypertension | 20 (48.8%) | 2 (4.9%) |
| Medication for diabetes mellitus | 13 (65.0%) | 2 (10.0%) |
Data are means ± standard deviation, geometric mean and range. These variables are shown in the original scale after analysis using log (natural)-transformed values.
BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment insulin resistance; LDL, low-density lipoprotein.
Bodyweight (P < 0.001), BMI (P < 0.001), systolic (P = 0.003) and diastolic (P < 0.001) BPs were significantly decreased in all participants after 24 weeks of ezetimibe treatment (Table2). Serum levels of triglycerides (P = 0.002), non-HDL-c (P = 0.001), LDL-c (P < 0.001) and HOMA-IR (P = 0.011) also decreased significantly after the observational period (Table3).
Table 2.
Anthropometric data before and after ezetimibe treatments
| Baseline | 24 weeks | P-value | |
|---|---|---|---|
| Body weight (kg) | 73.0 ± 10.4 | 71.4 ± 10.2 | <0.001 |
| BMI (kg/m2) | 27.8 ± 3.4 | 27.3 ± 3.4 | <0.001 |
| Waist circumference (cm) | 97.1 ± 8.5 | 95.8 ± 7.8 | 0.076 |
| Systolic blood pressure (mmHg) | 136.5 ± 13.6 | 130.8 ± 12.1 | 0.003 |
| Diastolic blood pressure (mmHg) | 85.2 ± 10.2 | 77.1 ± 9.3 | <0.001 |
| Carotid IMT (left) | 0.75 ± 0.17 | 0.77 ± 0.13 | 0.085 |
| Carotid IMT (right) | 0.74 ± 0.15 | 0.75 ± 0.16 | 0.244 |
BMI, body mass index; IMT, intimal-media thickness.
Table 3.
Laboratory findings before and after ezetimibe treatments
| Baseline | 24 weeks | P-value (95% CI) | |
|---|---|---|---|
| AST (IU/L) | 25.0 ± 11.3 | 26.8 ± 16.7 | 0.350 |
| ALT (IU/L) | 26.1 ± 14.7 | 26.8 ± 14.9 | 0.501 |
| γ-GTP (IU/L) | 54.9 ± 77.6 | 46.9 ± 63.9 | 0.054 |
| CPK (IU/L) | 143.5 ± 69.1 | 138.2 ± 81.9 | 0.726 |
| BUN (mg/dL) | 16.6 ± 5.0 | 17.3 ± 5.1 | 0.323 |
| Creatinine (mg/dL) | 0.81 ± 0.25 | 0.81 ± 0.26 | 0.324 |
| Uric acid (mg/dL) | 5.94 ± 1.46 | 5.95 ± 1.75 | 0.801 |
| Triglycerides (mg/dL)* | 188.7 (52–376) | 155.6 (54–777) | 0.002 (1.05 to 61.57) |
| HDL-cholesterol (mg/dL) | 50.0 ± 14.4 | 51.2 ± 15.6 | 0.444 |
| Non HDL-cholesterol (mg/dL) | 165.5 ± 37.1 | 134.8 ± 31.9 | 0.001 |
| LDL-cholesterol (mg/dL) | 131.8 ± 33.4 | 105.7 ± 28.4 | <0.001 |
| Fasting plasma glucose (mg/dL) | 105.3 ± 25.4 | 108.1 + 28.2 | 0.506 |
| Hemoglobin Alc (%) | 5.77 ± 0.61 | 5.69 ± 0.79 | 0.651 |
| Insulin (μU/mL)* | 14.5 (2.6–119.2) | 12.0 (2.4–270) | 0.211 (−1.87 to 7.67) |
| HOMA-IR* | 4.21 (0.65–40.2) | 3.08 (0.53–14.2) | 0.011 (−0.84 to 3.42) |
| White blood cell count (×102/mm3) | 6118 ± 1430 | 5710 ± 1316 | 0.403 |
| Hemoglobin (g/dL) | 14.3 ± 1.6 | 14.4 ± 1.7 | 0.324 |
| Platelet (×104/mm3) | 22.7 ± 4.8 | 22.8 ± 5.3 | 0.540 |
| High sensitivity CRP (mg/dL)* | 0.13 (0.011–1.200) | 0.11 (0.010–1.000) | 0.263 (−0.04 to 0.78) |
| RLP-c (mg/dL)* | 6.06 (1.6–18.9) | 7.52 (1.9–45.3) | 0.529 (−2.12 to 2.82) |
| Free fatty acid (mEq/dL) | 0.55 ± 0.28 | 0.47 ± 0.23 | 0.259 |
Data are means ± standard deviation, geometric mean and range. These variables are shown in the original scale after analysis using log (natural)-transformed values.
γ-GTP, γ-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, asparatate aminotransferase; BUN, blood urea nitrogen; CI, confidence interval; CPK, creatine phosphokinase; CRP, C-reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; RLP-c, remnant-like particle cholesterol.
In men (n = 41), the anthropometric data showed that ezetimibe treatment significantly decreased systolic (P = 0.001) and diastolic (P < 0.001) BPs (Table4). Although bodyweight, BMI or waist circumference were variable before and after the observational period, the treatment significantly decreased serum levels of triglycerides (P = 0.006), non-HDL-c (P = 0.002), LDL-c (P = 0.002), IRI (P = 0.002) and HOMA-IR (P = 0.001; Table5).
Table 4.
Anthropometric data before and after ezetimibe treatments in men
| Baseline | 24 weeks | P-value | |
|---|---|---|---|
| Body weight (kg) | 74.8 ± 7.9 | 73.0 ± 7.7 | 0.150 |
| BMI (kg/m2) | 27.2 ± 2.7 | 26.7 ± 2.5 | 0.193 |
| Waist circumference (cm) | 95.0 ± 6.4 | 94.0 ± 6.3 | 0.239 |
| Systolic blood pressure (mmHg) | 138.1 ± 12.9 | 130.7 ± 15.2 | 0.001 |
| Diastolic blood pressure (mmHg) | 87.3 ± 10.4 | 79.0 ± 8.9 | 0.001 |
| Carotid IMT (left) | 0.75 ± 0.17 | 0.78 ± 0.13 | 0.186 |
| Carotid IMT (right) | 0.74 ± 0.16 | 0.78 ± 0.18 | 0.145 |
BMI, body mass index; IMT, intimal-media thickness.
Table 5.
Laboratory findings before and after ezetimibe treatments in men
| Baseline | 24 weeks | P-value (95% CI) | |
|---|---|---|---|
| AST (IU/L) | 26.4 ± 12.9 | 25.8 ± 9.2 | 0.405 |
| ALT (IU/L) | 27.9 ± 16.2 | 27.5 ± 13.7 | 0.452 |
| γ-GTP (IU/L) | 68.2 ± 90.6 | 58.2 ± 75.6 | 0.294 |
| CPK (IU/L) | 151.6 ± 74.3 | 148.4 ± 87.1 | 0.429 |
| BUN (mg/dL) | 16.3 ± 4.9 | 17.1 ± 4.5 | 0.222 |
| Creatinine (mg/dL) | 0.88 ± 0.25 | 0.90 ± 0.26 | 0.362 |
| Uric acid (mg/dL) | 6.35 ± 1.22 | 6.48 ± 1.55 | 0.337 |
| Triglycerides (mg/dL)* | 172.4 (52–376) | 143.9 (56–777) | 0.006 (−14.4 to 65.4) |
| HDL-cholesterol (mg/dL) | 46.5 ± 10.5 | 47.3 + 11.5 | 0.372 |
| Non HDL-cholesterol (mg/dL) | 185.5 ± 95.6 | 139.3 ± 30.7 | 0.002 |
| LDL-cholesterol (mg/dL) | 128.1 ± 36.2 | 106.0 ± 29.8 | 0.002 |
| Fasting plasma glucose (mg/dL) | 108.5 ± 28.5 | 106.4 ± 26.7 | 0.366 |
| Hemoglobin Alc (%) | 5.7 ± 1.1 | 5.6 ± 0.7 | 0.312 |
| Insulin (μU/mL)* | 15.6 (2.7–119.2) | 7.8 (2.4–49.4) | 0.002 (−3.27 to 9.52) |
| HOMA-IR* | 6.00 (0.68–40.2) | 2.14 (0.53–14.2) | 0.001 (−1.43 to 5.02) |
| White blood cell count (×102/mm3) | 6358 ± 1438 | 5966 ± 1185 | 0.091 |
| Hemoglobin (g/dL) | 15.0 ± 1.4 | 15.0 ± 1.6 | 0.500 |
| Platelet (×104/mm3) | 22.3 ± 4.1 | 22.8 ± 4.6 | 0.302 |
| High sensitivity CRP (mg/dL)* | 0.09 (0.011–1.200) | 0.07 (0.010–1.000) | 0.132 (−0.05 to 0.12) |
| RLP-c (mg/dL)* | 5.15 (1.6–18.9) | 6.11 (1.9–45.3) | 0.376 (−3.12 to 3.42) |
| Free fatty acid (mEq/L) | 0.53 ± 0.28 | 0.47 ± 0.23 | 0.146 |
Data are means ± standard deviation, geometric mean and range. These variables are shown in the original scale after analysis using log (natural)-transformed values.
γ-GTP, γ-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, asparatate aminotransferase; BUN, blood urea nitrogen; CI, confidence interval; CPK, creatine phosphokinase; CRP, C-reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; RLP-c, remnant-like particle cholesterol.
In women (n = 20), the anthropometric data showed that ezetimibe treatment significantly decreased only diastolic BP (P = 0.005); Table6). Although bodyweight, BMI or waist circumference were variable before and after the observational period, the treatment significantly decreased serum levels of triglycerides (P = 0.038), non-HDL-c (P = 0.005), LDL-c (P = 0.001) and HOMA-IR (P = 0.047; Table7). There were no statistically significant differences between men and women in the effects of ezetimibe (data not shown).
Table 6.
Anthropometric data before and after ezetimibe treatments in women
| Baseline | 24 weeks | P-value | |
|---|---|---|---|
| Body weight (kg) | 69.5 ± 13.7 | 68.3 ± 13.6 | 0.391 |
| BMI (kg/m2) | 29.0 ± 4.4 | 28.5 ± 4.7 | 0.365 |
| Waist circumference (cm) | 101.6 ± 10.5 | 99.6 ± 9.6 | 0.267 |
| Systolic blood pressure (mmHg) | 133.3 ± 14.9 | 129.2 ± 11.7 | 0.170 |
| Diastolic blood pressure (mmHg) | 80.9 ± 8.6 | 73.4 ± 9.0 | 0.005 |
| Carotid IMT (left) | 0.75 ± 0.17 | 0.75 ± 0.12 | 0.500 |
| Carotid IMT (right) | 0.72 ± 0.14 | 0.69 ± 0.10 | 0.220 |
BMI, body mass index; IMT, intimal-media thickness.
Table 7.
Laboratory findings before and after ezetimibe treatments in women
| Baseline | 24 weeks | P-value (95% CI) | |
|---|---|---|---|
| AST (IU/L) | 21.9 ± 6.6 | 28.8 ± 25.9 | 0.128 |
| ALT (IU/L) | 22.6 ± 10.5 | 25.4 ± 17.2 | 0.269 |
| γ-GTP (IU/L) | 27.6 ± 23.5 | 25.0 ± 16.0 | 0.348 |
| CPK (IU/L) | 124.5 ± 52.0 | 118.4 ± 68.4 | 0.376 |
| BUN (mg/dL) | 17.3 ± 5.0 | 17.7 ± 6.3 | 0.413 |
| Creatinine (mg/dL) | 0.67 ± 0.19 | 0.63 ± 0.15 | 0.232 |
| Uric acid (mg/dL) | 5.11 ± 1.57 | 4.93 ± 1.69 | 0.365 |
| Triglycerides (mg/dL)* | 149.9 (64–371) | 108.9 (54–443) | 0.038 (−4.19 to 90.71) |
| HDL-cholesterol (mg/dL) | 57.4 ± 18.6 | 58.9 ± 19.6 | 0.403 |
| Non HDL-cholesterol (mg/dL) | 159.8 ± 43.7 | 128.2 ± 27.4 | 0.005 |
| LDL-cholesterol (mg/dL) | 136.8 ± 35.1 | 105.1 ± 26.4 | 0.001 |
| Fasting plasma glucose (mg/dL) | 100.4 ± 14.7 | 111.4 + 31.2 | 0.081 |
| Hemoglobin Alc (%) | 5.7 ± 0.4 | 5.6 ± 1.0 | 0.340 |
| Insulin (μU/mL)* | 11.8 (2.6–49.9) | 9.2 (3.5–110) | 0.140 (−4.59 to 9.41) |
| HOMA-IR* | 3.01 (0.7–10.2) | 2.11 (0.87–7.22) | 0.047 (−1.14–1.84) |
| White blood cell count (×102/mm3) | 5628 ± 1316 | 5213 ± 1444 | 0.174 |
| Hemoglobin (g/dL) | 13.1 ± 1.0 | 13.0 ± 1.0 | 0.377 |
| Platelet (×104/mm3) | 23.7 ± 6.1 | 22.9 ± 6.5 | 0.345 |
| High sensitivity CRP (mg/dL)* | 0.14 (0.023–0.194) | 0.13 (0.014–0.495) | 0.356 (−0.07 to 0.39) |
| RLP-c (mg/dL)* | 7.12 (2.1–14.7) | 7.47 (1.9–22.3) | 0.421 (−4.11 to 5.87) |
| Free fatty acid (mEq/L) | 0.58 ± 0.29 | 0.49 ± 0.23 | 0.142 |
Data are means ± standard deviation, geometric mean and range. These variables are shown in the original scale after analysis using log (natural)-transformed values.
γ-GTP, γ-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, asparatate aminotransferase; BUN, blood urea nitrogen; CI, confidence interval; CPK, creatine phosphokinase; CRP, C-reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; RLP-c, remnant-like particle cholesterol.
When the patients were divided into high- and low-HOMA-IR groups using the median of HOMA-IR, the high-HOMA-IR group (n = 30) showed a dramatically significant decrease (P = 0.007) of HOMA-IR, whereas the low-HOMA-IR group (n = 31) showed a mild, but significant, increase (P = 0.033; Figure2). Univariate regression analysis showed that BMI, waist circumference, systolic and diastolic BPs, LDL-c, triglycerides, HDL-c, and non-HDL-c were not significantly associated with the reduction of HOMA-IR (Table8). In the high HOMA-IR group, there were also no significant associations (Table9). Furthermore, none of the metabolic components (hypertension, dyslipidemia and diabetes mellitus) affected the reduction of HOMA-IR (Table10). The characteristics of the high-HOMA-IR group are shown in Table11. BMI (P = 0.002), diastolic BP (<0.001), triglycerides (P = 0.024), non-HDL-c (P < 0.001) and LDL-c (P < 0.001) were significantly decreased after the observational period.
Figure 2.
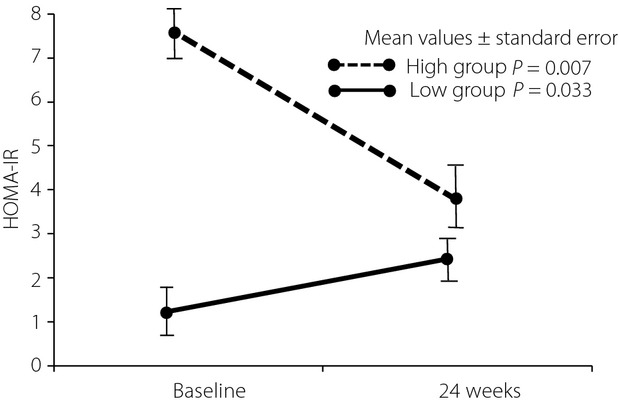
Differential improvement of homeostasis model assessment of insulin resistance (HOMA-IR) between high- and low-HOMA-IR groups. When the patients are divided into high- and low-HOMA-IR groups by using the median of HOMA-IR, the high group (n = 30) shows a dramatically significant decrease of HOMA-IR, whereas the low group (n = 31) shows a more modest, but still significant, increase of HOMA-IR.
Table 8.
Univariate regression analysis for the reduction of homeostasis model assessment of insulin resistance
| Metabolic components | β | SE | P-value |
|---|---|---|---|
| BMI (kg/m2) | 1.279 | 1.075 | 0.240 |
| Waist (cm) | 0.281 | 0.231 | 0.229 |
| Systolic blood pressure (mmHg) | −0.032 | 0.071 | 0.648 |
| Diastolic blood pressure (mmHg) | 0.039 | 0.095 | 0.683 |
| LDL-cholesterol (mg/dL) | −0.046 | 0.051 | 0.375 |
| Triglycerides (mg/dL)* | 0.007 | 0.009 | 0.454 |
| HDL-cholesterol (mg/dL) | −0.144 | 0.113 | 0.209 |
| Non HDL-cholesterol (mg/dL) | −0.026 | 0.045 | 0.563 |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
These variables are shown in the original scale after analysis using log (natural)-transformed values.
Table 9.
Univariate regression analysis for the reduction of homeostasis model assessment of insulin resistance in the high homeostasis model assessment of insulin resistance group
| Metabolic components | β | SE | P-value |
|---|---|---|---|
| BMI (kg/m2) | 1.753 | 2.192 | 0.433 |
| Waist (cm) | 0.421 | 0.416 | 0.322 |
| Systolic blood pressure (mmHg) | −0.001 | 0.128 | 0.998 |
| Diastolic blood pressure (mmHg) | 0.095 | 0.202 | 0.643 |
| LDL-cholesterol (mg/dL) | −0.059 | 0.094 | 0.535 |
| Triglycerides (mg/dL)* | 0.009 | 0.014 | 0.536 |
| HDL-cholesterol (mg/dL) | −0.361 | 0.262 | 0.182 |
| Non HDL-cholesterol (mg/dL) | −0.051 | 0.077 | 0.510 |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
These variables are shown in the original scale after analysis using log (natural)-transformed values.
Table 10.
Difference of metabolic factors before and after ezetimibe treatments for the reduction of homeostasis model assessment of insulin resistance
| Metabolic factors | HOMA-IR at baseline | HOMA-IR at 24 weeks | P-value |
|---|---|---|---|
| Hypertension (n = 57) | 2.10 | 2.11 | 0.879 |
| Dyslipidemia (n = 37) | 2.46 | 2.33 | 0.556 |
| Diabetes mellitus (n = 21) | 4.04 | 3.42 | 0.622 |
HOMA-IR, homeostasis model assessment of insulin resistance.
Table 11.
Changes of body mass index, blood pressure and lipids profiles before and after ezetimibe treatments in high homeostasis model assessment of insulin resistance group
| Metabolic factors | Baseline | 24 weeks | P-value |
|---|---|---|---|
| BMI (kg/m2) | 28.5 ± 3.4 | 27.8 ± 3.7 | 0.002 |
| Systolic blood pressure (mmHg) | 135.8 ± 14.6 | 132.9 ± 13.2 | 0.378 |
| Diastolic blood pressure (mmHg) | 86.8 ± 9.7 | 76.8 ± 9.5 | <0.001 |
| Triglycerides (mg/dL)* | 187.9 (68–371) | 140.5 (54–777) | 0.024 |
| HDL-cholesterol (mg/dL) | 46.5 ± 9.9 | 48.5 ± 10.7 | 0.193 |
| Non-HDL-cholesterol (mg/dL) | 166.6 ± 32.3 | 137.5 ± 32.7 | <0.001 |
| LDL-cholesterol (mg/dL) | 127.1 ± 26.9 | 104.7 ± 28.5 | <0.001 |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Data are means ± SD, geometric mean and range. These variables are shown in the original scale after analysis using log (natural)-transformed values.
Discussion
The major novel findings of the present study are that 24 weeks of ezetimibe treatment combined with standard diet and exercise therapy improved not only bodyweight and atherogenic lipid profiles, but also insulin resistance, blood pressure and anthropometric factors in local-dwelling Japanese with metabolic syndrome who were not being treated with lipid-lowering drugs, and that the improvement of HOMA-IR was not associated with that of other metabolic components, suggesting that ezetimibe treatment combined with standard diet and exercise therapy independently improved insulin resistance in this study population. To the best of our knowledge, this is the first report to examine the effects of ezetimibe on insulin resistance in patients with metabolic syndrome who were not being treated with lipid-lowering drugs.
Many investigators have shown that ezetimibe ameliorated atherogenic lipid profiles3–8,10–12; however, there are only a few reports regarding anthropometric data, and the results were inconsistent13,14,27. Although it has been shown that ezetimibe did not significantly change bodyweight, waist circumference and BMI13,27, Yagi et al.14 have shown that ezetimibe treatment significantly reduced bodyweight, BMI, waist circumference and blood pressure, as also shown in the present study. It has been previously shown that ezetimibe markedly reduced visceral fat assessed by abdominal computed tomography scan28, suggesting that ezetimibe might play a unique role in some specific populations, such as those with metabolic syndrome14,27,29.
Our present study showed that ezetimibe combined with standard diet and exercise therapy also ameliorated insulin resistance in addition to lipid profiles. This has been previously demonstrated in an experimental study, which indicated that ezetimibe improved insulin resistance in Zucker fatty rats, a model of obesity30. It has also been reported that combination therapy with low-dose statin (pravastatin) and ezetimibe improved insulin resistance markedly better than high-dose pravastatin monotherapy31, which suggested that the combined lipid-lowering therapy might be more favorable in high-risk patients with dyslipidemia and glucose intolerance. Furthermore, it has been demonstrated that ezetimibe significantly reduced the fasting insulin level (–12.8% reduction) and HbA1c (–3.4% reduction)27; however, results have been inconsistent. Kikuchi et al.32 reported that ezetimibe restored the postprandial dysregulation of lipid, but did not affect glucose metabolism in a double-blind randomized cross-over trial.
As it has been reported that statin therapy increased the risk of diabetes33, it is important to consider whether monotherapy (statin only) or combined therapy (ezetimibe plus statin) has a more favorable effect in patients with dyslipidemia complicated with glucose intolerance, as in metabolic syndrome with regard to insulin resistance. As shown in the present study, ezetimibe is a prime candidate for use in patients with metabolic syndrome and underlying insulin resistance.
It has been demonstrated that ezetimibe monotherapy ameliorates vascular function in patients with hypercholesterolemia through decreasing oxidative stress34, and that sex hormone concentrations are inversely associated with markers of inflammation and oxidative stress in men35. In the present study, IRI levels and HOMA-IR are higher in men than in women. We found no statistically significant sex differences in the effects of ezetimibe in anthropometric and laboratory data, probably because of the limited number of enrolled participants. Future studies with large numbers of patients are required to clarify any sex differences in the effects of ezetimibe, particularly with respect to sex hormone concentrations and oxidative stress.
In the present study, we confirmed that ezetimibe improves insulin resistance by reducing IRI and HOMA-IR in men and HOMA-IR in women. In particular, HOMA-IR was dramatically and significantly decreased in the high HOMA-IR group, as shown in Figure2. Furthermore, none of the metabolic components affected the reduction of HOMA-IR (Table10). In the present study population, we also recommended the standard diet and exercise therapies. To exclude the effects of the standard diet and exercise therapies, we examined the additional data in our other cohort. We enrolled 60 participants, who received health check-up examinations in our cohort carried out during 2001 and 2003 in Uku town in Japan, who took no medicine and who were recommended to follow standard diet and exercise therapies. Their HOMA-IR was 1.21 ± 0.84 at baseline in 2001 and 1.31 ± 0.87 at 2-year follow up in 2003. In this added data, there was no significant change in the insulin resistance (P = 0.272), suggesting that standard diet and exercise therapies have no significant effects on insulin resistance. However, the relationship between ezetimibe and the improvement of insulin resistance is still controversial. One of the potential mechanisms by which ezetimibe is able to improve insulin resistance is to inhibit the absorption of oxidized cholesterol36. Another potential mechanism could be the improvement of postprandial hyperlipidemia, because visceral fat cells store triglycerides under conditions of excessive calorie intake, and release free fatty acids and triglycerides after lipolysis under exercise and/or fasting conditions, which is able to affect insulin resistance23. It has been reported that ezetimibe combined with statin therapy improved insulin resistance, although the effect was not correlated with a decrease in the LDL-c level14, and that the effect of ezetimibe on the improvement of insulin resistance was shown only in ezetimibe monotherapy, but not in combined therapy with statin13. Taken together, ezetimibe monotherapy rather than combined therapy might be favorable to reduce LDL-c or non-HDL-c in regard to glucose intolerance, especially in patients with higher levels of HOMA-IR.
Several limitations should be mentioned for the present study. First, our study was a single-arm interventional study without a control group. Without a control group, the effect observed cannot be totally attributed to the intervention alone. There might have been a placebo or Hawthorne effect and regression to the mean. Second, the present study was carried out in Japan, where the incidence of obesity is low compared with Caucasians. Third, the number of attendants and the period of follow up might have been insufficient to elucidate the role of ezetimibe. Fourth, in the low-HOMA-IR group, the inhibition of the absorption of intra-intestinal lipid might not work in insulin resistance. The mechanisms are not still clear. Fifth, in the present study, there was no significant change in IMT by ezetimibe treatment, probably because of the limited number of participants with a short observational period. Sixth, high FPG overestimates HOMA-IR calculation, which might affect the results in the present study. Nevertheless, the pleiotropic effect of the monotherapy treatment is striking, and deserves further investigation. Additional studies of ezetimibe with a large sample size examining predictive measures of major atherosclerotic diseases are required37.
In conclusion, ezetimibe combined with standard diet and exercise therapy improves not only bodyweight and atherogenic lipid profiles, but also insulin resistance, blood pressure and anthropometric factors in metabolic syndrome in local-dwelling Japanese. Interestingly, the improvement of insulin resistance has no correlation with that of other metabolic components.
Acknowledgments
We are grateful to the members of the Japan Medical Association of Ukiha, the elected officials and residents of Tanushimaru, and the team of cooperating physicians for their help in carrying out the health examinations. This study was supported in part by the Kimura Memorial Heart Foundation (Fukuoka, Japan) and by Bayer Pharmaceutical Co., Ltd. We also certify that there are no financial or other relationships that could lead to a conflict of interest.
References
- Knopp RH, Dujovne CA, Le Beaut A, et al. Ezetimibe Study Group. Evaluation of efficacy, safety, and tolerability of ezetimibe in primary hypercholesterolaemia: a pooled analysis from two controlled phase III clinical studies. Int J Clin Pract. 2003;57:363–368. [PubMed] [Google Scholar]
- Denke M, Pearson T, Mcbride P, et al. Ezetimibe added to ongoing statin therapy improves LDL-C goal attainment and lipid profile in patients with diabetes or metabolic syndrome. Diab Vasc Dis Res. 2006;3:93–102. doi: 10.3132/dvdr.2006.020. [DOI] [PubMed] [Google Scholar]
- Hajer GR, Dallinga-Thie GM, van Vark-van der Zee LC, et al. The effect of statin alone or in combination with ezetimibe on postprandial lipoprotein composition in obese metabolic syndrome patients. Atherosclerosis. 2009;202:216–224. doi: 10.1016/j.atherosclerosis.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Winkler K, Schewe T, Pütz G, et al. Fluvastatin/fenofibrate vs. simvastatin/ezetimibe in patients with metabolic syndrome: different effects on LDL-profiles. Eur J Clin Invest. 2009;39:463–470. doi: 10.1111/j.1365-2362.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Ballantyne CM, Grundy SM, et al. Lipid-altering efficacy and safety of ezetimibe/simvastatin versus atorvastatin in patients with hypercholesterolemia and the metabolic syndrome (from the VYMET Study) Am J Cardiol. 2009;103:1694–1702. doi: 10.1016/j.amjcard.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Guyton JR, Mazzone T, et al. Relationships between metabolic syndrome and other baseline factors and the efficacy of ezetimibe/simvastatin and atorvastatin in patients with type 2 diabetes and hypercholesterolemia. Diabetes Care. 2010;33:1021–1024. doi: 10.2337/dc09-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conard S, Bays H, Leiter LA, et al. Ezetimibe added to atorvastatin compared with doubling the atorvastatin dose in patients at high risk for coronary heart disease with diabetes mellitus, metabolic syndrome or neither. Diabetes Obes Metab. 2010;12:210–218. doi: 10.1111/j.1463-1326.2009.01152.x. [DOI] [PubMed] [Google Scholar]
- Miller M, DiNicolantonio JJ, Can M, et al. The effects of ezetimibe/simvastatin versus simvastatin monotherapy on platelet and inflammatory biomarkers in patients with metabolic syndrome. Cardiology. 2013;125:74–77. doi: 10.1159/000347134. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Jimenez JG, Pirags V, et al. A comparison of efficacy and safety of an ezetimibe/simvastatin combination compared with other intensified lipid-lowering treatment strategies in diabetic patients with symptomatic cardiovascular disease. Diab Vasc Dis Res. 2013;10:277–286. doi: 10.1177/1479164112465212. [DOI] [PubMed] [Google Scholar]
- Bays HE, Shah A, Macdonell G, et al. Effects of coadministered ezetimibe plus fenofibrate in mixed dyslipidemic patients with metabolic syndrome. Metab Syndr Relat Disord. 2011;9:135–142. doi: 10.1089/met.2010.0068. [DOI] [PubMed] [Google Scholar]
- Averna M, Missault L, Vaverkova H, et al. Lipid-altering efficacy of switching to ezetimibe/simvastatin 10/20 mg versus rosuvastatin 10 mg in high-risk patients with and without metabolic syndrome. Diab Vasc Dis Res. 2011;8:262–270. doi: 10.1177/1479164111418136. [DOI] [PubMed] [Google Scholar]
- Ichimori S, Shimoda S, Goto R, et al. Ezetimibe improves glucose metabolism by ameliorating hepatic function in Japanese patients with type 2 diabetes. J Diabetes Invest. 2012;3:179–184. doi: 10.1111/j.2040-1124.2011.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki N, Ueno H, Morinaga Y, et al. Ezetimibe ameliorates atherosclerotic and inflammatory markers, atherogenic lipid profiles, insulin sensitivity, and liver dysfunction in Japanese patients with hypercholesterolemia. J Atheroscler Thromb. 2012;19:532–538. doi: 10.5551/jat.10835. [DOI] [PubMed] [Google Scholar]
- Yagi S, Akaike M, Aihara KI, et al. Ezetimibe ameliorates metabolic disorders and microalbuminuria in patients with hypercholesterolemia. J Atheroscler Thromb. 2010;17:173–180. doi: 10.5551/jat.2378. [DOI] [PubMed] [Google Scholar]
- Nochioka K, Tanaka S, Miura M, et al. Ezetimibe improves endothelial function and inhibits Rho-kinase activity associated with inhibition of cholesterol absorption in humans. Circ J. 2012;76:2023–2030. doi: 10.1253/circj.cj-12-0331. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Geleijnse JM, Adachi H, et al. Systolic blood pressure predicts cardiovascular mortality in a farming but not in a fishing community. - A 40-year follow up of the Japanese cohorts of the Seven Countries Study - Circ J. 2011;75:1890–1896. doi: 10.1253/circj.cj-10-0971. [DOI] [PubMed] [Google Scholar]
- Hino A, Adachi H, Toyomasu K, et al. Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis. 2004;176:145–149. doi: 10.1016/j.atherosclerosis.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Kumagai E, Adachi H, Jacobs DR, Jr, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58:1043–1048. doi: 10.1161/HYPERTENSIONAHA.111.180521. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Adachi H, Jacobs DR, Jr, et al. Serum hepatocyte growth factor and cancer mortality in an apparently healthy Japanese population. J Epidemiol. 2012;22:395–401. doi: 10.2188/jea.JE20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi K, Adachi H, Hirai Y, et al. Plasma endothelin-1 level is a predictor of 10-year mortality in a general population: the Tanushimaru study. Circ J. 2012;76:2779–2784. doi: 10.1253/circj.cj-12-0469. [DOI] [PubMed] [Google Scholar]
- Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurements with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kodama M, Nishizawa H, et al. Carotid intima-media thickness in Japanese type 2 diabetic subjects. Diabetes Care. 2000;23:1310–1315. doi: 10.2337/diacare.23.9.1310. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Saito T, Tamura A, et al. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta. 1993;223:53–71. doi: 10.1016/0009-8981(93)90062-9. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Magliano D, Matsuzawa Y, et al. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Metabolic syndrome – Definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12:301. doi: 10.5551/jat.12.301. [DOI] [PubMed] [Google Scholar]
- Hiramitsu S, Ishiguro Y, Matsuyama H, et al. The effects of ezetimibe on surrogate markers of cholesterol absorption and synthesis in Japanese patients with dyslipidemia. J Atheroscler Thromb. 2010;17:106–114. doi: 10.5551/jat.1578. [DOI] [PubMed] [Google Scholar]
- Takase H, Dohi Y, Okado T, et al. Effects of ezetimibe on visceral fat in the metabolic syndrome: a randomized controlled study. Eur J Clin Invest. 2012;42:1287–1294. doi: 10.1111/eci.12000. [DOI] [PubMed] [Google Scholar]
- Kotani K, Sakane N, Taniguchi N. Effect of ezetimibe on remnant-like particle cholesterol in subjects with metabolic syndrome. Med Princ Pract. 2012;21:134–138. doi: 10.1159/000332436. [DOI] [PubMed] [Google Scholar]
- Deushi M, Nomura M, Kawakami A, et al. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581:5664–5670. doi: 10.1016/j.febslet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Dagli N, Yavuzkir M, Karaca I. The effects of high dose pravastatin and low dose pravastatin and ezetimibe combination therapy on lipid, glucose metabolism and inflammation. Inflammation. 2007;30:230–235. doi: 10.1007/s10753-007-9041-3. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Nezu U, Inazumi K, et al. Double-blind randomized clinical trial of the effects of ezetimibe on postprandial hyperlipidemia and hyperglycaemia. J Atheroscler Thromb. 2012;19:1093–1101. doi: 10.5551/jat.12427. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurobe H, Aihara K, Higashida M, et al. Ezetimibe monotherapy ameliorates vascular function in patients with hypercholesterolemia through decreasing oxidative stress. J Atheroscler Thromb. 2011;18:1080–1089. doi: 10.5551/jat.9548. [DOI] [PubMed] [Google Scholar]
- Haring R, Baumeister SE, Völzke H, et al. Prospective inverse associations of sex hormone concentrations in men with biomarkers of inflammation and oxidative stress. J Androl. 2012;33:944–950. doi: 10.2164/jandrol.111.015065. [DOI] [PubMed] [Google Scholar]
- Sato K, Nakano K, Katsuki S, et al. Dietary cholesterol oxidation products accelerate plaque destabilization and rupture associated with monocyte infiltration/activation via the MCP-1-CCR2 pathway in mouse brachiocephalic arteries: therapeutic effects of Ezetimibe. J Atheroscler Thromb. 2012;19:986–998. doi: 10.5551/jat.13391. [DOI] [PubMed] [Google Scholar]
- Hayek S, Canepa Escaro F, Sattar A, et al. Effect of ezetimibe on major atherosclerotic disease events and all-cause mortality. Am J Cardiol. 2013;111:532–539. doi: 10.1016/j.amjcard.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


