Abstract
Prenatal stress (PS) impairs memory function; however, it is not clear whether PS-induced memory deficits are specific to spatial memory, or whether memory is more generally compromised by PS. Here we sought to distinguish between these possibilities by assessing spatial, recognition and contextual memory function in PS and nonstressed (NS) rodents. We also measured anxiety-related and social behaviors to determine whether our unpredictable PS paradigm generates a behavioral phenotype comparable to previous studies. Female Sprague-Dawley rats were exposed to daily random stress during the last gestational week and behavior tested in adulthood. In males but not females, PS decreased memory for novel objects and novel spatial locations, and facilitated memory for novel object/context pairings. In the elevated zero maze, PS increased anxiety-related behavior only in females. Social behaviors also varied with sex and PS condition. Females showed more anogenital sniffing regardless of stress condition. In contrast, prenatal stress eliminated a male-biased sex difference in nonspecific bodily sniffing by decreasing sniffing in males, and increasing sniffing in females. Finally, PS males but not females gained significantly more weight across adulthood than did NS controls. In summary, these data indicate that PS differentially impacts males and females resulting in sex-specific adult behavioral and bodily phenotypes.
Keywords: prenatal, stress, gestation, maternal, memory, novel object, environment, context, anxiety, social behavior, body weight, obesity, development, gender, sex differences, hippocampus, medial temporal lobe, psychopathology
1. Introduction
Spatial memory ability is diminished in the offspring of dams that were stressed during pregnancy. For example, prenatally stressed (PS) rodents show altered performance in spatial memory-dependent tasks such as the radial arm maze [1,2], T maze [3] and Morris water maze [4–13]. Lesion studies demonstrate that spatial memory is hippocampal based. Given that PS alters many aspects of hippocampal morphology including glucocorticoid receptor levels, neurogenesis, dendritic length, spine density, and overall hippocampal volume [13–16], these stress-induced changes may contribute to the effects of PS on spatial memory.
Although PS clearly impacts hippocampal-associated spatial memory function, the hippocampus is also important for recognition (visual memory for the features of specific objects) and contextual memory (the ability to recognize mismatched object and environmental context pairings). For example, many studies show impairments in recognition memory following hippocampal lesions [17–21], and contextual memory also depends on the integrity of the hippocampus [22]. Thus, it is surprising that studies of prenatal stress effects on adult memory have focused almost exclusively on spatial memory function. Indeed, we found only one published account on the effects of PS on recognition memory [23], and no studies of the effects of PS on contextual memory. The current study directly compares the effects of PS on recognition, spatial and contextual memory function in adult male and female rats. Three variations of the novel object recognition paradigm were employed to test these memory types. The primary advantage of modifying the same memory paradigm to test three different memory types is that levels of training, handling, and stress are held constant across spatial, recognition, and contextual memory trials [22]. We also measured anxiety-related and social behaviors to determine whether the unpredictable PS paradigm employed here generates sex-specific behavioral alterations comparable to previous literature reports.
2. Materials and Methods
2.1. Pregnant dams
Twelve timed-pregnant Sprague-Dawley rats were ordered from Charles Rivers Laboratories (Portage, MI) and were 2 days pregnant upon arrival. Pregnant females were singly housed in static clear polycarbonate cages with wire bar lids and microisolator air filtration covers. All animals had ad libitum access to food and water. Bedding (Tekfresh, Harlan Laboratories Inc., Indianapolis, IN), food (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories Inc., Indianapolis, IN), and filtered water were changed weekly. One day prior to parturition, the females were transferred to larger cages (40.6 × 30.5 × 20.3 cm) and extra bedding was provided as nesting material. Room conditions were maintained at 21 °C with a 12:12 light/dark cycle. All animals were treated in accordance with NIH guidelines and all protocols were approved by the IACUC of the University of Colorado Denver.
2.2 Prenatal stress procedure
Half of the pregnant females were randomly selected to experience unpredictable variable stress 2–3 times daily during the last week of gestation (prenatal days 14–21). The stressors were mild in nature and included restraint in cylindrical restrainers (30 min), swim in water at room temperature (15 min), exposure to a cold room at 4 °C (6 hours), social stress (5 rats/cage for 9 hours), and an overnight fast [24]. All stressed animals received the same schedule of stressors. The remaining 6 females served as controls and were exposed to only routine animal husbandry.
2.3 Litters
All pups were born on gestation day 22. Food and water continued to be replaced weekly following parturition, but the bedding and nests were left undisturbed until weaning at 22 days of age to minimize stress [as detailed in 24]. Cage cleanliness was closely monitored during this time, and additional bedding was provided if necessary. Upon weaning, weekly cage changing resumed, and animals were housed 2 per cage with same-sex littermates. It was not feasible to completely prevent litter effects by using only one representative pup from each litter [25], however, we tried to minimize litter effects by employing only two animals (of each sex) per litter (n=10/group; overall N=40).
2.4 Experimental timeline
Animals were acclimated to the test arena for 5 minutes on 4 consecutive days. On the first day animals were exposed only to the test arena, whereas on the 3 subsequent days animals were also habituated to toys. The toys used for habituation were not used again for testing. Memory testing (object and spatial trials) occurred in adulthood between 70–80 days of age, and novel object-context testing (context trials) occurred at 170 days of age. Additional testing for anxiety-related behaviors was performed in adulthood at 180 days of age using an elevated zero maze (San Diego Instruments, San Diego, CA). Social interaction testing was performed last at 187 days of age. During the object and spatial trials prenatally stressed animals appeared to be less anxious than nonstressed controls and spent more time in the center zone of the testing arena (data not presented here). In order to directly test this possibility we obtained the elevated zero apparatus, which necessarily caused a testing delay.
2.5 Behavioral testing
2.5.1 Apparatus and stimuli
Experimental tests with the exception of the elevated zero test were performed in arenas constructed of mat black expanded PVC (70 cm × 70 cm; wall height = 47.6 cm). To facilitate the spatial location tests (spatial trials), a false floor comprised of 4 separate square tiles was fitted into the test box. The individual floor tiles allowed for flexibility in object placement because each floor piece could be rotated to create the necessary spatial configuration. Testing for anxiety-related behaviors was performed using an elevated zero maze (San Diego Instruments, San Diego, CA). The elevated zero maze is constructed of black non-porous plastic, elevated 30 inches (76.2 cm) off the floor, and is circular in design (diameter of circle, 121.92 cm; width of runway, 20.32 cm) with adjacent open and closed sections.
The stimulus objects used in memory testing were vinyl dog toys (Lil’ Buddies) that varied in shape, texture, and color, but were all similar in size. Our design utilized nine total unique types of toys, and 4 identical copies of each toy type were used interchangeably to allow for faster cleaning between test phases. The types of objects encountered during trials and whether toys were used as novel or familiar objects was randomized for each animal. For object trials, the positions of objects were fixed during exploration and recognition phases, but whether novel objects were presented on the right or left side of the arena during the recognition phase was counterbalanced for each group. All objects were firmly secured to the individual segments of the arena’s false floor. Specifically, each floor piece had one inverted jar lid super-glued to its corner. The objects were attached via zip ties to the top of a plastic jar that screwed securely into place.
2.5.2 Procedures
All behavioral testing occurred in a dedicated behavior room during the light phase of the light/dark cycle. Rats were transported from their housing room into the adjacent behavior room one hour before testing began. Experimenters were blind to group assignment and the order in which individual animals were tested was randomized using a random sequence generator (www.random.org).
2.5.2.1 Object and spatial trials (Figure 1 and 2)
Figure 1.
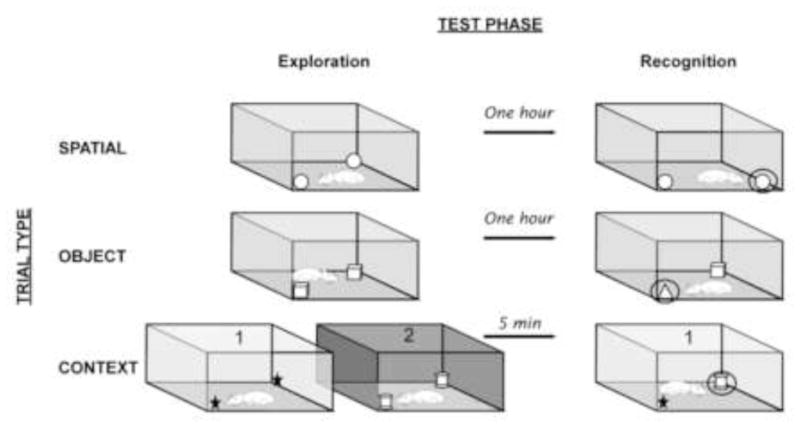
Schematic depicting the exploration, delay, and recognition phases of spatial, object, and context trials. For spatial and object trials, animals explored two identical objects for 5 min and were removed from the test area for 1 hour. During the recognition phase, either the object location (spatial trials) or object type (object trials) was changed. For context trials, animals sequentially explored two sets of context/object pairings for 5 min/context. The recognition phase occurred 5 minutes later. Even though all objects and contexts have been previously explored and are therefore familiar, animals typically show exploratory preferences for the mismatched object/context pairing during the recognition phase. The presentation order of environmental contexts and toys during the exploration phase was randomized for each animal, and whether the first or second context was used for the recognition phase was counterbalanced for each group.
Figure 2.
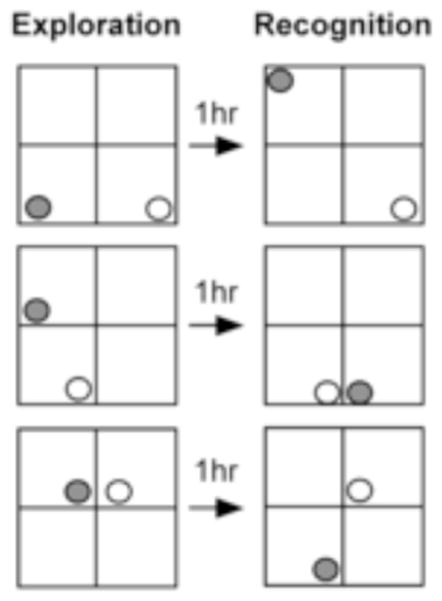
Spatial configurations employed during spatial trials. Three unique spatial configurations were designed to ensure the spatial arrangements of objects differed sufficiently between exploration and recognition phases. Animals were randomly assigned to experience one of the three possible spatial configurations. Shading illustrates which object changes locations between exploration and recognition phases of testing.
Experimental tests occurred over two days, but individuals experienced only one trial type per day. Whether animals first received object or spatial trials was counterbalanced such that half of each group received object trials first (day 1), and spatial trials second (day two), or vice versa. To minimize any effects time of day may have on behavior across a nine hour testing period, subject position within the testing schedule was also randomized. Figure 1 illustrates the testing procedure. A single trial consisted of 1) an exploration phase, 2) a delay phase, and 3) a recognition phase. During the exploration phase rats were placed into the arena and allowed to explore two identical stimuli for 5 min. Animals that explored objects for less than 10 seconds during the exploration phase were excluded from further analysis of that trial type. During the delay phase rats were removed from the arena and placed into their home cages for 1 hour. All objects were rinsed with a 70% ethanol solution, and then with an enzymatic solution designed to break down biological odors (Nature’s Miracle). The test arena walls and floor panels were also washed with a non-toxic deodorizing solution (Simply Green). During the recognition phase of object trials, one of the previously encountered objects was replaced with an unfamiliar novel object. During the recognition phase of spatial trials the objects did not change but one object changed spatial locations (Figure 2).
2.5.2.2 Novel object-context testing (Figure 1)
Novel object-context testing determines whether animals remember encountering a specific object in a specific environmental context [26]. Unique environmental contexts were achieved by adhering different patterns of contact paper onto the walls of each test chamber. To allow for easy visual discrimination between environmental contexts, contact paper types were chosen that varied significantly in pattern style and complexity. During the exploration phase, animals received 5 min exposure to two different environmental contexts (5 min/context; Figure 1). The presentation order of environmental contexts and toys during the exploration phase was randomized for each animal. Each environmental context contained two toys that were identical to each other but different from the toys encountered in the second context. Animals that explored objects for less than 10 seconds during the exploration phase were excluded from further behavioral analysis of the specific trial type. During the 5 min delay between exploration and recognition phases all objects and arenas were cleaned as described in the spatial and object trials. During the recognition phase animals were allowed to explore both previously encountered toy types within one of the two previous environmental contexts. Thus, although both objects had been previously encountered, only one of the objects was previously encountered in that particular environmental context. Whether the environmental context used for the recognition phase was the first or second context encountered during the exploration phase was counterbalanced for each group. Previous work has demonstrated that rats explore the novel object-context pairing more than the familiar object-context pairing [22].
2.5.2.3 Behavioral analyses for memory tests
Previous studies have found that object novelty quickly diminishes during the recognition phase [27], so our analyses were focused on the first 30 seconds of the recognition phase. However, while most animals visited the novel and familiar object within the first 30 seconds, a few animals’ preference for the novel object delayed investigation of the familiar object beyond the first 30 seconds of testing. Thus, to ensure an accurate latency measurement for these animals, data collection terminated when the familiar object was visited. An investigation bout was recorded by Topscan when the animal’s nose was oriented within at least 3 cm of an object. Time spent climbing or sitting on toys was not included as investigation. The latency, number and duration of visits to the novel or familiar object were recorded for each animal. From these measures, the proportion of total visits to the novel toy and proportion of total time spent investigating novel toys was calculated (novel/novel + familiar investigation).
2.5.2.4 Elevated zero
The elevated zero is a test of anxiety similar to the elevated plus maze, but is circular in shape allowing the rat to continuously investigate the maze without turning around, thereby reducing variability in the dataset. The elevated zero maze has been validated pharmacologically with anti-anxiety drugs [28], and also generates anxiety levels comparable to the elevated plus maze [29]. Similar to the elevated plus maze, the elevated zero maze allows indices of anxiety to be determined based on the amount of time spent in the open wall sections versus the amount of time spent in the closed wall sections. Time spent in the closed or open sections of the apparatus indicates more or less anxiety-related behavior, respectively [30]. Testing took place during the light phase of the dark/light cycle over a two day period. Animals were only tested once in the elevated zero, and whether subjects were tested on the first or second day was counterbalanced for each group. At the beginning of each test, an animal was placed in a closed section of the maze and allowed to investigate for a total of five minutes. The behavior analysis system measured the duration of time spent in both the open and closed sections and the distance traveled in the maze. The risk assessment behavior stretch-attend was quantified by analyzing the frequency and duration of sniffing the open sections from inside the closed sections (hind paws inside the closed section and front paws inside the open section). From this the bout durations (duration/frequency) of stretch-attend behavior were calculated.
2.5.2.5 Social interaction
Non-cage mates from the same stress condition were matched according to sex and weight. Animals were placed into the dimly lit behavioral arena simultaneously and allowed to interact for a total of ten minutes. Upon completion of testing, animals were removed from the arenas and placed back into their home cages. The test was recorded using video cameras placed over the test arenas and was analyzed by a single observer blind to experimental condition to quantify the frequency and duration of anogenital and nonspecific bodily sniffing of partners. Anogenital sniffing was defined as any instance in which the subject’s nose was in close proximity (<1 cm) or touching the partner’s anogenital region located underneath the tail. Non-specific bodily sniffing was defined as any instance in which the subject’s nose was in close proximity (<3 cm) and oriented toward the partner animal. As soon as its nose came within 3 cm of the partner, sniffing duration was started, if the animal turned or otherwise lost sniffing contact with its partner, sniffing duration was stopped.
2.6 Body weight measurements
Bodyweights were taken only in adulthood and not at earlier life stages to minimize stress to animals during development and prior to behavioral testing. Bodyweights were taken at three time points in adulthood 1) following open field testing at 82 days of age, 2) following elevated zero testing at 168 days of age, and 3) prior to sacrifice between 212–281 days of age. The age of animals range at the time of sacrifice because all animals underwent hippocampal electrophysiological procedures as part of a separate study. The time of sacrifice was counterbalanced across all groups to prevent potential age biases in any one group at sacrifice.
2.7 Statistical analysis
2.7.1 Sample sizes
Animals that spent < 10 seconds total toy investigation during the exploration phase of memory tests were excluded from further analysis of that particular trial type. This criterion led to the exclusion of 8 animals for spatial trials, 4 animals for object trials, and 2 animals for context trials. The elevated zero data for 5 animals were lost due to a temporary technical problem with the video recording.
2.7.2 Statistics
The overall number of males vs. females in litters was analyzed using a paired t-test. Using one-factor ANOVA we assessed the effects of PS on litter size and the effects of PS on the number of males or females in litters. Memory tests were analyzed by 3-factor mixed ANOVA treating sex and stress condition as between subject variables, and toy type (familiar or novel) as a within subject variable. One sample t-tests were also employed to assess whether rats discriminated between novel and familiar objects by comparing the proportion of novel visits and proportion of time investigating the novel object with what would be expected by chance (i. e. a ratio of ~ 0.50). Elevated zero and social interaction data were analyzed using a two-factor ANOVA treating sex and stress condition as independent variables (male vs. female, PS vs. NS). Body weight data were collected over time and therefore analyzed by 3-factor repeated measures ANOVA. Significant interactions were probed using Fischer’s PLSD. All statistical analyses were conducted using Statview Version 7. Differences were considered significant when p≤0.05.
3. Results
3.1 Litter size and sex ratio
No differences in litter size were found between PS and NS litters (PS = 13.00 ± 0.47 SEM; NS = 13.87 ± 0.47 SEM). Although litters were male-biased overall [t(1,11)=6.17, p<0.001], prenatal stress had no influence on the litter sex ratios (PS males = 7.83 ± 0.48 SEM; NS males = 8.17 ± 0.48 SEM; PS females = 5.17 ± 0.48 SEM; NS females = 5.50 ± 0.56 SEM).
3.2 Memory tests
3.2.1 Latency to novel spatial locations, object types, and object/context pairings
The latency to visit the novel or familiar object varied by sex and stress condition for both spatial [Figure 3A; F(1,28)=5.92, p<0.05] and object trials [Figure 3B; F(1,31)=4.03, p=0.05]. NS males were the only group to show significantly faster latencies to investigate the novel over the familiar objects [spatial, F(1,28)=5.36, p<0.05; object, F(1,31)=4.53, p<0.05]. For context trials a trend toward an interaction between stress condition and toy type (novel or familiar object/context pairing) was detected [Figure 3C; F(1,33)=3.81, p = 0.05]. Surprisingly, stressed animals showed significantly faster latencies to the novel than to the familiar object/context pairings [F(1,33)=10.98, p <0.01]. However, when stressed males and females were analyzed separately, only the stressed males showed significantly faster latencies to investigate the novel object/context pairings over the familiar object/context pairings [males, F(1,33)=9.52, p <0.01; females F(1,33)=2.56, p >0.05].
Figure 3.
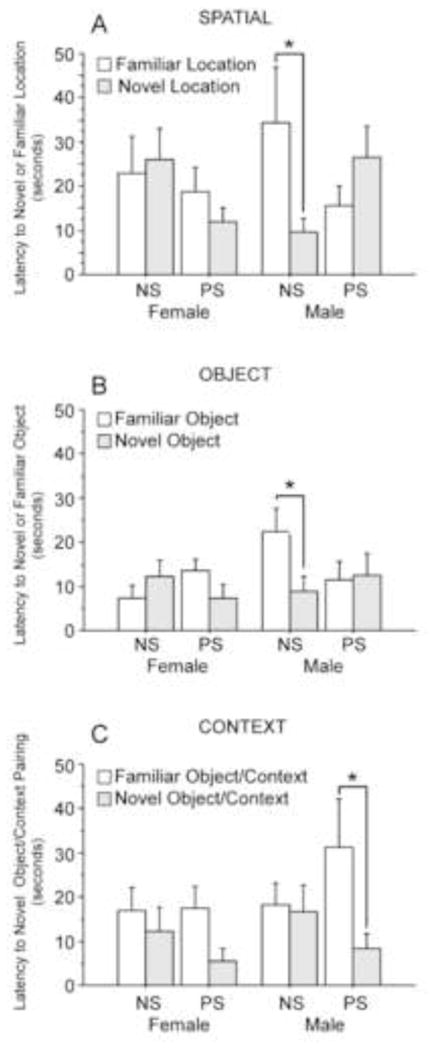
Effects of prenatal stress on latencies to investigate novel locations, object types, and object/context pairings. Only nonstressed (NS) males showed significantly faster latencies to investigate novel over familiar locations (A) and object types (B), suggesting that prenatal stress (PS) reduces memory for spatial locations in males. In contrast, PS showed significantly faster latencies to investigate novel object/context pairings over familiar object/context pairings, suggesting PS enhances memory for contextual memory in males (C). Latencies to novel or familiar locations, objects, or object/context parings did not differ in PS and NS females. NS females: spatial n=9; object n=8; context n=9. PS females: spatial n=8; object n=10; context n=10. NS males: spatial n=6; object n=9; context n=9. PS males: spatial n=9; object n=8; context n=9. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference (p<0.05) between groups.
3.2.2 Proportion of visits and time spent investigating novel locations, object types, and object/context pairings
For spatial trials, sex and condition interacted to influence the proportion of visits to the novel location (Figure 4A), and the proportion of time (Figure 4B) investigating the novel object locations [% novel visits, F(1,27)=7.44, p <0.01; % novel duration, [F(1,27)=5.12, p <0.05]. Stress significantly reduced the proportion of visits and time investigating novel locations in males [% novel visits, F(1,27)=9.92, p <0.01; % novel duration, F(1,27)=4.00, p <0.05]. No differences were found between stressed and NS females. One group t-tests confirmed that only NS males displayed above chance preferences for the novel spatial locations [Figure 4A, % novel visits, t=3.11, p <0.05; Figure 4B, % novel duration, t=2.43, p <0.05]. No significant effects of sex or stress condition were found for object or context trials. However, one group t-tests indicated that during object trials NS males displayed above chance preference for novel objects [Figure 4C, % novel visits, t=1.90, p < 0.05]. No other groups showed above chance preference for the novel toys for object or context trials (Figure 4E and 4F).
Figure 4.
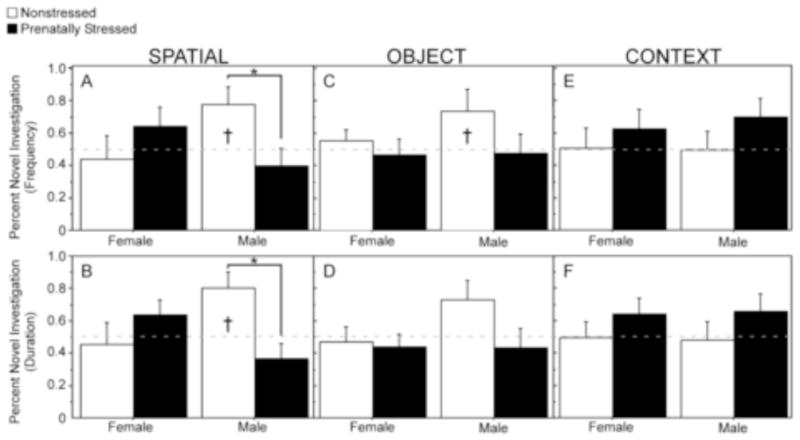
Effects of prenatal stress on novelty preferences during spatial, object, and context trials. For spatial trials prenatal stress (PS) significantly reduced the % of visits (A) and % time investigating (B) novel locations in males but not females. PS did not significantly affect the % of visits or % time investigating objects during object or context trials, however, nonstressed males displayed above chance preferences for novelty during object trials (†). NS females: spatial n=9; object n=8; context n=9. PS females: spatial n=8; object n=10; context n=10. NS males: spatial n=6; object n=9; context n=9. PS males: spatial n=9; object n=8; context n=9. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference (p<0.05) between groups. Cross (†) indicates an above chance preference for novelty (>50%) in that group.
3.3 Elevated zero
The bout durations (duration/frequency) of stretch-attend risk-assessment behavior (investigating the open arms from inside the closed arms) varied depending on sex and condition [Figure 5A; Interaction: F(1,31)=4.01, p=0.05]. When we compared the behavior of males and females separately, each stretch-attend bout was significantly longer for PS females than NS females [Figure 5A; F(1,31)=4.28, p<0.05], suggesting increased anxiety. In contrast, no significant differences between PS and NS males were found in stretch-attend bout durations. The duration of time spent in the open walls [Figure 5B; F(1,31)=5.00, p<0.05] and distance traveled [Figure 5C; F(1,31)=3.95, p=0.05] in the open arms of the elevated zero followed a similar pattern of interaction. Specifically, PS females spent significantly less time [5B; F(1,31)=6.89, p<0.05] and traveled shorter distances [5C; F(1,31)=4.40, p<0.05] in the open arm than NS females, whereas these behaviors were unaffected by PS in males (p > 0.05).
Figure 5.
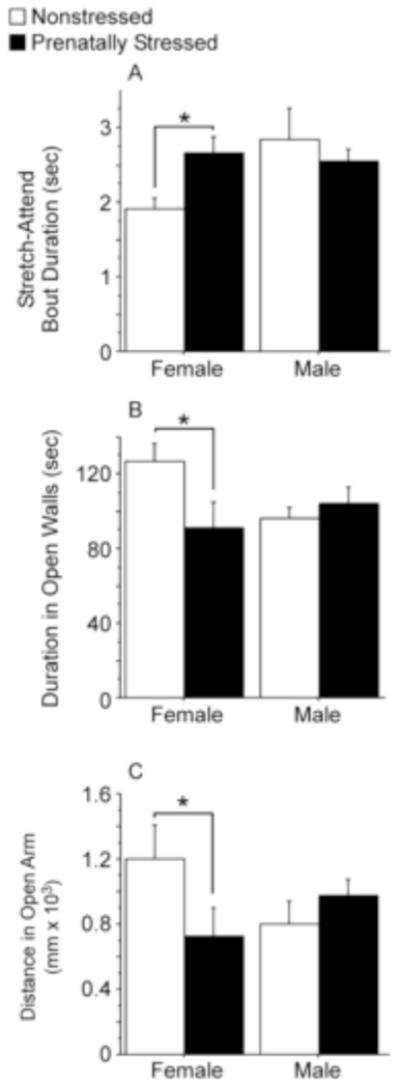
Investigatory behavior in the elevated zero maze. (A) Prenatally stressed (PS) females displayed longer bout durations of stretch-attend (risk assessment) than did nonstressed (NS) females. No differences in stretch-attend bout durations were observed between PS and NS males. (B) PS females spent less time in the open walls of the elevated zero than NS females, whereas no differences were observed between PS and NS males. (C) PS females traveled less in the open walls than did NS females, whereas no difference in distance traveled in open arms was found between PS and NS males. NS females, n=9; stressed PS females, n=9; NS males, n=9; PS males, n=8. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference (p<0.05) between groups.
3.4 Social interaction
Females engaged in significantly more anogenital sniffing than males overall [Figures 6A and 6B; F(1,36)=12.22, p<0.002]. No effects of stress condition or interactions between stress condition and sex were found, but anogenital sniffing significantly decreased across the 10 min test[F(1,144)=7.72, p<0.0001]. In contrast to female-biased anogenital sniffing, males engaged in more nonspecific sniffing than females [Figure 6C and 6D; F(1,36)=3.84, p=0.05]. Sex also interacted with stress condition [Interaction: F(1,36)=4.32, p<0.05]. Post-hoc analyses indicated that NS males sniffed partners more than nonstressed females (Figure 6C), whereas PS males and females did not differ in nonspecific sniffing (Figure 6D). A significant main effect of time during testing was also found for nonspecific sniffing, such that sniffing generally decreased across the 10 min test [Figure 6; F(1,144)=11.80, p<0.0001].
Figure 6.
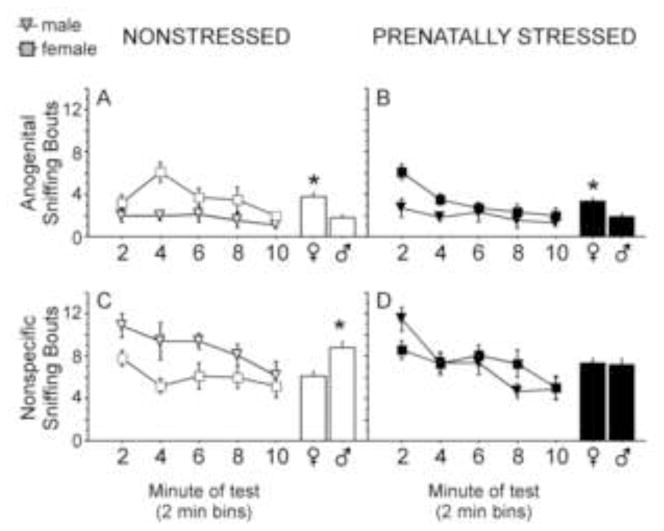
Anogenital and nonspecific bodily sniffing of same-sex and -stress condition partners across a 10 min test. (A &B) Females engaged in more anogenital sniffing of partners than males, regardless of stress condition (histograms illustrate main effect of sex; prenatal stress=PS; nonstressed=NS). (C) Males engaged in more nonspecific sniffing of partners than did females, but only in the NS condition (histogram illustrates main effect of sex). (D) No sex differences in nonspecific sniffing were observed in prenatally stressed animals. Both anogenital and nonspecific sniffing decreased significantly across the 10-min test. NS females, n=10; PS females, n=10; NS males, n=10; PS males, n=10. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference (p<0.05) between groups.
3.5 Body weight
Sex and condition interacted to influence body weight [Figure 7; F(1,36)=6.20, p<0.02]. Specifically, stress significantly increased body weight in males [F(1,36)=11.30, p<0.004] but not females [F(1,36)=0.004 p=0.96]. Age also significantly influenced body weight [F(1,72)=465.6, p<0.0001], and age and sex significantly interacted to affect body weight [F(1,72)=97.58 p<0.0001]. Post-hoc analyses indicated significant weight gains at each age tested, and for both sexes (82 days, 168 days, and the final weights ranging from 212–281 days). However, males displayed proportionally greater gains than females between 168 days of age and the final weight [males: 168 vs. final weight = p<0.0001; females: 168 vs. final weight= p<0.02].
Figure 7.
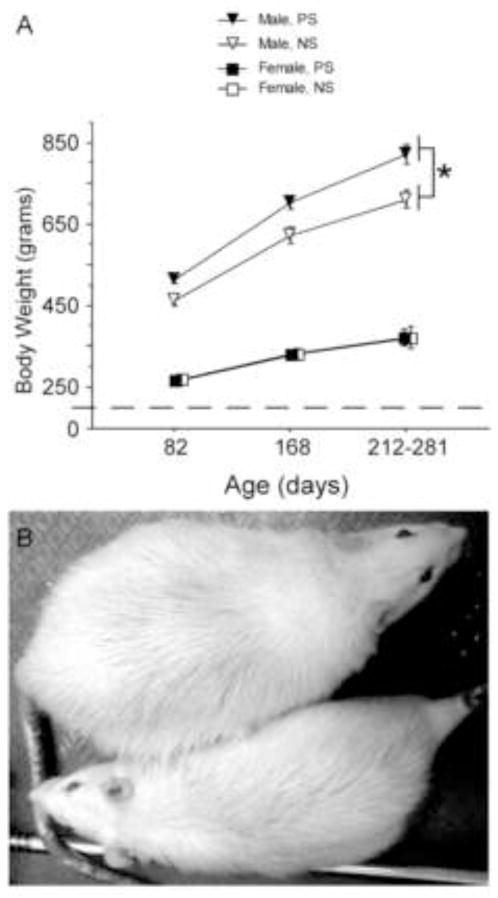
Effects of prenatal stress on body weight across adulthood. (A) Prenatal stress (PS) significantly increased the body weight of males but not females, and PS males gained the most weight across time. (B) Photo of representative PS (top) and nonstressed (NS, bottom) males from this experiment. Note that the body weights of PS and NS females are completely overlapping, so the symbols for the NS female groups were shifted to the right to allow for visual inspection. NS females, n=10; PS females, n=10; NS males, n=10; PS males, n=10. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference (p<0.05) between groups.
4. Discussion
The primary aim of this study was to determine whether PS influences hippocampal-associated memory function in rats. Three types of memory were tested using spatial, recognition, and contextual variants of the novel object recognition task [22]. PS impaired memory for spatial locations and novel object recognition in males, but modestly facilitated memory for object/context associations in males. In contrast, PS did not affect female performance on these measures. These findings suggest that PS impacts hippocampal-associated forms of memory, but this relationship depends on the sex of animals and the specific type of memory being tested.
Our findings corroborate and extend previous work investigating the effects of stress during gestation on the memory performance of adult offspring. Like previous studies, we found that PS impairs spatial memory performance in males [2,4,5,7,10–13,23]. However, to our knowledge, this is the first demonstration of prenatal stress-induced spatial memory deficits using the novel object spatial location paradigm. The strength of using variants of the novel object recognition paradigm is that spatial, recognition, and contextual memory can be compared while controlling for the amount of training, handling, and the levels of stress experienced during testing. Thus, while previous studies have also shown PS-induced spatial memory deficits using the Morris water maze [4–13], radial arm maze [1,2], T-maze [3,6], or Y-maze [3,6], here we extend their findings by also testing recognition and contextual memory function in the same animals. Another report found no effect of PS on recognition memory [23]. In this study rats were acclimated to the behavioral arena and received 4 novel object testing sessions before data collection occurred. In contrast, our rats were acclimated to the behavioral arena and were not repeatedly tested in any given trial-type (object, spatial, or context). Thus, it is possible that testing experiences mitigate the initial negative effects of PS on recognition memory. Additional experiments manipulating both stress and testing experience will help determine whether these factors interact to influence recognition memory.
PS also impacted contextual memory function. Surprisingly, prenatally stressed males showed significantly shorter latencies than NS males to investigate novel object/context pairings, suggesting PS improves contextual memory. However, while PS males showed significantly faster latencies to investigate novel object/context pairings, the proportion of visits and time spent with the novel object/context did not significantly differ between PS and NS males. Thus, while these initial findings are intriguing, experimental replication will help to determine the extent to which PS affects contextual memory.
Prenatal stress only affected memory performance in males. However, even NS females did not show a preference for novel objects. The reason for this is not clear, especially since females showed definite novelty preferences in our pilot experiments (data not presented here). One possibility is that the female subjects were in the low-estrogen and –progesterone estrous cycle phase diestrus when memory performance is decreased relative to proestrus and estrus phases [31–33]. This possibility is only speculative given that we did not track estrous cycle phase. While most studies investigating the effects of prenatal stress on behavior have not controlled for estrous cycle phase [but see 15,34,35], our future experiments will account for potential estrous cycle phase effects on memory performance.
Given that spatial, recognition, and contextual memory are all hippocampal-associated, it is surprising that PS may impair spatial and recognition memory while facilitating contextual memory. An interesting possibility is that different subcomponents of the hippocampus are important for different hippocampal-associated memory types. For example, studies have found that adult adrenalectomy results in a selective loss of cells in the dentate gyrus [36,37]. When spatial, recognition, and contextual memory were assessed following adrenalectomy and corticosterone replacement, only contextual memory impairments were found [37]. Thus, it is possible that in the current study elevated corticosterone levels during gestational stress impacted dentate gyrus development differently than other hippocampal subregions, leading to dissociations in hippocampal-associated memory functions in adulthood.
While the effects of PS on memory function were male-biased, PS preferentially affected the anxiety-related behaviors of females. Specifically, PS decreased the time females spent in the open arms of the elevated zero maze, and significantly increased female bout durations of stretch-attend behavior (i. e. risk assessment), suggesting that PS females were more anxious than controls. These data are in agreement with previous studies demonstrating PS preferentially increases female anxiety-related behavior in the elevated plus maze. Richardson et al., found that only PS female CD rats reduced the proportion of time spent in the open arm of the elevated plus maze [15]. Similarly, PS female Wistar rats showed increased anxiety-related behavior when tested in the elevated plus maze [12,38]. Thus, our finding that PS increases anxiety in females supports previous research, and validates the elevated zero for use in PS animals. It should be noted, however, that other studies employing the elevated plus maze have found anxiety increases in both male and female PS rats [39,40]. These differences between studies may reflect differences in the implementation of the conditions surrounding the elevated plus maze test such as lighting conditions during testing, or possibly differences in maze configuration (circle vs. plus).
Social interactions were affected by sex and stress condition in a behavior-specific manner. Females engaged in more anogenital sniffing than males, irrespective of stress condition. In contrast, males engaged in more nonspecific bodily sniffing than females. However, bodily sniffing was only male-biased in NS animals. PS eliminated the male-biased sex difference by decreasing nonspecific bodily sniffing in males, and increasing bodily sniffing in females. Thus, while previous studies have also demonstrated prenatal stress-decreases social investigation in male rats [41,42], here we extend those findings by demonstrating that PS increases female social investigation. Interestingly, PS females displayed increased anxiety-related behavior in the elevated zero yet displayed increased bodily sniffing of partners, suggesting that the effects of PS on anxiety-related behavior do not extend to social interactions. Likewise, given that PS males were not anxious in the elevated zero maze, reduced nonspecific bodily sniffing in PS males is not simply attributable to increased anxiety. These dissociations between anxiety-related behavior and social interactions suggest that altered social behaviors are not secondary to PS-induced changes in the neural circuitry regulating anxiety, but rather, PS may independently organize neural circuits underlying the motivation to engage in social interactions.
Exposure to gestational stress also influenced body mass in a sex-specific manner. Specifically, PS significantly increased the bodyweight of male rats, whereas female bodyweights were less affected by stress. In fact, PS males were on average 100 grams larger than NS males. These data are consistent with a recent report that PS increases body weight in male and female rats [43]. Although PS did not increase female body weights in our study, this discrepancy between studies is likely due to differences in the fat content of the post-weaning diet. Tamashiro and colleagues [43] fed animals a high fat diet at weaning (60% kcal from fat), whereas our animals were fed a standard rodent diet at weaning (17% of kcal from fat). The species of rodent is another important factor mediating the effects of gestational stress on bodyweight. Stress experienced early in gestation decreases bodyweight and adiposity in male and female C57Bl/6:129 mice [44]. Taken together, these studies highlight the complexity of the relationships between gestational stress, species, sex, and diet.
Animal models of human disorders linked to the prenatal period are necessary for the development of appropriate preventions and treatments for conditions such as schizophrenia, anxiety, depression, autism, and obesity. These disorders are also highly gender-specific in the incidence and/or expression of symptoms. Although PS in rodents generally induces behavioral and bodily phenotypes analogous to those found in these human conditions, determining the nature and direction of stress-induced sex differences has proved difficult. The rodent species, strain, timing of stress and testing environment have all been linked to the sex-specific expression of behavior, but the direction of sex-specific changes varies by study [45,46]. In the current study, PS induced female anxiety-related behavior in the elevated zero maze. This finding maps nicely onto female-biased disorders such as depression and anxiety in humans. In addition, the PS-induced social deficits in male rats may be analogous to the male-biased social withdrawal experienced in conditions such as schizophrenia and autism [47–50]. In contrast, the stress-induced and male-specific increases in bodyweight did not recapitulate the female-biased incidence of obesity in the general population [51]. However, whether PS affects obesity in a gender-dependent fashion in humans is not yet known. Future studies focused on the mechanisms by which PS induces these bodily and behavioral phenotypes may help elucidate the mechanisms by which PS increases susceptibility to disorders with etiological links to the prenatal period.
Acknowledgments
This work was supported by T32 MH15442-30, MH073725 to KES, MH081177, DA09457 and Veterans Affairs Merit Review to SL.
We thank Drs. Kristin Wildeboer-Andrud, Randy Ross, Heather Richardson, and Kaliris Salas-Ramirez for critical feedback on early drafts of this manuscript. We also thank Joan Yonchek for valuable technical assistance, and Dr. Michael Hall for constructing the testing chambers for memory testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowman RE. Stress-induced changes in spatial memory are sexually differentiated and vary across the lifespan. Journal of Neuroendocrinology. 2005;17:526–535. doi: 10.1111/j.1365-2826.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 2.Son GH, Geum D, Chung SY, Kim EJ, Jo JH, Kim CM, et al. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. Journal of Neuroscience. 2006;26:3309–3318. doi: 10.1523/JNEUROSCI.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gue M, Bravard A, Meunier J, Veyrier R, Gaillet S, Recasens M, et al. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behavioural Brain Research. 2004;150:149–157. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- 4.Yaka R, Salomon S, Matzner H, Weinstock M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behavioural Brain Research. 2007;179:126–132. doi: 10.1016/j.bbr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 6.Meunier J, Gue M, Recasens M, Maurice T. Attenuation by a sigma(1) (sigma(1)) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br J Pharmacol. 2004;142:689–700. doi: 10.1038/sj.bjp.0705835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JL, Han HL, Cao J, Li LJ, Xu L. Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus. 2006;16:431–436. doi: 10.1002/hipo.20181. [DOI] [PubMed] [Google Scholar]
- 8.Meek LR, Burda KM, Paster E. Effects of prenatal stress on development in mice: maturation and learning. Physiology & Behavior. 2000;71:543–549. doi: 10.1016/s0031-9384(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor A, Kostaki A, Janus C, Matthews SG. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behavioural Brain Research. 2009;197:144–149. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Van Waes V, Enache M, Zuena A, Mairesse J, Nicoletti F, Vinner E, et al. Ethanol Attenuates Spatial Memory Deficits and Increases mGlu1a Receptor Expression in the Hippocampus of Rats Exposed to Prenatal Stress. Alcoholism-Clinical and Experimental Research. 2009;33:1346–1354. doi: 10.1111/j.1530-0277.2009.00964.x. [DOI] [PubMed] [Google Scholar]
- 11.Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiology & Behavior. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- 12.Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behavioural Brain Research. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 15.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal Stress Alters Spine Density and Dendritic Length of Nucleus Accumbens and Hippocampus Neurons in Rat Offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- 17.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learning & Memory. 2010;17:794–800. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- 20.Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ. Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Natl Acad Sci U S A. 2004;101:5064–5068. doi: 10.1073/pnas.0308528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res. 2006;167:183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 24.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, et al. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behavioural Brain Research. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 27.Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behavioural Brain Research. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni SK, Singh K, Bishnoi M. Elevated zero maze: A paradigm to evaluate antianxiety effects of drugs. Methods Find Exp Clin Pharmacol. 2007;29:343–348. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni SK, Singh K, Bishnoi M. Comparative behavioural profile of newer antianxiety drugs on different mazes. Indian J Exp Biol. 2008;46:633–638. [PubMed] [Google Scholar]
- 30.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neuroscience & Biobehavioral Reviews. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 31.Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapoor A, Matthews SG. Prenatal Stress Modifies Behavior and Hypothalamic-Pituitary-Adrenal Function in Female Guinea Pig Offspring: Effects of Timing of Prenatal Stress and Stage of Reproductive Cycle. Endocrinology. 2008;149:6406–6415. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- 35.Saal FSV, Even MD, Quadagno DM. Effects of maternal stress on puberty, fertility, and aggressive-behavior of female mice from different intrauterine positions. Physiology & Behavior. 1991;49:1073–1078. doi: 10.1016/0031-9384(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 36.Spanswick SC, Epp JR, Keith JR, Sutherland RJ. Adrenalectomy-induced granule cell degeneration in the hippocampus causes spatial memory deficits that are not reversed by chronic treatment with corticosterone or fluoxetine. Hippocampus. 2007;17:137–146. doi: 10.1002/hipo.20252. [DOI] [PubMed] [Google Scholar]
- 37.Spanswick SC, Sutherland RJ. Object/context-specific memory deficits associated with loss of hippocampal granule cells after adrenalectomy in rats. Learning & Memory. 2010;17:241–245. doi: 10.1101/lm.1746710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behavioural Brain Research. 2005;163:70–77. doi: 10.1016/j.bbr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 40.Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sciences. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- 41.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Research. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koenig JI, Shelton S, Taylor A. Paliperidone improves cognitive but not social defects in animal models of schizophrenia. Schizophrenia Bulletin. 2007;33:474–474. [Google Scholar]
- 43.Tamashiro KLK, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal Stress or High-Fat Diet Increases Susceptibility to Diet-Induced Obesity in Rat Offspring. Diabetes. 2009;58:1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pankevich DE, Mueller BR, Brockel B, Bale TL. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiology & Behavior. 2009;98:94–102. doi: 10.1016/j.physbeh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochemical Research. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Yamasue H, Kuwabara H, Kawakubo Y, Kasai K. Oxytocin, sexually dimorphic features of the social brain, and autism. Psychiatry Clin Neurosci. 2009;63:129–140. doi: 10.1111/j.1440-1819.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 48.Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neuroscience and Biobehavioral Reviews. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rydell AM, Diamantopoulou S, Thorell LB, Bohlin G. Hyperactivity, shyness, and sex: Development and socio-emotional functioning. Br J Dev Psychol. 2009;27:625–648. doi: 10.1348/026151008x346996. [DOI] [PubMed] [Google Scholar]
- 50.Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatrica Scandinavica. 1999;100:105–118. doi: 10.1111/j.1600-0447.1999.tb10831.x. [DOI] [PubMed] [Google Scholar]
- 51.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]


