Abstract
Cellular apoptosis is of major importance in the struggle between virus and host. Although many viruses use various strategies to control the cell death machinery by encoding anti-apoptotic virulence factors, it is now becoming clear that, in addition to their role in inhibiting apoptosis, these factors function in multiple immune and metabolic pathways to promote fitness and pathogenesis. In this Progress article, we discuss novel functions of viral anti-apoptotic factors in the regulation of autophagy in the nuclear factor-κB (NF-κB) pathway and in interferon signalling, with a focus on persistent and oncogenic gammaherpesviruses. If viral anti-apoptotic proteins are to be properly exploited as targets for antiviral drugs, their diverse and complex roles should be considered.
Escape from apoptosis is a common hallmark of viral infection that contributes to viral replication and propagation. Apoptosis proceeds via a caspase-activation cascade that is triggered either extrinsically (death receptor-mediated) or intrinsically (mitochondria-dependent), which results in the collapse of cellular infrastructure, mitochondrial membrane potential and cell membrane integrity (reviewed in REF. 1). To evade elimination via apoptosis, viruses, including many oncogenic viruses, target key regulatory steps in the apoptotic pathway2, including the inhibition of death receptor-mediated apoptotic signalling, as has been shown for viral FLICE-like inhibitory protein (vFLIP; also known as caspase 8-inhibitory protein); the modulation of mitochondrial permeability, as exemplified by viral homologues of the apoptosis regulator B cell lymphoma 2 (BCL-2); and interference with caspase activation by viral inhibitor of apoptosis (vIAP). In addition, viruses can inhibit apoptosis by targeting the pro-apoptotic tumour suppressor p53.
Importantly, a growing body of evidence shows that, in addition to their role in inhibiting apoptosis, these virally encoded proteins function in several immune and metabolic pathways, including autophagy, activation of nuclear factor-κB (NF-κB), interferon (IFN) signalling and cytoskeleton remodelling. As loss of homeostatic balance and immune activation promote the progression of viral infection and underlie pathogenic mechanisms in many virus-induced pathologies, a comprehensive understanding of these novel properties of viral apoptotic inhibitors could aid in the development of new strategies to prevent and treat multiple viral infectious diseases. In this Progress article, we discuss recent studies that have provided insights into novel functions of viral anti-apoptotic factors in the regulation of autophagy, in the NF-κB pathway and in IFN signalling. We mainly focus on oncogenic gammaherpesviruses (BOX 1), especially the human pathogen Kaposi's sarcoma-associated herpesvirus (KSHV) and a close relative of KSHV, mouse gammaherpesvirus 68 (γ-HV68), as the molecular or physiological functions of their anti-apoptotic factors have been most extensively studied and are likely to be conserved among other persistent viruses.
Box 1. Oncogenic gammaherpesviruses.
The gamma subfamily of herpesviruses comprises two major genera: the lymphocryptoviruses (also known as gamma-1), which include human Epstein–Barr virus; and rhadinoviruses (also known as gamma-2), which include human Kaposi's sarcoma-associated herpesvirus (KSHV). A striking feature that is shared by members of the gammaherpesvirus family is their ability to establish life-long latency in their hosts, with intermittent periods of lytic replication, which are associated with the development of several types of malignancies, including B cell lymphomas6. Viral proteins that are expressed during both the latent and lytic phases contribute considerably, albeit differently, to gammaherpesvirus-associated oncogenesis. Evasion of host immune responses that would otherwise eliminate the virus is crucial to the ability of the virus to maintain persistent infection. Via co-evolution with their host, gammaherpesviruses have evolved elaborate strategies to subvert the host defence mechanisms; for example, almost 50% of the KSHV genome is dedicated to modulating the host immune response, including blockage of innate apoptotic effects6. Intriguingly, most of these viral immune modulators, such as B cell lymphoma 2 (vBCL-2), viral FLICE-like inhibitory protein (vFLIP), viral inhibitor of apoptosis (vIAP) and viral interferon regulatory factor (vIRF), seem to be ‘pirated’ from the host and expressed as native host proteins or as homologues of host proteins to avoid being targeted by a particular branch of the immune system. These evasion strategies contribute substantially to the incidence of viral malignancies and their resistance to immune control.
Modulation of autophagy
Initially discovered as a cellular response to nutrient deficiency, macroautophagy (hereafter referred to as autophagy) has since been proven to be a fundamental homeostatic process in every eukaryotic organism3. In contrast to the apoptotic (‘self-killing’) programme, autophagy (‘self-eating’) involves lysosome-dependent bulk degradation of cytoplasmic proteins and organelles in a characteristic double-membrane-coated vesicle, known as the autophagosome3. The process of autophagy includes induction, nucleation of the phagophore, elongation, fusion and degradation. As part of its intrinsic role in maintaining homeostasis, autophagy is a cellular defence mechanism against infections by various pathogens (for more details, see REF. 4). The cytoplasmic localization of autophagosomes enables them to target intracellular pathogens or pathogen components for lysosomal degradation. In addition, autophagic degradation releases signals for viral recognition, type I IFN secretion, as well as antigen presentation4. Hence, autophagy is important in antiviral immunity and is therefore blocked by certain viruses to establish successful infection5. This is particularly pertinent in KSHV, which establishes long-term latent infection in lymphoid or endothelial cells, in which it can be reactivated, sometimes frequently, to cause recurrent disease and viral spreading, without being cleared by the host immune system6. Almost every stage of the autophagy pathway is targeted by KSHV proteins that were originally identified as inhibitors of apoptosis. Such a dual role in both apoptosis and autophagy reflects the substantial crosstalk between the host apoptotic and autophagic machineries during viral infection. For clarity, we discuss the many interactions between viral anti-apoptotic factors and the host autophagy pathway in relation to the linear cascade of the autophagic progress (FIG. 1).
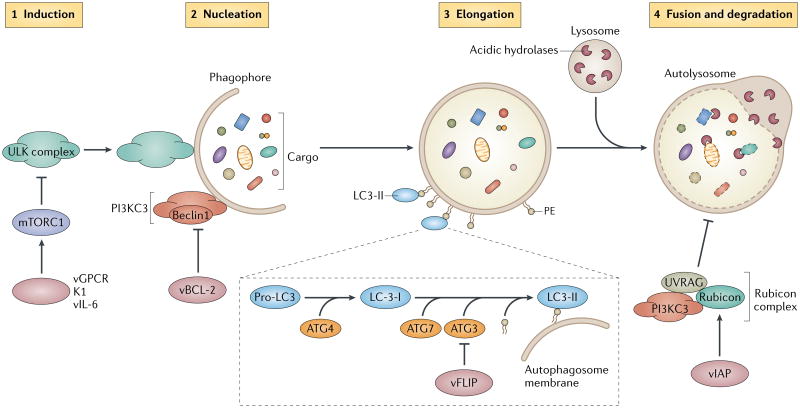
Figure 1. Viral anti-apoptotic factors target autophagy.
Autophagy proceeds through a series of steps, which include induction, phagophore nucleation, elongation and fusion of the autophagosome with the lysosome for degradation of the sequestered cargoes. Mammalian target of rapamycin complex 1 (mTORC1) is a negative regulator of autophagy that, under nutrient-rich conditions, prevents the assembly and the activation of the autophagy-initiating ULK complex (which comprises ULK1, ATG13 and FIP200) (step 1). mTORC1 is inactivated in response to various stimuli (for example, starvation) and autophagy is induced. Viral G protein-coupled receptor (vGPCR), K1 and viral interleukin-6 (vIL-6) of Kaposi's sarcoma-associated herpesvirus (KSHV) promote the constitutive activation of mTORC1, which may suppress autophagy in infected cells. Phagophore nucleation is driven by the class III phosphoinositol 3-kinase complex (PI3KC3), which includes Beclin1 (step 2). The activity of this lipid kinase complex is inhibited by the anti-apoptotic viral B cell lymphoma 2 (vBCL-2) of gammaherpesviruses, which strongly sequesters Beclin1 to suppress phagophore nucleation. Growth and closure of the autophagosome membrane involve the conjugation of the autophagy marker LC3 to phosphatidylethanolamine in the membrane, which is sequentially processed by ATG4, ATG7 and ATG3 (step 3). KSHV FLICE-like inhibitory protein (vFLIP) competes with LC3 for binding to ATG3 to inhibit expansion of the autophagosome. The fusion of the autophagosome with the lysosome to form a single membrane-bound auto-lysosome signifies the maturation stage of the autophagy pathway (step 4). Consequently, the encapsulated contents, together with the inner membrane of the autophagosome, undergo breakdown by lysosomal hydrolases. Maturation is negatively regulated by the host Rubicon complex, which comprises Rubicon, UVRAG (UV irradiation resistance-associated protein), Beclin1 and other members of the PI3KC3 complex. KSHV viral inhibitor of apoptosis (vIAP) forms a complex with Rubicon and enhances Rubicon-mediated inhibition of autophagosome maturation.
Targeting induction
Gammaherpesviruses interfere with upstream autophagy signalling by targeting the ‘nutrient sensor’, mammalian target of rapamycin complex 1 (mTORC1), which integrates metabolic signals from the microenvironment of the cell and represses the autophagy-initiating ULK1 complex7–9 (FIG. 1). Viral proteins, including K1, viral interleukin-6 (vIL-6) and a viral G protein-coupled receptor (vGPCR) of KSHV, have been shown to promote the activation of the phosphoinositide 3-kinase (PI3K)–AKT–mTORC1 pathway, which is required for the development of the KSHV-associated vascular tumour Kaposi's sarcoma7,8. More recent findings further established that the activation of the ERK2–RSK1–TSC2 pathway by the KSHV protein ORF45 also contributes to activation of mTORC1; however, surprisingly, this only occurred in lymphatic endothelial cells and not in blood vessel endothelial cells10, which suggests that there is a tissue-specific pathogenic mechanism. It remains to be determined whether inhibition of autophagy is involved in KSHV-associated sarcomagenesis; however, constitutive stimulation of mTORC1 signalling may be an important viral mechanism to suppress autophagy in infected cells and promote long-term survival of the virus and oncogenic transformation.
Targeting nucleation
Nucleation of the phagophore is driven by the activation of the Beclin1-associated class III PI3K (PI3KC3) complex3 (FIG. 1). This process is suppressed by the anti-apoptotic protein BCL-2, which binds to Beclin1 to prevent autophagosome formation11. Virus-encoded orthologues of BCL-2 (vBCL-2) contribute to immune evasion in all gammaherpesviruses, including Epstein–Barr virus (EBV), KSHV, herpesvirus saimiri and γ-HV68. Initial studies on vBCL-2 focused on its ability to prevent the mitochondrial release of apoptogenic factors, which enables completion of viral replication12. Paradoxically, loss of vBCL-2 in γ-HV68 and KSHV did not affect acute infection but instead severely impaired chronic infection in the host13,14, which suggests that its inhibitory effect on apoptosis is not the sole action of vBCL-2 and that other targets are also involved in vBCL-2-mediated virulence. It is now clear that, analogous to the cellular counterpart, the vBCL-2 proteins of KSHV and γ-HV68 attenuate autophagy via direct interaction with host Beclin1 and confer even more robust inhibition than the host BCL-2 (REF. 15). In addition, the affinity of vBCL-2 proteins for Beclin1 is higher than that of pro-apoptotic BCL-2 family members such as BAX or BAK, which suggests that Beclin1 is the main target of vBCL-2 proteins of KSHV and γ-HV68 during chronic infection15. Consistent with this, targeted elimination of the anti-autophagic activity of vBCL-2 severely attenuated the ability of γ-HV68 to maintain a latent reservoir in splenocytes, which is a prerequisite for persistent infection and transmission, whereas the mutant virus that lacks the anti-apoptotic property of vBCL-2 had reduced viral reactivation frequency16. These findings suggest that vBCL-2-mediated inhibition of autophagy and evasion of apoptosis have different roles in the γ-HV68 life cycle — maintenance of the latent reservoir and reactivation, respectively16. As persistent infection is crucial for gammaherpesvirus-associated malignancy, vBCL-2-mediated inhibition of autophagy might contribute to the oncogenic potential of these viruses13. It is not currently clear whether the two viral BCL-2 homologues (BHRF1 and BALF1) that are encoded by EBV also have the capability to inhibit autophagy; however, owing to their structural and functional homology to cellular BCL-2, it seems likely that BHRF1 and/or BALF1 may also function as anti-autophagic factors. Importantly, Beclin1-mediated autophagy is also targeted by other anti-apoptotic proteins from other herpesviruses; for example, the neurovirulence factor, ICP34.5, which prevents apoptosis in herpes simplex virus 1 (HSV-1)-infected cells, directly binds to Beclin1 and thereby blocks virus-induced autophagy17.
Notably, the control of autophagy by vBCL-2 is both complex and context specific, as the interaction of the adenoviral BCL-2 homologue E1B19K with Beclin1 results in the induction of autophagy, which might be required for lytic replication18.
Inhibiting autophagosome elongation
In addition to targeting autophagy induction and nucleation, gammaherpesviruses have evolved a second strategy to subvert this host process by expressing latency-associated vFLIP. This viral protein contains two death effector domains and was originally described to inhibit death receptor-mediated caspase activation19. However, recent data suggest that vFLIP from KSHV can induce the NF-κB pathway (see below) and impair elongation of the autophagosome membrane in latently infected cells19,20. Autophagosome elongation involves two ubiquitin-like conjugation reactions, one in which ATG12 is covalently attached to ATG5 and one in which LC3 (mammalian ATG8) is covalently attached to phosphatidylethanolamine (reviewed in REF. 3) (FIG. 1). Conjugation of LC3 to autophagosome-bound phosphatidylethanolamine is sequentially processed by ATG7 (an E1-like enzyme) and ATG3 (an E2-like enzyme)3. A study showed that both KSHV vFLIP and cellular FLIP (cFLIP) compete with LC3 for binding to ATG3, but vFLIP has evolved to be a more potent inhibitor of autophagosome expansion than cFLIP20. In support of this finding, expression of vFLIP inhibits rapamycin-induced autophagic cell death of KSHV-infected B lymphocytes20. Despite these advances in our understanding of the role of vFLIP in the regulation of autophagy, the exact function of vFLIP-mediated inhibition of autophagy during viral infection remains unclear. It was speculated that the antagonism of host autophagy by vFLIP may promote chronic infection. It is also worth noting that persistent suppression of autophagy could have important non-cytolytic oncogenic roles; for example, it may be involved in antagonizing autophagy-related antigen presentation of viral latent proteins to escape immune detection. Strikingly, a peptide derived from KSHV vFLIP has the opposite effect by binding to vFLIP itself and thereby preventing the vFLIP–ATG3 interaction, which leads to a substantial induction of autophagy-related death of KSHV-infected cells20. The potent effects and functional specificity of the gammaherpesvirus-derived antiviral peptides might be useful in the treatment or prevention of infection.
Modulating autophagosome maturation
Mammalian viruses express structural homologues of IAP, which, similarly to cellular IAP, have anti-apoptotic function; for example, KSHV vIAP, which is encoded by the ORF K7 and localizes to mitochondria in infected cells, has been shown to inhibit apoptosis in response to several stimuli21. In addition to the diverse anti-apoptotic roles of vIAP, a distinct function for this viral protein in the late stage of autophagosome maturation was recently identified22, and this has not been found in its cellular counterpart. The autophagosome matures by fusion with the late endosome and the lysosome, which is a process that is negatively regulated by Rubicon (RUN domain Beclin1-interacting and cysteine-rich containing protein) in complex with UVRAG (UV irradiation resistance-associated protein), Beclin1 and other members of the PI3KC3 complex23 (FIG. 1). Overexpression of Rubicon results in the accumulation of autophagic cargoes, whereas Rubicon depletion induces their degradation, consistent with a role in the inhibition of autophagy23. KSHV vIAP interacts with Rubicon and increases its inhibitory effect on autophagosome maturation in lytically infected cells22. Although the in vivo relevance of vIAP-mediated inhibition of autophagic degradation has not been investigated, it is likely that it may promote survival of the virus in host cells.
In summary, the involvement of different viral anti-apoptotic proteins in various steps of autophagy suggests that interference with this cellular process is a common strategy that is used by viral pathogens and could therefore be a potential therapeutic target for the treatment of virally associated malignancies in gammaherpesvirus infection.
Regulating NF-κB signalling
The NF-κB pathway is a prime target for viral pathogens as many of the protein products of NF-κB target genes profoundly influence the host cell microenvironment and the immune response24. Whereas transient activation of NF-κB is an integral part of normal physiological regulation, excessive and persistent activation of NF-κB signalling is often promoted by tumorigenic viruses to optimize their intracellular stage and to induce chronic inflammation and oncogenic transformation25. In addition, some viruses modulate the NF-κB pathway to either evade host apoptosis to increase the production of progeny virions, or conversely, to trigger cell lysis to increase viral spread. Thus, the context of NF-κB signalling is an important consideration; the same virulence factor may have opposite effects depending on when and where it is deployed.
Members of the mammalian NF-κB family of transcription factors form heterodimers and activate or inhibit the expression of several genes, including the genes that encode pro-inflammatory cytokines, chemokines and their respective receptors, as well as genes that regulate cell survival and proliferation. In most cell types, NF-κB complexes are sequestered in the cytoplasm by a family of inhibitory proteins known as inhibitors of NF-κB (IκBs). A remarkably diverse range of stimuli and viral factors, including Tax from human T cell leukaemia virus type 1 (HTLV1), Tat from HIV-1, LMP1 from EBV, UL31 from HSV1, UL144 from human cytomegalovirus (HCMV) and HBx from hepatitis B virus (reviewed in REF. 25), function via distinct mechanisms to activate NF-κB, but most of these mechanisms converge on the upstream IκB kinase (IKK) complex24. The IKK complex consists of two catalytic subunits, IKKα and IKKβ, and the non-catalytic scaffold and regulatory subunit, NF-κB essential modifier (NEMO), and is activated by cytosolic adaptors (for example, the TRAF family of proteins)24. IKK-mediated phosphorylation subsequently targets IκB for protein degradation, which enables NF-κB to translocate to the nucleus and regulate target gene expression. Together with IKKα and IKKβ, NEMO functions as a molecular switch to activate the canonical NF-κB pathway, which has been shown to be modulated by KSHV vFLIP to promote viral latency and pathogenesis.
vFLIP targets NF-κB signalling
As mentioned in the previous sections, the importance of vFLIP for viral infection has only recently been understood, concomitant with an appreciation for the crucial role of NF-κB activation and the regulation of autophagy in chronic gammaherpesvirus infection and pathogenesis19,26,27. Although vFLIP was initally shown to inhibit caspase activation and Fas-mediated apoptosis28, this finding was not confirmed in vFLIP-expressing transgenic mice19. Instead, vFLIP expression was found to induce NF-κB-regulated cytokine expression and secretion and to contribute to the pro-inflammatory microenvironment of Kaposi's sarcoma29. In addition to the secretion of cytokines, the constitutive activation of NF-κB signalling by vFLIP is mechanistically associated with the spindle cell morphological transformation of endothelial cells, which is a hallmark of Kaposi's sarcoma lesions30. Of note, deregulated activation of NF-κB by vFLIP also induces the transcriptional activation of several host anti-apoptotic factors, such as BCL-2 and BCL-XL, cIAP-1 and cIAP-2, which promotes the resistance of virally infected cells to programmed cell death31,32. In accordance with this, RNA interference (RNAi)-mediated silencing of vFLIP or treatment with specific NF-κB inhibitors triggered apoptosis of KSHV-transformed B cells and impaired tumorigenesis in mice with KSHV-transformed cells33,34. Thus, it is conceivable that vFLIP blocks host apoptosis either directly via caspase inactivation or indirectly via the activation of NF-κB-dependent transcription factors that induce anti-apoptotic target genes, which is essential for the survival and transformation of infected cells.
Unlike c-FLIP, which is cleaved by active caspase 8 and then interacts with TRAF or NEMO to activate NF-κB35, KSHV vFLIP-stimulated NF-κB signalling bypasses the upstream mediators (such as TRAF family proteins) and directly binds to NEMO to assemble the vFLIP-responsive IKK signalosome, which leads to the continuous activation of NF-κB36–38 (FIG. 2a). In agreement with this, a recent observation39 indicates that binding of KSHV vFLIP to NEMO induces a conformational shift in NEMO from a helical bundle to an open conformation, which activates the catalytic subunits of IKKα and IKKβ. Indeed, KSHV vFLIP can induce both the canonical and non-canonical NF-κB pathway via an upstream adaptor-independent and NEMO- and IKKα-dependent mechanism. In addition, the crosstalk of other parallel signalling pathways may affect the vFLIP-mediated NF-κB response during different stages of the viral life cycle. A recent study40 showed that vFLIP also inhibits NF-κB: expression of vFLIP was found to induce robust phosphorylation of NEMO at Y374 and S377, which normally confers a negative feedback loop for tumour necrosis factor (TNF)-induced NF-κB activation. Furthermore, A20 binds to vFLIP and blocks vFLIP-induced activation of NF-κB, which generates a negative feedback loop to regulate vFLIP-mediated NF-κB signalling41. Although it remains unclear whether the anti-inflammatory roles of vFLIP result from a viral-specific process or from the secondary effects of host defences, the ability of vFLIP to induce — as well as to repress — NF-κB suggests that activation of NF-κB is not always the default pathway for host immunity during viral infection. Strategies to modulate, rather than to induce or inhibit, NF-κB could promote a beneficial outcome for the virus, which should also be considered for the therapeutic targeting of the NF-κB pathway.
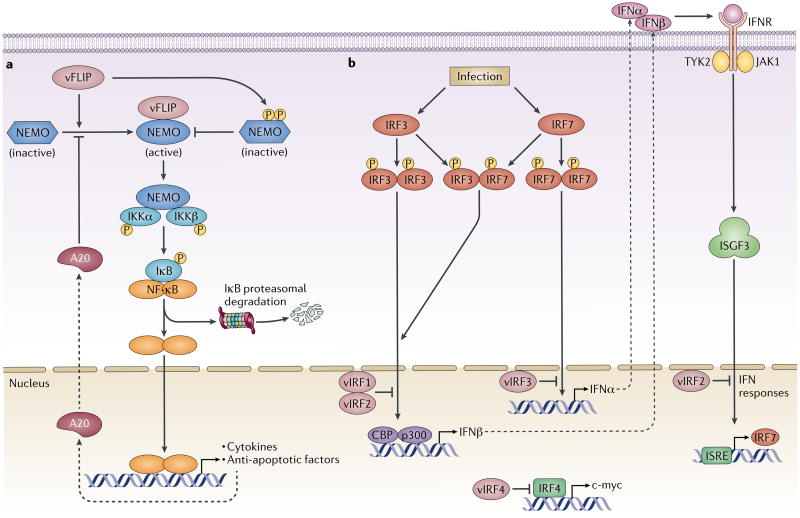
Figure 2. Interference with the NF-kB and IFN pathways by viral anti-apoptotic factors.
a| The Kaposi's sarcoma-associated herpesvirus (KSHV) anti-apoptotic protein viral FLICE-like inhibitory protein (vFLIP) activates nuclear factor- κB (NF-κB) signalling by targeting NF-κB essential modifier (NEMO), which is a central regulatory subunit of the upstream inhibitor of NF-κB (IκB) kinase (IKK) complex. Following stimulation by extracellular stress signals or viral pathogens, the IKK complex is activated and phosphorylates IκBs, which leads to their proteosomal degradation and the release of NF-κB dimers. Binding of KSHV vFLIP to NEMO induces and stabilizes a conformational change in the host protein that enables the recruitment of IKKα and IKKβ and the continuous activation of NF-κB. NF-κB induces the expression of inflammatory, proliferative and anti-apoptosis genes, including the gene that encodes A20. A20 binds to vFLIP and blocks vFLIP-induced NF-κB activation, which generates a negative feedback loop. In addition, expression of vFLIP enhances the phophorylation of NEMO at Y374 and S377, which negatively regulates its function. The dual regulation of NF-κB signalling by vFLIP suggests that the virus modulates this pathway to promote survival. b| KSHV encodes a cluster of four viral homologues of interferon (IFN) regulatory factors (IRFs) (vIRF1–4). In addition to suppressing p53-mediated apoptosis, vIRFs also function as negative regulators of type I IFN responses. Transcription of type I IFNα and IFNβ following viral infection is primarily controlled by IRF3 and IRF7. Cytoplasmic IRF3 and IRF7 undergo phosphorylation, homodimerization and heterodimerization, followed by nuclear translocation to induce the expression of type I IFNs. Binding of IFNs to the type I IFN receptor (IFNR) induces the expression of IFN-stimulated genes (ISGs), which initiate an intracellular antiviral programme to restrict viral replication and spread. Both vIRF1 and vIRF2 inhibit IRF3-mediated transcriptional activation and the production of IFNβ, whereas vIRF2 can also inhibit IFNα-induced induction of IFN-stimulated response element (ISRE)-containing genes. vIRF3 specifically interacts with, and inhibits, IRF7 and thereby abrogates the production of IFNα and IFN-mediated immunity. vIRF4 is an inhibitor of IRF4, which unlike other vIRFs, mainly inhibits IRF4-mediated expression of the proto-oncogene c-MYC to facilitate viral lytic replication and/or reactivation. ISGF3, IFN-stimulated gene factor 3; JAK, Janus kinase; TYK2, non-receptor tyrosine kinase 2.
Interference with the IFN response
Although apoptosis is a formidable defence mechanism against viral infection, it is also costly to the host. This is particularly true for irreplaceable cells such as neurons. The non-cytolytic antiviral programmes that are presented by IFNs can arrest the viral life cycle, which leads to the potent attenuation of viral spread without killing cells. Type I IFNs (that is, IFNα and IFNβ) are produced in response to viral infection in nearly every cell type and are transcriptionally controlled by cellular IFN regulatory factors (IRFs) (reviewed in REF. 42). Among the nine IRF family members that have been identified so far (IRF1 to IRF9), IRF3 and IRF7 are the key regulators of IFNA and IFNB gene expression in response to viral infection42. Unlike IRF3, IRF7 expression in most cells is low but is strongly induced by type I IFN-mediated signalling, which suggests that IFNA and IFNB gene induction occurs sequentially42. The initial IRF3-mediated IFNβ induction (known as the first phase) triggers a positive feedback upregulation of IFN-inducible IRF7, which can in turn activate both IFNA and IFNB (known as the second phase)42. The large set of genes that are induced by IFNα and IFNβ ultimately establishes the ‘antiviral state’ to arrest the infection, which in some circumstances can also promote apoptosis of infected cells. Viruses have undoubtedly evolved various strategies to circumvent the IFN response: viral structural homologues of cellular IRFs block the cellular activities of IRFs and block IFN gene transcription in a dominant-negative manner in addition to targeting the cellular IRF-induced anti-apoptotic programme43. A cluster of four viral IRF derivatives (vIRF1–4) are encoded by KSHV to elicit distinct evasion of IFNs and apoptosis43.
vIRFs modulate antiviral defence
A key mechanism by which KSHV establishes infection and malignancy is the inhibition of p53-mediated apoptosis. Indeed, all KSHV vIRFs have evolved to independently subvert the p53 pathway by targeting p53 itself or by targeting its modulators, such as ATM, HAUSP and MDM2, as well as by modulating pro-death molecules or activating caspases43.
In addition to inhibiting apoptosis during in vivo infection, vIRFs are equally — if not more — crucial for the dysregulation of virus-mediated induction of IFNs for a sustained infection and for immune evasion (FIG. 2b). KSHV vIRF1, vIRF2 and vIRF3, but not vIRF4, have all been shown to inhibit the induction of IFN and IFN-stimulated genes (ISGs) in infected cells via interference with IRFs43. Unlike vIRF1 and vIRF2, which mainly target the IRF3-mediated IFN and inflammatory response, vIRF3 interacts with IRF7 and inhibits its DNA-binding activity, thereby abrogating the production of IFNα43. A recent study44 showed that vIRF4 functions as a dominant inhibitor of cellular IRF4, but instead of decreasing IFN signalling, vIRF4 inhibits IRF4-dependent expression of the c-MYC proto-oncogene, which is probably needed for productive viral reactivation. Although different vIRFs often target the same cellular pathways, they may function differently depending on the cellular context and the stage of the viral life cycle to promote viral replication and oncogenicity. Collectively, the involvement of vIRFs in both IFN- and apoptosis-mediated antiviral defence suggests that these two processes are tightly linked and are regulated in a coordinated manner, which makes vIRFs promising therapeutic targets for viral infection control.
Conclusions
Disruption of cellular apoptosis is a common strategy of viruses. The apoptosis pathway in host cells does not exist in isolation and is integrated with other cell signalling networks. This crosstalk reshapes the biological functions of viral anti-apoptotic factors during virus–host co-evolution. As outlined in this Progress article, viral anti-apoptotic factors have evolved to exploit multiple metabolic and immune pathways, including, but not limited to, autophagy and the IFN and NF-κB signalling pathways, by directly interacting with key factors of these host pathways. Intriguingly, their diverse roles are often independent of their anti-apoptotic properties; for example, a recent study has shown that, in addition to blocking apoptosis at the mitochondrial checkpoint, the HCMV homologues of mitochondrial inhibitor of apoptosis (vMIA) can co-opt the host antiviral protein Viperin to disrupt the cytoskeleton, which in turn promotes replication of the virus45. However, how these new immune-modulatory functions of virulence factors are fine-tuned in host–pathogen interactions and how they relate to the regulation of the viral life cycle and pathogenesis, as well as how they relate to host pathological conditions, remain unknown. Therefore, an understanding of the complexity and specificity of the viral strategy to subvert apoptosis and how this is integrated with other signalling networks has the potential to inform appropriate therapeutic strategies and provides an opportunity for the effective exploitation of these proteins in the treatment of different infectious diseases in the future.
Acknowledgments
C.L. is funded by the by American Cancer Society (grant RSG-11-121-01-CCG) and the US National Institutes of Health (NIH) (grants CA140964 and CA161436). B.-H.O is funded by the GRL Program (K20815000001) and KAIST Institute Cancer Metastasis Control Center (grant N1014002). J.U.J is funded by the NIH (grants CA082057, CA31363, CA115284, CA180779, AI073099, AI105809 and HL110609), the Global Research Laboratory (GRL) Program (K20815000001) from National Research Foundation of Korea, the Hastings Foundation and the Fletcher Jones Foundation.
Footnotes
Competing interests statement: The authors declare no competing interests.
Contributor Information
Chengyu Liang, Department of Molecular Microbiology and Immunology, University of Southern California, Los Angeles, California 90033, USA.
Byung-Ha Oh, Department of Biological Sciences, KAIST Institute for the Biocentury, Korea Advanced Institute of Science and Technology, Daejeon 305–701, Korea.
Jae U. Jung, Department of Molecular Microbiology and Immunology, University of Southern California, Los Angeles, California 90033, USA
References
- 1.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nature Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 2.Fuentes-Gonzalez AM, Contreras-Paredes A, Manzo-Merino J, Lizano M. The modulation of apoptosis by oncogenic viruses. Virol J. 2013;10:182. doi: 10.1186/1743-422X-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signalling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgin HW, Levine B. Autophagy genes in immunity. Nature Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orvedahl A, Levine B. Autophagy in mammalian antiviral immunity. Curr Top Microbiol Immunol. 2009;335:267–285. doi: 10.1007/978-3-642-00302-8_13. [DOI] [PubMed] [Google Scholar]
- 6.Liang C, Lee JS, Jung JU. Immune evasion in Kaposi's sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol. 2008;18:423–436. doi: 10.1016/j.semcancer.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodhi A, et al. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10:133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt AP, Damania B. AKTivation of PI3K/AKT/ mTOR signalling pathway by KSHV. Front Immunol. 2012;3:401–417. doi: 10.3389/fimmu.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esclatine A, Chaumorcel M, Codogno P. Macroautophagy signalling and regulation. Curr Top Microbiol Immunol. 2009;335:33–70. doi: 10.1007/978-3-642-00302-8_2. [DOI] [PubMed] [Google Scholar]
- 10.Chang HH, Ganem D. A unique herpesviral transcriptional program in KSHV-infected lymphatic endothelial cells leads to mTORC1 activation and rapamycin sensitivity. Cell Host Microbe. 2013;13:429–440. doi: 10.1016/j.chom.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Cuconati A, White E. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- 13.Tarakanova VL, Kreisel F, White DW, Virgin HW., 4th Murine gammaherpesvirus 68 genes both induce and suppress lymphoproliferative disease. J Virol. 2008;82:1034–1039. doi: 10.1128/JVI.01426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh J, et al. A surface groove essential for viral Bcl-2 function during chronic infection in vivo. PLoS Pathog. 2005;1:e10. doi: 10.1371/journal.ppat.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku B, et al. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008;4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiaofei E, et al. Viral Bcl-2-mediated evasion of autophagy aids chronic infection of gammaherpesvirus 68. PLoS Pathog. 2009;5:e1000609. doi: 10.1371/journal.ppat.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orvedahl A, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Piya S, et al. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS ONE. 2011;6:e29467. doi: 10.1371/journal.pone.0029467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chugh P, et al. Constitutive NF-κB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci USA. 2005;102:12885–12890. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, et al. FLIP-mediated autophagy regulation in cell death control. Nature Cell Biol. 2009;11:1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi's sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602–2615. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Q, et al. Kaposi's sarcoma-associated herpesvirus K7 modulates Rubicon-mediated inhibition of autophagosome maturation. J Virol. 2013;87:12499–12503. doi: 10.1128/JVI.01898-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Y, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nature Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 25.Sun SC, Cesarman E. NF-κB as a target for oncogenic viruses. Curr Top Microbiol Immunol. 2011;349:197–244. doi: 10.1007/82_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Q, Matta H, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-κB activation. Blood. 2003;101:1956–1961. doi: 10.1182/blood-2002-07-2072. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Matta H, Chaudhary PM. Kaposi's sarcoma associated herpes virus-encoded viral FLICE inhibitory protein activates transcription from HIV-1 Long Terminal Repeat via the classical NF-κB pathway and functionally cooperates with Tat. Retrovirology. 2005;2:9. doi: 10.1186/1742-4690-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Djerbi M, et al. The inhibitor of death receptor signalling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q, Zachariah S, Chaudhary PM. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-κB activation. J Biol Chem. 2003;278:52437–52445. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann C, Podgrabinska S, Skobe M, Ganem D. Activation of NF-κB by the latent vFLIP gene of Kaposi's sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J Virol. 2006;80:7179–7185. doi: 10.1128/JVI.01603-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-κB pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 32.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Godfrey A, Anderson J, Papanastasiou A, Takeuchi Y, Boshoff C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood. 2005;105:2510–2518. doi: 10.1182/blood-2004-08-3052. [DOI] [PubMed] [Google Scholar]
- 34.Keller SA, et al. NF-κB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood. 2006;107:3295–3302. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozturk S, Schleich K, Lavrik IN. Cellular FLICE-like inhibitory proteins (c-FLIPs): fine-tuners of life and death decisions. Exp Cell Res. 2012;318:1324–1331. doi: 10.1016/j.yexcr.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Matta H, et al. Kaposi's sarcoma associated herpesvirus encoded viral FLICE inhibitory protein K13 activates NF-κB pathway independent of TRAF6, TAK1 and LUBAC. PLoS ONE. 2012;7:e36601. doi: 10.1371/journal.pone.0036601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, et al. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the IκB kinase complex. J Biol Chem. 2002;277:13745–13751. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- 38.Field N, et al. KSHV vFLIP binds to IKK-γ to activate IKK. J Cell Sci. 2003;116:3721–3728. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- 39.Bagneris C, et al. Crystal structure of a vFlip-IKKγ complex: insights into viral activation of the IKK signalosome. Mol Cell. 2008;30:620–631. doi: 10.1016/j.molcel.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, et al. Novel phosphorylations of IKKγ/ NEMO. mBio. 2012;3:e00411–12. doi: 10.1128/mBio.00411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matta H, et al. A20 is induced by Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 and blocks K13-induced nuclear factor-κB in a negative feedback manner. J Biol Chem. 2011;286:21555–21564. doi: 10.1074/jbc.M111.224048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honda K, Takaoka A, Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Baresova P, Pitha PM, Lubyova B. Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer. J Virol. 2013;87:9398–9410. doi: 10.1128/JVI.03315-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HR, et al. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 4 (vIRF4) targets expression of cellular IRF4 and the Myc gene to facilitate lytic replication. J Virol. 2014;88:2183–2194. doi: 10.1128/JVI.02106-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein Viperin to enhance infectivity. Science. 2011;332:1093–1097. doi: 10.1126/science.1202007. [DOI] [PubMed] [Google Scholar]