Summary
Background
Antenatal corticosteroids for pregnant women at risk of preterm birth are among the most effective hospital-based interventions to reduce neonatal mortality. We aimed to assess the feasibility, effectiveness, and safety of a multifaceted intervention designed to increase the use of antenatal corticosteroids at all levels of health care in low-income and middle-income countries.
Methods
In this 18-month, cluster-randomised trial, we randomly assigned (1:1) rural and semi-urban clusters within six countries (Argentina, Guatemala, India, Kenya, Pakistan, and Zambia) to standard care or a multifaceted intervention including components to improve identification of women at risk of preterm birth and to facilitate appropriate use of antenatal corticosteroids. The primary outcome was 28-day neonatal mortality among infants less than the 5th percentile for birthweight (a proxy for preterm birth) across the clusters. Use of antenatal corticosteroids and suspected maternal infection were additional main outcomes. This trial is registered with ClinicalTrials.gov, number NCT01084096.
Findings
The ACT trial took place between October, 2011, and March, 2014 (start dates varied by site). 51 intervention clusters with 47 394 livebirths (2520 [5%] less than 5th percentile for birthweight) and 50 control clusters with 50 743 livebirths (2258 [4%] less than 5th percentile) completed follow-up. 1052 (45%) of 2327 women in intervention clusters who delivered less-than-5th-percentile infants received antenatal corticosteroids, compared with 215 (10%) of 2062 in control clusters (p<0·0001). Among the less-than-5th-percentile infants, 28-day neonatal mortality was 225 per 1000 livebirths for the intervention group and 232 per 1000 livebirths for the control group (relative risk [RR] 0·96, 95% CI 0·87–1·06, p=0·65) and suspected maternal infection was reported in 236 (10%) of 2361 women in the intervention group and 133 (6%) of 2094 in the control group (odds ratio [OR] 1·67, 1·33–2·09, p<0·0001). Among the whole population, 28-day neonatal mortality was 27·4 per 1000 livebirths for the intervention group and 23·9 per 1000 livebirths for the control group (RR 1·12, 1·02–1·22, p=0·0127) and suspected maternal infection was reported in 1207 (3%) of 48 219 women in the intervention group and 867 (2%) of 51 523 in the control group (OR 1·45, 1·33–1·58, p<0·0001).
Interpretation
Despite increased use of antenatal corticosteroids in low-birthweight infants in the intervention groups, neonatal mortality did not decrease in this group, and increased in the population overall. For every 1000 women exposed to this strategy, an excess of 3·5 neonatal deaths occurred, and the risk of maternal infection seems to have been increased.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Introduction
The use of antenatal corticosteroids for pregnant women at high risk of preterm delivery is among the most effective hospital-based interventions to reduce neonatal mortality associated with preterm birth, a leading cause of childhood mortality.1–6 A systematic review3 of 21 randomised controlled trials of antenatal corticosteroids showed a 31% relative reduction in neonatal mortality (relative risk [RR] 0·69, 95% CI 0·58–0·81) and an even larger reduction in severe neonatal morbidity. However, a non-significant increased risk of puerperal sepsis (1·35, 0·93–1·95) was noted from eight studies.3 All of the trials were done in hospitals with neonatal intensive care and respiratory support. Similar reductions in neonatal mortality were seen in trials in both high-income countries and middle-income countries (Brazil, Jordan, South Africa, and Tunisia).3
On the basis of this strong evidence, the use of antenatal corticosteroids in hospitals for women at high risk of preterm birth is widely recommended by national and international health organisations.1,6 Antenatal corticosteroids have been included in the UN list of life-saving commodities for women and children,7 and WHO has recommended dexamethasone for women at risk of preterm birth.7,8
Whereas 80% of the women at high risk of preterm birth in high-income countries currently receive antenatal corticosteroids, less than 10% of women at risk in low-income countries receive the treatment, and proportions in middle-income countries range from 30% to 50%.6,9–12 An important determinant is that less than half of births in low-income countries occur in hospitals with antenatal corticosteroids available.13,14 Although institutional delivery is increasing, access to tertiary care similar to that in hospitals in middle-income or high-income countries is poor for most women in low-income countries. Thus, to increase coverage of antenatal corticosteroids for women at risk in low-income countries, they would need to be made available in primary care facilities or through community strategies. So far, evidence for the reduction of neonatal mortality from antenatal corticosteroids comes solely from clinical trials done in hospitals with neonatal intensive care. Whether similar reductions would occur in settings, such as primary health-care clinics, in which intensive care for preterm infants might not be available and in which risk of preterm birth might be less accurately assessed, is unclear. Questions have also been raised about risks of infectious morbidity for women and their infants delivered in community settings related to the use of antenatal corticosteroids.15,16
Many barriers limit effective coverage of antenatal corticosteroids in low-income countries. Estimation of gestational age can be suboptimum in these settings because of low availability of ultrasound, frequent uncertainty about the date of last menstrual period, and inadequate training in the assessment of gestational age.15–18 Birth attendants in low-resource settings might not have the skills necessary to assess risk of preterm birth or to safely administer antenatal corticosteroids, even when authorised to do so by health authorities.18,19 Additionally, birth attendants might be unaware of antenatal corticosteroids as a treatment and health-care facilities might have poor or sporadic access to the necessary supplies.19
We aimed to assess the feasibility, effectiveness, and safety of a multifaceted intervention designed to increase the use of antenatal corticosteroids at all levels of health care. The intervention included components to improve identification of women at risk of preterm birth and to facilitate appropriate use of antenatal corticosteroids.20
Methods
Trial design and participants
The Antenatal Corticosteroids Trial (ACT) was an 18-month, two-arm, parallel, cluster-randomised trial done in geographical clusters at seven sites of the Global Network for Women's and Children's Health Research.20,21 Clusters were distinct geographical rural and semi-urban settings in Argentina, Zambia, Guatemala, Belgaum (India), Nagpur (India), Pakistan, and Kenya, described elsewhere.21,22 Clusters that had established an effective birth registry with at least 300 births annually in the defined catchment area, whether at homes or facilities, were eligible for inclusion.
Ethics review committees of the sites, partner institutions, and the WHO approved the trial (appendix p 12). Registry administrators obtained informed consent from eligible women for data collection. All women eligible to receive antenatal corticosteroids provided consent, except where their use was standard care (eg, hospitals). The protocol ethics were in accordance with the Ottawa Statement.23 An independent data monitoring committee appointed by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) reviewed the progress of the trial, as specified in the protocol. Stopping rules were predefined for neonatal mortality on the basis of O'Brien-Fleming boundaries.24
Randomisation and masking
The data coordinating centre (RTI International, Durham, NC, USA) randomly assigned eligible clusters (1:1) to intervention or control using a stratified randomisation procedure to account for Global Network site, neonatal mortality, and treatment group in Global Network Emergency Obstetric and Neonatal Care trial.25 Pretrial data from the Global Network's Maternal and Neonatal Health Registry (MNH Registry)—an ongoing, prospective, population-based registry of pregnancies at the seven Global Network sites—were used to create strata of two or four clusters. Staff at the data coordinating centre informed investigators at each site of the randomisation allocation during the preparatory period to allow time for staff training for the intervention before the start of the trial. The nature of the trial precluded masking of group allocation. To reduce bias, the MNH Registry team obtained outcome data independently of the intervention teams.
Procedures
After randomisation but before the intervention, a survey was done in all study clusters to identify participating health facilities and birth attendants. Intervention clusters received a multifaceted intervention that consisted of health-provider training, posters, pregnancy disc, and uterine height tape to facilitate identification of women at risk of preterm birth, and kits for provision of antenatal corticosteroids. All health providers in intervention clusters were trained to identify women presenting before 36 weeks' gestation with signs of labour, preterm premature rupture of membranes, pre-eclampsia or eclampsia, or obstetric haemorrhage as at high risk of preterm birth and potential candidates for antenatal corticosteroids. Providers were trained to assess gestational age by use of an algorithm that includes last menstrual period and estimated delivery date, or uterine height if neither last menstrual period nor estimated delivery date were known. To facilitate this training, posters were displayed at facilities in the intervention group and pregnancy discs were designed and distributed to calculate gestational age on the basis of last menstrual period or estimated delivery date. Additionally, a colour-coded tape was developed and validated to measure uterine height, with a red zone indicating an estimated gestational age younger than 36 weeks and 0 days (unpublished). The discs and tapes also served as reminders about signs of risk (appendix p 6).
Intervention providers were trained to administer one course of four doses of 6 mg of dexamethasone every 12 h to women identified as at risk of preterm birth from 24 to less than 36 weeks' gestational age.26 Repeated courses were not recommended. Ready-to-use preterm kits containing four vials of 1·5 mL dexamethasone, reuse-prevention syringes, gloves, and instructions for administration were distributed.20 Each site obtained dexamethasone from local suppliers and followed local administration regimens (table 1). An independent laboratory at the data coordinating centre tested a sample of each site's product to confirm the presence of active drug. A product in use at one field site for 11 months had a much lower than expected concentration of active drug; when this issue was identified, this drug was immediately removed from the field and replaced with an active stock of dexamethasone.
Table 1. Trial intervention and general characteristics by site.
| South Asia | Sub-Saharan Africa | Central and South America | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| India (Belgaum) | India (Nagpur) | Pakistan | Zambia | Kenya | Guatemala | Argentina | |
| Location of birth (n/N [%]) | |||||||
| Hospital | 11 548/18 175 (64%) | 6465/10 079 (64%) | 3422/12 422 (28%) | 438/7107 (6%) | 963/9069 (11%) | 1370/5269 (26%) | 2815/2855 (99%) |
| Clinic | 5142/18 175 (28%) | 2592/10 079 (26%) | 2796/12 422 (23%) | 3147/7107 (44%) | 2291/9069 (25%) | 131/5269 (2%) | 6/2855 (<1%) |
| Home or other | 1485/18 175 (8%) | 1022/10 079 (10%) | 6204/12 422 (50%) | 3522/7107 (50%) | 5815/9069 (64%) | 3768/5269 (72%) | 34/2855 (1%) |
| Antenatal care (n/N [%]) | 18 162/18 196 (100%) | 10 076/10 078 (100%) | 9917/12 402 (80%) | 7030/7108 (99%) | 8551/9069 (94%) | 5149/5269 (98%) | 2659/2837 (94%) |
| Identification (signs of preterm birth) | |||||||
| Location | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Clinic, hospital |
| Provider | CHW, nurse, physician | Self-identification, nurse, physician | Self-identification, TBA, CHW, nurse, physician | TBA, nurse, physician | CHW, nurse | Self-identification, TBA, nurse, physician | Nurse, physician |
| Identification (gestational age assessment) | |||||||
| Location | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Clinic, hospital |
| Provider | CHW, nurse, physician | TBA, CHW, nurse, physician | TBA, nurse, physician | TBA, nurse, physician | Nurse | TBA, nurse, physician, | Nurse, physician |
| Antenatal corticosteroid administration* | |||||||
| Location | Community, clinic, hospital | Clinic, hospital | Community, clinic, hospital | Clinic, hospital | Community, clinic, hospital | Community, clinic, hospital | Clinic, hospital |
| Provider | Nurse, physician | Nurse, physician | CHW, nurse, physician | Nurse, physician | Nurse | Nurse | Nurse, physician |
| Steroid type† | |||||||
| Name (manufacturer) | Dexona (Zydus Cadila) | Decamycin (Ranbaxy) and Decacin (Natco) | Decadron (OBS Pakistan) | Dexona (Zydus Cadila) | Dexamethasone (Dawa) | Dexamethasone (Caplin Point Laboratories) | Trofinan (Biol) |
| Description | 8 mg per 2 mL vial | 4 mg per 1 mL vial | 4 mg per 1 mL vial | 8 mg per 2 mL vial | 4 mg per 1 mL ampoule | 8 mg per 2 mL ampoule | 8 mg per 2 mL ampoule |
| Trial period | April 10, 2012–Oct 10, 2013, and May 1, 2012–Oct 31, 2013 | June 11, 2012–Dec 11, 2013 | June 5, 2012–Dec 5, 2013 | April 14, 2012–Oct 14, 2013 | April 30, 2012–Oct 30, 2013 | Sept 20, 2012–March 20, 2014 | Oct 1, 2011–March 31, 2013 |
Data for location of birth and antenatal care are from before the trial (2010). Nurses includes auxiliary nurses, nurses, nurse midwives, and similar. TBA=traditional birth attendant. CHW=community health workers (term encompasses a range of health workers specific to countries).
Community-level administration by study staff or nurse (Guatemala); auxiliary nurse midwife (Belgaum, India); vaccinators (Pakistan); and study nurses or clinical officers (Kenya).
All sites used dexamethasone sodium phosphate injection.
A third intervention component was referral recommendation for women identified as at high risk of preterm birth. However, neither transport nor strategies to improve referral were included. Training in essential newborn care was provided in both intervention and control clusters.27 No other interventions were provided to the control group.
The intervention was pragmatic by design. First, site ACT coordination teams received intervention training at a local venue. Following each site's in-country central training, each site team developed a detailed implementation plan. For example, at all sites, apart from Argentina (because of few non-hospital deliveries), community health workers or traditional birth attendants providing obstetric care were trained to identify women at risk and all birth attendants at health facilities were trained to identify women at risk and administer corticosteroids. The trial started upon completion of the training.
Outcomes
The primary outcome was 28-day neonatal mortality among infants less than the 5th percentile for birthweight. The less-than-5th-percentile birthweight group (referred to as less-than-5th-percentile infants) was a proxy for preterm birth and, in view of the differences in birthweight distributions across the sites, was established separately for each site on the basis of birthweight data for the pretrial year. Site-specific cutoffs were 2450 g for Argentina, 2400 g for Zambia, 2267 g for Guatemala, 2000 g for Belgaum, India, 2150 g for Pakistan, 2000 g for Nagpur, India, and 2500 g for Kenya. Infants were classified as less than 5th percentile on the basis of measured birthweights. Estimated weights by clinical assessment were used when measured weights were unavailable; those missing both estimated and measured weights were classified as less than 5th percentile (since based on historical data, most of the missing data were for preterm infants). We used birthweight rather than gestational age for the primary analysis subgroup because many women in the registry had missing or uncertain gestational age, ultrasound was often unavailable, and the intervention was designed to improve estimation of gestational age, which could potentially bias gestational age-based analyses. All births, including multiple births, are included in infant outcomes.
Maternal safety was assessed through the frequency of suspected maternal infection, a composite of process outcomes including receipt of antibiotics plus hospital admission or referral, and receipt of intravenous fluids, surgery, or other treatment related to infection. The definition also included evidence of antepartum or post-partum infection for mothers with infants with a birthweight less than 2500 g. Additionally, use of antenatal corticosteroids, neonatal and perinatal mortality, and suspected maternal infection were measured for all births, irrespective of birthweight.
All mortality outcomes were obtained via the MNH Registry based in each study cluster.21 Maternal and perinatal outcomes were measured and included for all consenting pregnant women residing in clusters during the trial. Briefly, registry administrators (community health workers and nurses) aimed to enrol and obtain birth outcomes for all pregnant residents of the defined catchment area by 20 weeks' gestation. In addition to the enrolment visit, registry administrators visited participants within 3 days of delivery and at 6 weeks post partum to obtain pregnancy outcomes and information about use of basic health-care services. Registry administrators interviewed family members and birth attendants, and reviewed available medical records. Clinical causes of death were reported by the health provider. The ACT team assessed the identification of women at high risk of preterm birth as well as the use of the ACT kits, the use of corticosteroids, and other process measures. Data for use of antenatal corticosteroids and indicators were obtained for women identified as at risk of preterm birth and eligible to receive antenatal corticosteroids.
Data were entered into password-protected servers and securely transmitted to the data coordinating centre. We used data entry software to do range and consistency checks, and cross-form edits were done at the data coordinating centre and resolved locally. We used double data entry to assess data keying errors for a random sample of 5% of data forms.
Statistical analysis
The trial was powered to detect a 30% reduction in 28-day neonatal mortality among infants born at less than the 5th percentile of birthweight, based on previous research3,4 and an expected increase from 10% to 50% in the use of antenatal corticosteroids among women at risk of preterm birth in the intervention group. With an average of 33 less-than-5th-percentile infants per cluster over an 18-month period, an intraclass correlation of 0·01–0·02, and a baseline neonatal mortality of 160–200 per 1000 livebirths (based on historical data), a total of 50 clusters per treatment group would provide 88–95% power to detect the hypothesised difference for a two-sample test (α=0·05).
We assessed the primary outcome using an intention-to-treat approach, with a model-based adaptation of the permutation test.28 First, we fitted an individual-level linear model with 28-day neonatal mortality as a function of site and randomisation strata, nested within the sites, and computed the residual for each individual and mean cluster-level residuals. Next, we used an ANOVA model to test for treatment differences between the mean residuals for the intervention and control clusters. For secondary analyses of the primary outcome and analyses of secondary outcomes we used a generalised linear model with generalised estimating equations to estimate parameters while controlling for cluster correlations.29 Model-generated measures of risk and p values were adjusted for randomisation strata, unless otherwise noted. Because of small numbers of maternal morbidity events, we calculated odds ratios (ORs) with corresponding 95% CIs and p values for these outcomes using a Cochran-Mantel-Haenszel test, controlling for randomisation strata. Analyses were done with SAS version 9.3 (SAS Institute, Cary, NC, USA).
This trial is registered with ClinicalTrials.gov, number NCT01084096.
Role of the funding source
Staff from the funder (NICHD) had input into the study design and data interpretation and reviewed and approved the report. However, the authors' views do not necessarily represent those of the NICHD. EMM, VT, SM, DDW, and FA had access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
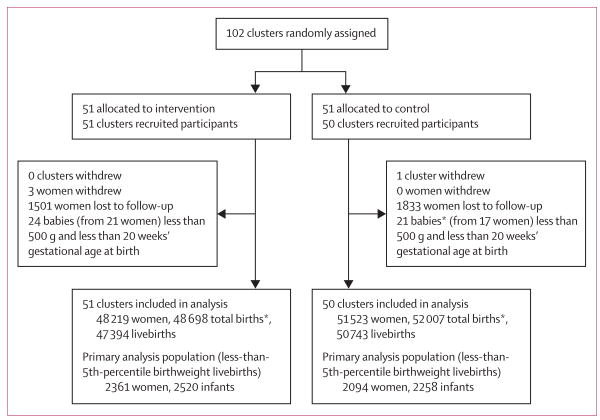
The ACT trial took place between October, 2011, and March, 2014, with start dates varying by site. 102 clusters were eligible for the study—six in Argentina, ten in Zambia, ten in Guatemala, 20 in Belgaum, India, 20 in Nagpur, India, 20 in Pakistan, and 16 in Kenya. We randomly assigned 51 clusters to the intervention group and 51 to control. One Guatemalan cluster assigned to the control group withdrew because of unrest and staff concerns about safety (unrelated to the trial). Therefore, 51 intervention clusters (48 219 pregnant women and 47 394 livebirths) and 50 control clusters (51 523 women and 50 743 livebirths) remained in the study and completed follow-up (figure 1). Overall, 349 health facilities with deliveries served intervention clusters, compared with 360 for control clusters; most (260 in each group) were clinics (health centres and other non-hospital facilities); the remainder were primary health centres, community health clinics, or dispensaries.
Figure 1. Trial profile.
*Includes stillbirths.
Of the livebirths, 2361 (5%) of 48 219 women in the intervention group delivered 2520 (5%) of 47 394 less-than-5th-percentile infants and 2094 (4%) of 51 523 women in the control group delivered 2258 (4%) of 50 743 less-than-5th-percentile infants. Few of the total births (620 [1%] of 48 698 in the intervention group and 661 [1%] of 52 007 in the control group) had estimated or missing birthweights (treated as less than 5th percentile).
In the year before the trial, fewer women in the intervention clusters than in the control clusters had deliveries attended by physicians, and more deliveries in the intervention clusters than in the control clusters were attended by nurses (appendix p 1). Additionally, compared with the control clusters, fewer deliveries in the intervention clusters took place at hospital or at home, more occurred at clinics.
For the trial period, results are presented for all enrolled women and for those with an infant of less than 5th percentile birthweight (table 2). For all women, the intervention and control groups were similar with respect to most maternal and perinatal characteristics. However, the proportion of less-than-5th-percentile livebirths was greater in the intervention group than in the control group. Maternal and perinatal characteristics of the less-than-5th-percentile infants were similar for the intervention and control groups, apart from slightly lower proportions of women younger than 20 years and nulliparous women in the intervention group.
Table 2. Enrolment characteristics by treatment arm for trial period (2011–14)*.
| Women with a less-than-5th-percentile infant† | All women | |||
|---|---|---|---|---|
|
|
|
|||
| Intervention (n=2361) | Control (n=2094) | Intervention (n=48 219) | Control (n=51 523) | |
| Maternal age group (years) | ||||
| <20 | 303/2358 (13%) | 344/2094 (16%) | 5412/48 156 (11%) | 6622/51 455 (13%) |
| 20–35 | 1937/2358 (82%) | 1673/2094 (80%) | 41 003/48 156 (85%) | 42 938/51 455 (83%) |
| >35 | 118/2358 (5%) | 77/2094 (4%) | 1741/48 156 (4%) | 1895/51 455 (4%) |
| Maternal education | ||||
| No formal school | 787/2335 (34%) | 687/2077 (33%) | 11 005/47 976 (23%) | 11 258/51 256 (22%) |
| Primary | 796/2335 (34%) | 700/2077 (34%) | 17 686/47 976 (37%) | 19 515/51 256 (38%) |
| Secondary | 640/2335 (27%) | 567/2077 (27%) | 15 528/47 976 (32%) | 16 170/51 256 (32%) |
| University | 112/2335 (5%) | 123/2077 (6%) | 3757/47 976 (8%) | 4313/51 256 (8%) |
| Parity | ||||
| 0 | 899/2348 (38%) | 867/2088 (42%) | 16 366/47 897 (34%) | 17 901/51 434 (35%) |
| 1 | 553/2348 (24%) | 508/2088 (24%) | 13 961/47 897 (29%) | 14 599/51 434 (28%) |
| 2 | 896/2348 (38%) | 713/2088 (34%) | 17 570/47 897 (37%) | 18 934/51 434 (37%) |
| Previous pregnancy loss | 208/1525 (14%) | 170/1300 (13%) | 2567/33 684 (8%) | 2449/36 098 (7%) |
| Received antenatal care | 2209/2333 (95%) | 1963/2073 (95%) | 46 763/47 979 (97%) | 50 071/51 182 (98%) |
| Multiple pregnancy | 280/2360 (12%) | 274/2094 (13%) | 508/48 202 (1%) | 500/51 515 (1%) |
Data are n/N (%).
Dates vary by study site.
Cutoffs for the less-than-5th-percentile birthweight groups were determined from 2011 data.
In the intervention group, 6214 (13%) of 48 219 women were identified as being at high risk of preterm birth; 3741 (60%) were identified at the community level. 4789 (77%) of 6214 high-risk women were identified because of signs of preterm labour; 3031 (50%) of 6026 with data available were identified at 33–36 weeks' gestation, and 2190 (36%) at 28–32 weeks' gestation (table 3). Of the women identified as being at risk of preterm birth, 6109 (98%) of 6214 received antenatal corticosteroids, mainly by ACT kit, with most women receiving a full course (table 3). 14 women received more than one course of antenatal corticosteroids. Of all women who received antenatal corticosteroids in the intervention group, 976 (16%) of 6109 had delivered a less-than-5th-percentile infant.
Table 3. Intervention process measures among women identified as at high risk for preterm birth in intervention clusters.
| n/N (%) | |
|---|---|
| Women identified as at high risk for preterm birth by the intervention | 6214/48 219* (13%) |
| Location of identification | |
| Community level | 3741/6214 (60%) |
| Primary health care | 1666/6214 (27%) |
| Hospital | 807/6214 (13%) |
| Location of first dose administration | |
| Community level | 1209/6109 (20%) |
| Primary health care | 3853/6109 (63%) |
| Hospital | 1047/6109 (17%) |
| Maternal conditions at time of antenatal corticosteroid use | |
| Signs of preterm labour | 4789/6214 (77%) |
| Preterm premature rupture of membranes | 1204/6214 (19%) |
| Haemorrhage | 432/6214 (7%) |
| Hypertension | 902/6214 (15%) |
| Other | 186/6214 (3%) |
| Estimated gestational age at identification (weeks) | |
| 20–23 | 8/6026 (<1%) |
| 24–27 | 778/6026 (13%) |
| 28–32 | 2190/6026 (36%) |
| 33–36 | 3031/6026 (50%) |
| 37–39 | 19/6026 (<1%) |
| Received antenatal corticosteroids | 6109/6214 (98%) |
| Antenatal corticosteroid kits with dexamethasone doses (6 mg) used | |
| 1 dose | 1316/5973 (22%) |
| 2 doses | 317/5973 (5%) |
| 3 doses | 149/5973 (2%) |
| 4 doses (complete course) | 4191/5973 (70%) |
The denominator for all percentages apart from the first is the number of women identified as at high risk for preterm birth by the intervention, excluding missing data (varies by characteristic).
48 219 is the total number of pregnant women enrolled in intervention clusters.
Among women who delivered less-than-5th-percentile infants for whom data were available, 1052 (45%) of 2327 in the intervention group and 215 (10%) of 2062 in the control group received at least one dose of antenatal corticosteroids (p<0·0001). Among all women with livebirths, 5571 (12%) of 45 439 in the intervention group and 746 (2%) of 48 187 in the control group received antenatal corticosteroids (p<0·0001; table 4). Women in the intervention group were more likely to be attended by nurses than those in the control group (18 166 [38%] of 48 215 vs 15 366 [30%] of 51 519) and less likely to be attended by physicians (19 122 [40%] of 48 215 vs 23 233 [45%] of 51 519). More women in the intervention group than in the control group delivered in clinics (13 593 [28%] of 48 217 vs 11 675 [23%] of 51 519), and fewer had hospital deliveries (23 798 [49%] of 48 217 vs 27 345 [53%] of 51 519). Similar patterns were also seen in women with less-than-5th-percentile infants, and trends were similar to those noted in the pretrial period. The proportion of newborn babies referred to higher levels of care was similar in the intervention and control groups (1860 [4%] of 48 498 vs 2191 [4%] of 51 771). Comparing use of antenatal corticosteroids by treatment group and randomisation strata, all strata had higher use in the intervention clusters than in the control clusters, with the exception of one stratum in Argentina (appendix pp 7–8).
Table 4. Antenatal corticosteroid use and delivery care by intervention group.
| Women with a less-than-5th-percentile infant | All women | |||
|---|---|---|---|---|
|
|
|
|||
| Intervention (n=2361) | Control (n=2094) | Intervention (n=48 219) | Control (n=51 523) | |
| Antenatal corticosteroids provided antepartum | 1052/2327 (45%) | 215/2062 (10%) | 5571/45 439 (12%) | 746/48 187 (2%) |
| Delivery attendant | ||||
| Physician | 1027/2360 (44%) | 1007/2094 (48%) | 19 122/48 215 (40%) | 23 233/51 519 (45%) |
| Nurse* | 764/2360 (32%) | 547/2094 (26%) | 18 166/48 215 (38%) | 15 366/51 519 (30%) |
| TBA | 461/2360 (20%) | 443/2094 (21%) | 8581/48 215 (18%) | 10 434/51 519 (20%) |
| Family or unattended | 108/2360 (5%) | 97/2094 (5%) | 2346/48 215 (5%) | 2486/51 519 (5%) |
| Delivery location | ||||
| Hospital | 1194/2360 (51%) | 1208/2094 (58%) | 23 798/48 217 (49%) | 27 345/51 519 (53%) |
| Clinic | 613/2360 (26%) | 401/2094 (19%) | 13 593/48 217 (28%) | 11 675/51 519 (23%) |
| Home or other | 553/2360 (23%) | 485/2094 (23%) | 10 826/48 217 (22%) | 12 499/51 519 (24%) |
| Delivery mode | ||||
| Vaginal or vaginal (assisted) | 1972/2360 (84%) | 1731/2094 (83%) | 41 085/48 218 (85%) | 43 865/51 520 (85%) |
| Caesarean section | 388/2360 (16%) | 363/2094 (17%) | 7133/48 218 (15%) | 7655/51 520 (15%) |
Data are n/N (%). TBA=traditional birth attendant.
Includes auxiliary nurses, nurses, nurse midwives, and similar.
Among the less-than-5th-percentile infants, 28-day neonatal mortality was 225 per 1000 livebirths for the intervention group and 232 per 1000 livebirths for the control group (RR 0·96, 95% CI 0·87–1·06, p=0·65; table 5). The frequency of stillbirths was 229 per 1000 births in the intervention group, compared with 247 per 1000 births in the control group (RR 0·99, 95% CI 0·90–1·09, p=0·81), and perinatal mortality was 368 per 1000 births for the intervention group compared with 391 per 1000 births for the control group (RR 0·97, 95% CI 0·91–1·04, p=0·46). Results were similar for male and female infants.
Table 5. Perinatal and 28-day neonatal outcomes by intervention group.
| Less-than-5th-percentile birthweight births | All births | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Intervention (n=3268) | Control (n=2997) | RR (95% CI) | p value | Intervention (n=48 698) | Control (n=52 007) | RR (95% CI) | p value | |
| Stillbirths | 748 (23%) | 739 (25%) | ·· | ·· | 1304 (3%) | 1264 (2%) | ·· | ·· |
| Livebirths | 2520 (77%) | 2258 (75%) | ·· | ·· | 47 394 (97%) | 50 743 (98%) | ·· | ·· |
| Number of neonatal deaths before 28 days (frequency, per 1000 livebirths) | 566 (224·6) | 524 (232·1) | 0·96 (0·87-1·06) | 0·65* | 1300 (27·4) | 1211 (23·9) | 1·12 (1·02-1·22) | 0·0127 |
| Number of neonatal deaths before 7 days (frequency, per 1000 livebirths) | 455 (180·6) | 433 (191·8) | 0·94 (0·84-1·06) | 0·30 | 1036 (21·9) | 969 (19·1) | 1·12 (1·02-1·22) | 0·0155 |
| Number of stillbirths (frequency, per 1000 births) | 748 (228·9) | 739 (246·6) | 0·99 (0·90-1·09) | 0·81 | 1304 (26·8) | 1264 (24·3) | 1·11 (1·02-1·22) | 0·0181 |
| Number of perinatal deaths (frequency, per 1000 births) | 1203 (368·1) | 1172 (391·1) | 0·97 (0·91-1·04) | 0·46 | 2339 (48·0) | 2233 (42·9) | 1·11 (1·04-1·19) | 0·0031 |
| Male infants only | ||||||||
| Livebirths | 1211 | 1138 | ·· | ·· | 24 415 | 26 231 | ·· | ·· |
| Number of neonatal deaths before 28 days (frequency, per 1000 livebirths) | 294 (242·8) | 281 (246·9) | 0·99 (0.88-1.12) | 0·90 | 708 (29·0) | 643 (24·5) | 1·16 (1·06-1·27) | 0·0013 |
| Female infants only | ||||||||
| Livebirths | 1303 | 1116 | ·· | ·· | 22 966 | 24 506 | ·· | ·· |
| Number of neonatal deaths before 28 days (frequency, per 1000 livebirths) | 270 (207·2) | 240 (215·1) | 0·95 (0·84-1·07) | 0·37 | 590 (25·7) | 565 (23·1) | 1·08 (0·96-1·21) | 0·2011 |
Data are n or n (%), unless otherwise indicated. Unless otherwise indicated, relative risks (RRs) with corresponding 95% CIs and p values were calculated from generalised linear models accounting for the cluster-level variance and adjusted for randomisation strata.
Calculated from a t test (cluster-level, 62 degrees of freedom [101 clusters–37 strata–2 treatment groups]).
Neonatal mortality across all livebirths (irrespective of birthweight) was 27·4 per 1000 for the intervention group and 23·9 per 1000 for the control group (RR 1·12, 95% CI 1·02–1·22, p=0·0127). Stillbirths were more common in the intervention group than in the control group (26·8 vs 24·3 per 1000 births; RR 1·11, 95% CI 1·02–1·22, p=0·0181) and perinatal mortality was higher for intervention than for control (48·0 vs 42·9 per 1000 births; RR 1·11, 95% CI 1·04–1·19, p=0·0031). We noted similar patterns in neonatal mortality for male and female infants; however, the effect among male infants was larger and statistically significant (male infant RR 1·16, 95% CI 1·06–1·27, p=0·0013; female infant RR 1·08, 95% CI 0·96–1·21, p=0·20). These mortality results were fairly consistent across all randomisation strata, in both less-than-5th-percentile infants and all births (appendix pp 9–10). Infant mortality at 42 days showed similar trends (data not shown).
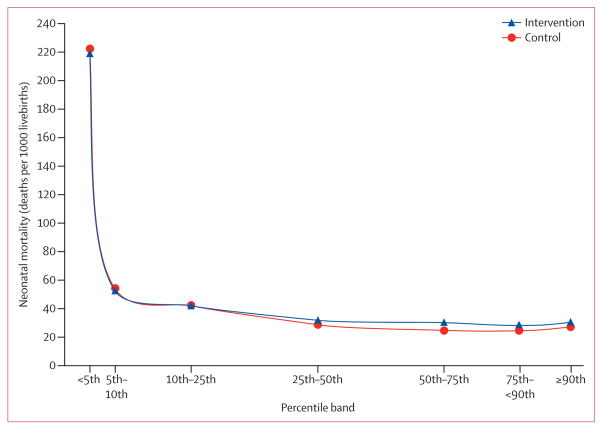
Because the findings among all livebirths were unexpected, we did further post-hoc analyses of mortality, in accordance with CONSORT guidelines and the recommendations of the data monitoring committee.30,31 Because the proportion of less-than-5th-percentile infants was higher in the intervention group than in the control group, we assessed 28-day neonatal mortality by birthweight percentile bands (figure 2, appendix p 2) by fitting a model for 28-day neonatal mortality with the treatment group and percentile bands as predictors. The interaction between treatment and birthweight percentile bands was significant (p<0·0001). These analyses showed that mortality values for the intervention and control groups were similar in infants less than the 25th percentile for birthweight, but were 30% higher in the intervention group than in the control group for birthweight bands at or above the 25th percentile. Antenatal corticosteroids were used in 2322 (7%) of 33 870 of higher birthweight (≥25th percentile) births in the intervention group, compared with 279 (1%) of 36 511 in the control group.
Figure 2. 28-day neonatal mortality by birthweight percentile band.
Neonatal mortality is adjusted for site and randomisation stratum. Only babies with measured birthweight are represented (ie, those with estimated or missing birthweights are excluded).
We also explored the effects of gestational age on 28-day neonatal mortality (<37 weeks vs ≥37 weeks), using an algorithm to determine gestational age on the basis of estimated delivery date, last menstrual period, and site-specific 95th percentile for birthweight at gestational age 36 weeks. Using this classification, 3779 (60%) of 6265 less-than-5th-percentile births were estimated to be born at a gestational age younger than 37 weeks (preterm; denominator includes livebirths and stillbirths). Among all livebirths, the proportions of preterm infants were similar in the intervention and control groups (5530 [12%] of 47 371 vs 5329 [11%] of 50 372). Although 28-day neonatal mortality in the preterm infants did not differ between the intervention and the control groups (RR 0·96, 95% CI 0·86–1·08, p=0·497), a higher mortality was seen in the intervention group for infants born at a gestational age of 37 weeks or older (RR 1·21, 95% CI 1·07–1·36, p=0·0018; appendix p 3). Among infants born at a gestational age of 37 weeks or older, 3198 (8%) of 38 594 in the intervention group and 424 (1%) of 41 385 in the control group received antenatal corticosteroids.
We noted similar trends for births at hospitals, clinics, and home, in analyses stratified by delivery location, for both less-than-5th-percentile and all infants (data not shown). Reported causes of neonatal mortality were similar in both groups (appendix p 4).
We attempted to determine whether there was enrolment bias (ie, more high-risk women enrolled in the intervention group). The proportions of births with exact and estimated birthweights and the average time between study enrolment and delivery did not differ between the groups. Additionally, the number of women enrolled in intervention and control groups was similar to the number of women who gave birth in the pretrial period (data not shown).
In the total sample, the use of medical care was similar in the intervention and control groups: 3392 (7%) of 48 211 women in the intervention group and 3100 (6%) of 51 523 in the control group were admitted to hospital, 27 309 (57%) women in the intervention group and 28 227 (55%) in the control group received antibiotics, and fluids were provided to 25 077 (52%) women in the intervention group and 21 245 (41%) women in the control group. Suspected maternal infection was reported in 1207 (3%) of 48 219 women in the intervention group and 867 (2%) of 51 523 in the control group (OR 1·45, 95% CI 1·33–1·58, p<0·0001; appendix p 5). Among women who delivered less-than-5th-percentile infants, the suspected maternal infection was reported in 236 (10%) of 2361 in the intervention group and 133 (6%) of 2094 in the control group (OR 1·67, 95% CI 1·33–2·09, p<0·0001). The maternal mortality ratios in all women were 106 per 100 000 livebirths in the intervention group and 97 per 100 000 livebirths in the control group.
The intracluster correlation value for 28-day neonatal mortality was 0·001. An interaction test showed treatment effects did not differ by site (p=0·40). In a post-hoc sensitivity analysis in which we excluded the period of enrolment when 202 women received the suboptimum drug at one site (Nagpur, India), trial results did not change (data not shown).
Discussion
In this study, we assessed the health effects of a population-based multifaceted strategy to identify women at high risk of preterm birth and administer antenatal corticosteroids in low-resource settings. The intervention effectively increased the use of antenatal corticosteroids to 45% of women delivering infants less than the 5th percentile for birthweight, compared with about 10% in control clusters. However, we also identified overtreatment with antenatal corticosteroids in the intervention group. Of the 13% of pregnant women in intervention clusters who were identified by the intervention and received antenatal corticosteroids, only 16% delivered a less-than-5th-percentile infant. Furthermore, despite nearly half of the less-than-5th-percentile infants receiving antenatal corticosteroids, neonatal mortality did not decrease in this subgroup. Among the entire population, the intervention resulted in a significant increase in neonatal deaths of 3·5 per 1000 livebirths and an increase in perinatal deaths of 5·1 per 1000 births. This harmful effect was concentrated among infants at and above the 25th percentile for birthweight. Additionally, there was a larger proportion of less-than-5th-percentile infants in the intervention clusters than in the control clusters. Finally, the intervention was associated with a significant 3·6% absolute increase in suspected infection among mothers of less-than-5th-percentile infants and a significant 0·8% increase among all women (panel).
This study had several strengths, including the participation of seven sites in six countries with almost 100 000 women, resulting in enough power to detect small variations in mortality among the whole population. The experimental design was rigorous and achieved similar groups through stratified randomisation. Use of the independent Maternal and Neonatal Health Registry, ongoing since 2009, prevented observer bias of the mortality outcomes and led to follow-up data being available for a very high proportion of women and infants. Finally, the population-based assessment strategy was a successful, pragmatic approach to integrate the intervention within existing health systems, suggesting that the reported effects might be similar in a programme to scale up antenatal corticosteroids with these components.
Nonetheless, the study has limitations. The use of birthweight percentile instead of gestational age to define the target subgroup for the primary analysis misclassified some preterm infants as term infants. Disentangling the contribution of each specific component of the packaged intervention to the overall effect is challenging and the analyses of unanticipated outcomes should be considered with caution. Because of the pragmatic design, we did not systematically collect process data on the use or non-use of other potential co-interventions that might have affected outcomes. Additionally, this approach precluded collection of high-quality data for causes of mortality and morbidity to address the causal mechanisms.
The observed effects are unlikely to be accounted for by selection bias, since the mortality data were obtained from an independent, well established registry. Participant characteristics, enrolment performance, and the proportions of preterm birth in the intervention and control groups were similar both before the trial and during the enrolment period.
The absence of a positive effect on mortality in the less-than-5th-percentile infants could have several explanations. Although almost half of the less-than-5th-percentile infants in the intervention group received antenatal corticosteroids, these might have little effect in settings without neonatal intensive care.15,20,34 However, the direction of our results was similar in hospitals and clinics, although most hospitals in this study did not have high-quality neonatal intensive care.
Although a substantial proportion of the subgroup of less-than-5th-percentile infants was probably born at term, this issue is unlikely to account for the findings by a dilution of beneficial effects; the gestational age-based analysis showed similar results in the preterm infants. However, the preterm group probably included many late preterm infants for whom no effect of antenatal corticosteroids has been shown so far.4,33 Unfortunately, our data for gestational age were not reliable enough for further analysis by gestational age categories. Finally, the intervention did not modify the delivery location of these infants compared with the control group, and postnatal referrals did not differ between groups.
An unexpected and unfortunate finding was that the intervention resulted in an 11–12% relative increase in neonatal and perinatal mortality in the whole population. This harmful effect was concentrated in infants at and above the 25th birthweight percentile, in whom the relative increase in mortality was 30%. Gestational age-based analysis showed a similar effect for term infants. A possible explanation is that the study screening method used to determine risk of preterm birth was fairly non-specific, identifying some women who delivered at term as at risk of preterm birth, leading to potentially harmful use of antenatal corticosteroids for infants not delivered preterm. The mistaken prediction of preterm birth has raised concerns about overtreatment—eg, of term infants.35 On the basis of only three studies that included 500 participants and 13 perinatal deaths, investigators of a systematic review3 reported a non-significant 2·6-times increase in neonatal deaths and non-significant 3·3-times increase in perinatal deaths in infants receiving antenatal corticosteroids and born at a gestational age of 36 weeks or older. The remaining trials in the review did not report results for women who delivered at term. In our trial, the intervention promoted the use of antenatal corticosteroids in women identified as being at high risk of preterm birth, and 84% of these women delivered an infant at or above the 5th percentile for birthweight. The recommendation to use antenatal corticosteroids up to 36 weeks' gestational age probably contributed to this high proportion of women who probably delivered at term.
An alternative explanation could be that mistaken identification of women at risk who ultimately delivered a term baby adversely affected the quality of perinatal care and thereby increased perinatal mortality. However, we did not collect process data to test such a hypothesis.
Findings from both animals and human beings suggest that antenatal corticosteroids can affect fetal growth, especially if several courses are used.34,36 Additionally, reductions in birthweight associated with antenatal corticosteroids have been reported in infants born at a gestational age of 36 weeks or older in at least one study.3 The higher proportion of less-than-5th-percentile infants in the ACT intervention group suggests possible effects of antenatal corticosteroids on fetal growth that should be further explored.
Our results for maternal post-partum infection should be interpreted cautiously, because these outcomes were defined by process outcomes related to infection. However, our results are consistent with a significant 1·7-times increase in puerperal sepsis reported for women enrolled in trials that used dexamethasone that were included in the Cochrane review.3 Beneficial effects of antenatal corticosteroids on maternal health were not expected at any gestational age.
In summary, this intervention strategy was not only ineffective at reducing neonatal mortality in less-than-5th-percentile infants, but also increased mortality in the population overall. For every 1000 women exposed to the multifaceted strategy at all levels of care, an excess of five perinatal deaths occurred compared with standard care. Furthermore, the strategy seemed to increase the risk of maternal infectious morbidity. These results do not support the scale-up of this population-based multifaceted strategy to identify women at high risk of preterm birth at all levels of care and administer antenatal corticosteroids in low-resource settings. Caution should be used in the deployment of similar interventions in similar settings.
Exploration of the potential causal pathways of our findings will be crucial to advance understanding about how and to whom antenatal corticosteroids can be safely and effectively delivered in low-resource settings. Questions that could be explored include whether antenatal corticosteroids were the direct cause of the harmful effects seen in a subgroup of babies and the potential mechanisms involved. Scale-up strategies should explore the minimum maternal and neonatal care needed to attend infants exposed to antenatal corticosteroids in such settings. Furthermore, pragmatic and accurate methods to identify women at risk of preterm birth, including assessment of gestational age where ultrasound is unavailable, are needed.
Supplemental Material
Panel: Research in context.
Systematic review
We searched the Cochrane Library for systematic reviews published in any language up to Sept 1, 2014, using the terms “antenatal steroids” OR “prenatal steroids” OR “antenatal corticosteroids” OR “prenatal corticosteroids”. We excluded reviews that compared multiple with single courses of steroids, or different types of corticosteroids. We identified one systematic review3 of randomised controlled trials investigating the effects of steroids versus placebo or no treatment on perinatal and maternal outcomes in women at high risk of preterm birth, published in 2006. The review included 21 trials; 18 included data for neonatal mortality and showed a 31% reduction in babies who received either dexamethasone or betamethasone antenatally. Additionally, we searched PubMed for systematic reviews or reports of trials published in any language between Jan 1, 2007, and Sept 1, 2014, using the same terms AND “meta-analysis” OR “randomized controlled trial”. We identified two additional systematic reviews,2,32 which supported the findings and conclusions of the 2006 Cochrane review.3 We also identified one placebo-controlled trial done in a tertiary hospital in Brazil, which assessed the effects of betamethasone given to women at high risk of preterm birth with pregnancies between 34 and 36 weeks' gestational age.33 The results showed no beneficial effect for a reduction in respiratory distress syndrome of betamethasone compared with placebo. We identified no cluster-randomised trials to assess population-based comprehensive strategies to implement antenatal corticosteroid treatment for women at risk of preterm birth in low-income and middle-income countries.
Interpretation
To our knowledge, this is the first study done to assess the effects of a population-based multifaceted strategy to identify women at high risk of preterm birth and administer antenatal corticosteroids in low-resource settings. The well established beneficial effects of antenatal corticosteroids in preterm neonates seen in the efficacy trials when given in hospitals with newborn intensive care were not confirmed in our study in low-income and middle-income countries. Several factors might account for these differences: not all preterm or small babies received steroids, half of them were likely to be late preterm, and no neonatal intensive care was available for the vast majority. Additionally, to increase the use of antenatal corticosteroids for preterm babies, antenatal corticosteroids were given to many women identified as at risk who ultimately did not deliver a small or preterm baby. This strategy increased neonatal and perinatal mortality in the population overall for reasons still to be explored.
Acknowledgments
This trial was funded by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD058322, U01 HD040477, U01 HD043464, U01 HD040657, U01 HD042372, U01 HD040607, U01 HD058326, and U01 HD040636). Support was also provided by the WHO Department of Reproductive Health and Research. Syringes were donated by Becton Dickinson. Members of the ACT group are listed in the appendix (pp 11–12).
Footnotes
See Online for appendix
Contributors: FA, JMB, and PMB had the original idea and designed the initial protocol. FA and JH-F wrote the final protocol in collaboration with all members of the steering committee (PMB, JMB, WAC, EC, ALG, NFK, KMH, RJD, SSG, BSK, OP, SS, RLG, AP PLH, FE, EAL, AHJ, and EMM). FA, JH-F, AMaz, and MB prepared manuals in collaboration with the members of staff from the data coordinating centre. FA, JH-F, KS, VT, EMM, JMB, PMB, and MK-T coordinated the overall execution of the trial. FA, MB, AMaz, AC, SSG, BSK, NSM, SMD, GMK, MCM, AMJ, MBB, NVH, OP, SS, SAl, FH, FE, PN, SAy, ALG, LF, AP, AB, MW, EC, AMw, MC, AMan, and SP coordinated the implementation of the study at country level. SM wrote the plan of analysis in collaboration with DDW, VT, and FA. VT and SM did the statistical analysis in collaboration with DDW. FA, EMM, VT, and SM wrote the report with input from all authors, especially JMB, DDW, WAC, RLG, AMJ, and MK-T.
Declaration of interests: We declare no competing interests.
Contributor Information
Fernando Althabe, Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina.
José M Belizán, Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina.
Elizabeth M McClure, RTI International, Durham, NC, USA.
Jennifer Hemingway-Foday, RTI International, Durham, NC, USA.
Mabel Berrueta, Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina.
Agustina Mazzoni, Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina.
Alvaro Ciganda, Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina; UNICEM, Montevideo, Uruguay.
Prof Shivaprasad S Goudar, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Prof Bhalachandra S Kodkany, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Prof Niranjana S Mahantshetti, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Prof Sangappa M Dhaded, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Geetanjali M Katageri, S Nijalingappa Medical College, Bagalkot, Karnataka, India.
Prof Mrityunjay C Metgud, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Anjali M Joshi, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Prof Mrutyunjaya B Bellad, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Narayan V Honnungar, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, Karnataka, India.
Prof Richard J Derman, Department of Obstetrics and Gynecology, Christiana Health Care Services, Newark, DE, USA.
Sarah Saleem, Department of Community Health Sciences, Aga Khan University, Karachi, Pakistan.
Omrana Pasha, Department of Community Health Sciences, Aga Khan University, Karachi, Pakistan.
Sumera Ali, Department of Community Health Sciences, Aga Khan University, Karachi, Pakistan.
Farid Hasnain, Department of Community Health Sciences, Aga Khan University, Karachi, Pakistan.
Prof Robert L Goldenberg, Department of Obstetrics and Gynecology, Columbia University, New York, NY, USA.
Fabian Esamai, Moi University School of Medicine, Eldoret, Kenya.
Paul Nyongesa, Moi University School of Medicine, Eldoret, Kenya.
Silas Ayunga, Moi University School of Medicine, Eldoret, Kenya.
Edward A Liechty, School of Medicine, Indiana University, Indianapolis, IN, USA.
Ana L Garces, Fundación para la Alimentación y Nutrición de Centro América y Panamá, Guatemala City, Guatemala; Francisco Marroquin University, Guatemala City, Guatemala.
Lester Figueroa, Fundación para la Alimentación y Nutrición de Centro América y Panamá, Guatemala City, Guatemala.
Prof K Michael Hambidge, University of Colorado School of Medicine, Denver, CO, USA.
Nancy F Krebs, University of Colorado School of Medicine, Denver, CO, USA.
Prof Archana Patel, Lata Medical Research Foundation, Nagpur, India; Indira Gandhi Government Medical College, Nagpur, India.
Anjali Bhandarkar, Lata Medical Research Foundation, Nagpur, India.
Manjushri Waikar, Lata Medical Research Foundation, Nagpur, India.
Prof Patricia L Hibberd, Massachusetts General Hospital, Boston, MA, USA.
Prof Elwyn Chomba, University Teaching Hospital, Lusaka, Zambia.
Prof Waldemar A Carlo, University of Alabama at Birmingham, Birmingham, AL, USA.
Angel Mwiche, University Teaching Hospital, Lusaka, Zambia.
Melody Chiwila, Centre for Infectious Disease Zambia, Lusaka, Zambia.
Albert Manasyan, University of Alabama at Birmingham, Birmingham, AL, USA.
Sayury Pineda, Fundación para la Alimentación y Nutrición de Centro América y Panamá, Guatemala City, Guatemala.
Sreelatha Meleth, RTI International, Durham, NC, USA.
Vanessa Thorsten, RTI International, Durham, NC, USA.
Kristen Stolka, RTI International, Durham, NC, USA.
Dennis D Wallace, RTI International, Durham, NC, USA.
Marion Koso-Thomas, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Prof Alan H Jobe, Cincinnati Children's Hospital, Cincinnati, OH, USA.
Prof Pierre M Buekens, Tulane School of Public Health and Tropical Medicine, New Orleans, LA, USA.
References
- 1.National Institutes of Health. The effect of corticosteroids for fetal maturation on perinatal outcomes. Consensus Development Conference Statement; Feb 28–March 2, 1994; [accessed June 1, 2014]. http://consensus.nih.gov/1994/1994AntenatalSteroidPerinatal095html.htm. [PubMed] [Google Scholar]
- 2.Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol. 2010;39(suppl 1):i122–33. doi: 10.1093/ije/dyq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Katz J, Lee AC, Kozuki N, et al. the CHERG Small-for-Gestational-Age-Preterm Birth Working Group Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–25. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn JE, Blencowe H, Oza S, et al. for The Lancet Every Newborn Study Group Every Newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Das JK, Bahl R, et al. for The Lancet Newborn Interventions Review Group and The Lancet Every Newborn Study Group Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–70. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 7.WHO Expert Committee. The selection and use of essential medicines: report of the WHO Expert Committee, 2013 (including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essentials Medicines for Children) Geneva: World Health Organization; 2013. [Google Scholar]
- 8.UN Commission on Life-Saving Commodities for Women and Children. [accessed July 15, 2014];Commissioners' report September 2012. http://www.everywomaneverychild.org/images/UN_Commission_Report_September_2012_Final.pdf.
- 9.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L, for the Lancet Neonatal Survival Steering Team Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 10.Vargas-Origel A, Leon Ramirez D, Zamora-Orozco J. Corticoesterides antenatales: empleo y actitud del personal medico ginecoobstetra. Ginecol Obstet Mex. 2000;68:291–95. [PubMed] [Google Scholar]
- 11.Riganti AA, Cafferata ML, Althabe F, et al. Use of prenatal corticosteroids for preterm birth in three Latin American countries. Int J Gynaecol Obstet. 2010;108:52–57. doi: 10.1016/j.ijgo.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva LK, Costa TP, Reis AF, Iamada NO, Azevedo AP, Albuquerque CP. Assessment of quality of obstetric hospital care: use of corticoid in preterm labor. Cad Saude Publica. 1999;15:817–29. doi: 10.1590/s0102-311x1999000400016. (in Portuguese) [DOI] [PubMed] [Google Scholar]
- 13.Darmstadt GL, Kinney MV, Chopra M, et al. for The Lancet Every Newborn Study Group Who has been caring for the baby? Lancet. 2014;384:174–88. doi: 10.1016/S0140-6736(14)60458-X. [DOI] [PubMed] [Google Scholar]
- 14.Darmstadt GL, Lee AC, Cousens S, et al. 60 million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynaecol Obstet. 2009;107(suppl 1):S89–112. doi: 10.1016/j.ijgo.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClure EM, de Graft-Johnson J, Jobe AH, et al. A conference report on prenatal corticosteroid use in low- and middle-income countries. Int J Gynaecol Obstet. 2011;115:215–19. doi: 10.1016/j.ijgo.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad K, Costello A. Extreme caution is needed before scale-up of antenatal corticosteroids to reduce preterm deaths in low-income settings. Lancet Glob Health. 2014;2:e191–92. doi: 10.1016/S2214-109X(14)70020-8. [DOI] [PubMed] [Google Scholar]
- 17.Jehan I, Zaidi S, Rizvi S, et al. Dating gestational age by last menstrual period, symphysis-fundal height, and ultrasound in urban Pakistan. Int J Gynaecol Obstet. 2010;110:231–34. doi: 10.1016/j.ijgo.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garces A, McClure EM, Chomba E, et al. Home birth attendants in low income countries: who are they and what do they do? BMC Pregnancy Childbirth. 2012;12:34. doi: 10.1186/1471-2393-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson KE, Simen-Kapeu A, Kinney MV, et al. for The Lancet Every Newborn Study Group Every Newborn: health-systems bottlenecks and strategies to accelerate scale-up in countries. Lancet. 2014;384:438–54. doi: 10.1016/S0140-6736(14)60582-1. [DOI] [PubMed] [Google Scholar]
- 20.Althabe F, Belizán JM, Mazzoni A, et al. Antenatal corticosteroids trial in preterm births to increase neonatal survival in developing countries: study protocol. Reprod Health. 2012;9:22. doi: 10.1186/1742-4755-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goudar SS, Carlo WA, McClure EM, et al. The Maternal and Newborn Health Registry study of the Global Network for Women's and Children's Health Research. Int J Gynecol Obstet. 2012;118:190–93. doi: 10.1016/j.ijgo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belizán JM, McClure EM, Goudar SS, et al. Neonatal death in low- to middle-income countries: a global network study. Am J Perinatol. 2012;29:649–56. doi: 10.1055/s-0032-1314885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weijer C, Grimshaw JM, Eccles MP, et al. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9:e1001346. doi: 10.1371/journal.pmed.1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 25.Pasha O, Goldenberg RL, McClure EM, et al. Communities, birth attendants and health facilities: a continuum of emergency maternal and newborn care (the Global Network's EmONC trial) BMC Pregnancy Childbirth. 2010;10:82. doi: 10.1186/1471-2393-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmeyr GJ. Antenatal corticosteroids for women at risk of preterm birth: RHL commentary (last revised Feb 2, 2009) Geneva: World Health Organization Reproductive Health Library; 2009. [accessed July 6, 2014]. http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/index.html. [Google Scholar]
- 27.WHO Department of Reproductive Health and Research. Integrated management of pregnancy and childbirth Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. Geneva: World Health Organization; 2003. [accessed Oct 8, 2014]. http://whqlibdoc.who.int/publications/2003/924159084X.pdf. [Google Scholar]
- 28.Gail MH, Mark SD, Carroll RJ, Green SB, Pee D. On design considerations and randomization-based inference for community intervention trials. Stat Med. 1996;15:1069–92. doi: 10.1002/(SICI)1097-0258(19960615)15:11<1069::AID-SIM220>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 30.Ioannidis JP, Evans SJ, Gøtzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781–88. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 31.Campbell MK, Piaggio G, Elbourne DR, Altman DG, for the CONSORT Group Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 32.Onland W, de Laat MW, Mol BW, Offringa M. Effects of antenatal corticosteroids given prior to 26 weeks' gestation: a systematic review of randomized controlled trials. Am J Perinatol. 2011;28:33–44. doi: 10.1055/s-0030-1262509. [DOI] [PubMed] [Google Scholar]
- 33.Porto AM, Coutinho IC, Correia JB, Amorim MM. Efectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ. 2011;342:d1696. doi: 10.1136/bmj.d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blencowe H, Cousens S, Chou D, et al. on behalf of the Born Too Soon Preterm Birth Action Group Born Too Soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myles TD. Steroids—plenty of benefits, but not without risk. Obstet Gynecol. 2011;117:429–30. doi: 10.1097/AOG.0b013e31820711f0. [DOI] [PubMed] [Google Scholar]
- 36.Vermillion ST, Soper DE, Newman RB. Neonatal sepsis and death after multiple courses of antenatal betamethasone therapy. Am J Obstet Gynecol. 2000;183:810–14. doi: 10.1067/mob.2000.108838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.