Abstract
Pruritus is one of the cardinal symptoms found in patients with leukemic cutaneous T cell lymphoma (CTCL). The nature of the pruritus experienced by CTCL patients is complex, involving different pathways and cell mediators, thus making it poorly responsive to conventional anti-itch therapies. Recent reports highlight the role of interleukin 31 (IL-31) as a novel cytokine involved in the pathogenesis of pruritus in atopic dermatitis and CTCL. Here we provide both in vivo and in vitro evidence suggesting that histone deacetylase (HDAC) inhibitors may mitigate itch through lowering of levels of IL-31-expressing T cells. Furthermore, we demonstrate that chemokine receptor type-4 (CCR4)-bearing T cells are a main source of IL-31 in CTCL, and that neutralizing the IL-31 pathway through targeting of the CCR4-expressing T cells may represent a promising therapeutic strategy for symptomatic relief in CTCL.
Keywords: pruritus, IL-31, immunotherapy, CTCL
1. Introduction
Cutaneous T cell lymphoma (CTCL) is an unusual group of primary non-Hodgkin's T cell dyscrasias of skin-homing lymphocytes that involve the skin, peripheral blood and lymph node compartments [1]. In advanced stages, the great majority of CTCL patients complain of chronic intractable pruritus, an often debilitating and life-limiting symptom. Due to the complex pathogenesis of itch in CTCL, anti-histamines, gamma-aminobutyric acid (GABA) analogs or anti-depressants are only partially effective in relieving pruritus; however, eradication of the malignant clone effectively suppresses pruritus [2].
Patients with leukemic CTCL exhibit an immunologic signature enriched by type-2 (Th2) helper cytokines including interleukin-4 (IL-4), IL-5 and IL-13, which drive eosinophilia and IgE production [3]. In addition, recent reports have demonstrated that CTCL patients have increased expression of IL-31, a short-chain 4-helix-bundle cytokine and member of the IL-6 family [4], whose expression could be triggered by IL-4 and Staphylococcus aureus superantigens, both of which are present in the lesional milieu of CTCL patients [5, 6]. Notably, we have previously shown that IL-31 serum levels directly correlate with the degree of itch in leukemic CTCL patients[4]. Furthermore, CD4+ T cells lacking CD26 expression, which is the phenotype of the malignant cells, as well as clonal cells identified by antibodies against the beta chain variable region (Vβ) of the T cell receptor appear to be the primary source of this cytokine in CTCL [4].
In this report, we show that in vitro treatment of peripheral blood mononuclear cells (PBMCs) from Stage IV CTCL patients with the histone deacetylase inhibitor (HDACi), vorinostat, effectively lowers IL-31 expression. These observations are further validated by analysis of blood samples from Stage IV CTCL patients before and after treatment with the HDACi, romidepsin, in which we demonstrate diminished pruritus, tumor burden, and IL-31 expression after treatment. In addition, we show that IL-31 is specifically produced by a subset of the malignant T cells (CD4+/CD26-) which expresses the skin homing chemokine receptor type-4 (CCR4), and that treatment with the fully humanized anti-CCR4 monoclonal antibody, mogamulizumab, results in contraction of the malignant population with subsequent reduction of IL-31 and markedly diminished pruritus.
Collectively, our data suggest that disruption of IL-31 production, either through pro-apoptotic/antiproliferative mechanisms or immunotherapeutic agents targeting T cells with epidermotropic potential, may translate into effective anti-itch treatments for leukemic CTCL patients.
2. Materials and Methods
2.1 Human Subjects
All studies were conducted in accordance with the Declaration of Helsinki and approved by the University of Pennsylvania's Institutional Review Board (IRB). Written consent was obtained from all patients prior to sample collection. Blood and skin samples were obtained from patients with leukemic Stage IIIB or IVA CTCL (Erythrodermic mycosis fungoides and Sezary Syndrome) at the University of Pennsylvania, as depicted in Table 1. Sezary Syndrome was diagnosed on the clinical, histopathologic and immunohistologic criteria [7]. All patients were stage IIIB or IVA CTCL in accordance with the Tumor-Node-Metastasis-Blood (TNMB) 2007 and the European Organization of Research and Treatment of Cancer (EORTC) revised classification system. Circulating malignant cells were assessed by the absence of CD26 surface expression on CD4+ T cells. The intensity of pruritus was measured subjectively with a numerical analogue scale, where a score of 0 reflects no symptoms and a 10 reflected the worst possible symptoms.
Table 1. Clinic and phenotypical patient characteristics.
| Age/ gender | TNMB Stage | Flow cytometry features | %IL-31+ CD3+/ CD4+/CD26- | Functional assay |
|---|---|---|---|---|
| 80sM | Stage IVA, T4NXM0B2 | CD4:CD8 of 101, discrete subset of CD3+/CD4+/CD26- :61 % (6146 cells/μl) | 4.19% | Dexamethasone suppression |
| 70sM | Stage IVA, T4N0M0B2 | CD4:CD8 of 31.3, discrete subset of CD3+/CD4+/CD26-: 87% (9369 cells/μl) | 6.32% | Dexa/Vorinostat suppression |
| 60sM | Stage IVA2 T4N3M0B2 | CD4:CD8 of 4.9, discrete subset of CD3+/CD4+/CD26-: 41 % (1020 cells/μl) | 12% | Vorinostat suppression |
| 50sM | Stage IIIB T4NXM0B1 | CD4:CD8 of 4.1, discrete subset of CD3+/CD4+/CD26-23% (435 cells/μl) | 2.67% | Dexamethasone suppression |
| 70sF | Stage IVA, T2NXM0B2 | CD4:CD8 of 64.2, discrete subset of CD3+/CD4+/CD26-64% (5833 cells/μl) | 3.34% | Vorinostat suppression |
| 20sM | Stage IIIB T4N2M0B1 | CD4:CD8 of 4.9, discrete subset of CD3+/CD4+/CD26-30% (164 cells/μl) | 1.03% | Dexa/Vorinostat suppression |
| 60sM | Stage IVA, T2NXM0B2 | CD4:CD8 of 22, discrete subset of CD3+/CD4+/CD26-80% (843 cells/μl) | 4.73% | Anti-CCR4 trial |
| 70sM | Stage IVA, T4NXM0B2 | CD4:CD8 of 132.9, discrete subset of CD3+/CD4+/CD26- of 96% (33251 cells/μl) | 9.17% | In vivo Romidepsin |
| 50sM | Stage IVA, T4NXM0B2 | CD4:CD8 of 30, discrete subset of CD3+/CD4+/CD26- of 91 % (7012 cells/μl) | Unknown High IL-31 mRNA expression by PCR | In vivo Romidepsin |
2.2 Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Sigma) gradient centrifugation. Viability was assessed by Trypan blue (Sigma) exclusion. PBMCs were stained with fluorophore-conjugated anti-CD3, CD4, CCR4 and CD26 mAbs (BD Biosciences). For intracellular cytokine staining, cells were cultured in complete RPMI 20% FCS (Sigma) in the presence of phorbol myristate acetate (PMA) /ionomycin, and brefeldin A for a total of 5 hours, followed by staining of surface antigens, fixation and permeabilization (Invitrogen) followed by incubation with biotin-conjugated anti-human IL-31 (R&D Systems; BAF2824) followed by streptavidin-APC, as previously reported. Cells were analyzed in a FACSCanto (Becton Dickinson) followed by FlowJo version 9.4.4 (Treestar, Inc). 100,000 events were collected for analysis.
2.3 In vitro treatments
PBMCs from leukemic CTCL patients were treated with dexametasone (n=4) 100nM, 1μM vorinostat (n=4) or diluent controls for 12 hours. The cells were then stimulated, stained and analyzed by flow cytometry as described above.
2.4 Real-time RT-PCR
PBMCs were obtained pre and post romidepsin treatment from a Stage IV CTCL patient and incubated for 5 hours in the presence PMA and ionomycin. Total RNA was extracted using the phenol chloroform method as previously described. Complementary DNA (cDNA) was further synthesized using the High Capacity RNA to cDNA kit (Applied Biosystems) for further analysis. Quantitative reverse transcriptase real time PCR was performed using β-actin as a housekeeping gene on a AB 7500 RT PCR System (Applied Biosystems). Relative IL-31 mRNA expression was quantified by the ΔΔCt normalized against the Non-CTCL samples.
3. Results
3.1. Pro-apoptotic and epigenetic control of the malignant population reduce the levels of IL-31 in vitro
Since their introduction over 50 years ago, topical and systemic corticosteroids have been consistently used as anti-pruritic agents [8]. Malignant and activated T cells are particularly sensitive to cortisone-mediated pro-apoptotic and antiproliferative effects, and thus corticosteroids are commonly used as adjuvant therapy in localized and systemic CTCL [9]. In order to validate the effects of corticosteroids in the suppression of IL-31 expression, we treated PBMCs from pruritic Stage IIIB and IVA CTCL patients with 100 nM dexamethasone or diluent control for a total of 12 hours and measured their expression of intracellular IL-31 by flow cytometry. As expected, after 12 hours of dexamethasone treatment we noticed an 8-fold reduction in IL-31 expression of the malignant (CD4+, CD26-) population as compared to the diluent control (Figure 1a). The reduction of IL-31-producing CD4+/CD26- T cells ranged between 29% and 81% compared to the control group (Figure 1B).
Figure 1. In vitro treatment with the corticosteroid, dexamethasone, or the HDAC inhibitor, vorinostat reduces IL-31 expression in samples from advanced CTCL patients.
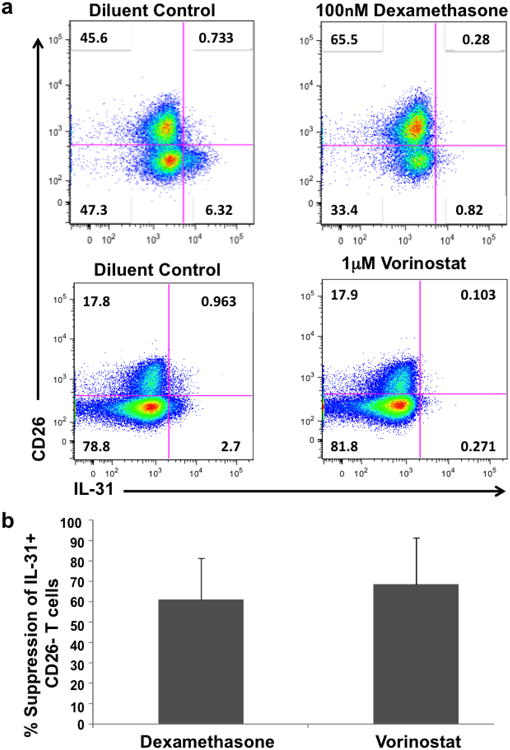
PBMCs from pruritic advanced CTCL patients were treated with 100nM dexamethaxone (n=4) or 1μM vorinostat (n=4) or their corresponding diluent controls for 12 hours. Cells were then stimulated with PMA/ionomycin/brefeldin A for a total of 5 hours, stained with fluorophore-conjugated monoclonal antibodies against surface-bound CD3, CD8, CD26 and intracellular IL-31 and further analyzed by flow cytometry. Representative plots show pre-gated cells on CD3+/CD8- (a). Analysis of the percentage in reduction of IL-31 expression in CD26- T cells after dexamethasone or vorinostat treatment is shown as mean value (n=4). Error bars indicate standard deviation of the mean (b).
Similarly, we performed a parallel in vitro experiment using the histone deacetylase inhibitor (HDACi) vorinostat. HDACi are a novel class of therapeutic agents recently approved by the Food and Drug Administration (FDA) for Stage IB and advanced CTCL [10]. In their respective trials, both vorinostat and romidepsin, recently approved HDACi for CTCL demonstrated significant efficacy in decreasing CTCL pruritus [11, 12]. The main mechanism of action of HDACi relies upon the disruption of the epigenetic control of chromatin assembly and packaging process through histone formation, which occurs more dynamically in rapidly dividing cells including malignant cells and those undergoing rapid turnover such as in erythropoiesis or myelopoiesis [13]. Beyond their epigenetic control, HDACi sensitize rapidly dividing cells to undergo apoptosis through caspase activation involving the intrinsic and extrinsic pathways [13]. In order to investigate whether HDACi may decrease IL-31 expression, we treated PBMCs from pruritic leukemic CTCL patients with 1 μM vorinostat for 12 hours and then analyzed intracellular IL-31 expression. Similar to dexamethasone treatments, vorinostat induced between 39%-90% reduction in the number of malignant T cells producing IL-31 (Figures 1a and 1b).
3.2. Therapy with romidepsin effectively suppresses pruritus and abrogates the expression of IL-31 in treated patients
Similar to vorinostat, romidepsin is another recently approved HDACi for the control of advanced stage CTCL. We collected PBMCs from Stage IVA CTCL patients before and after a single infusion of IV romidepsin. Our data reveals that both pruritus and circulating lymphocyte IL-31 mRNA expression were diminished after romidepsin treatment (Figure 2a). To further validate this observation at the cellular level, we analyzed by flow cytometry PBMCs before and after receiving romidepsin IV infusion. Intracellular IL-31 expression is dramatically reduced, but also, restoration of the normal T cell population (CD3+/CD4/CD26+) is evident after a single treatment (Figure 2b).
Figure 2. Reduced pruritus and lower IL-31 expression are observed in a CTCL patient after treatment with Romidepsin.
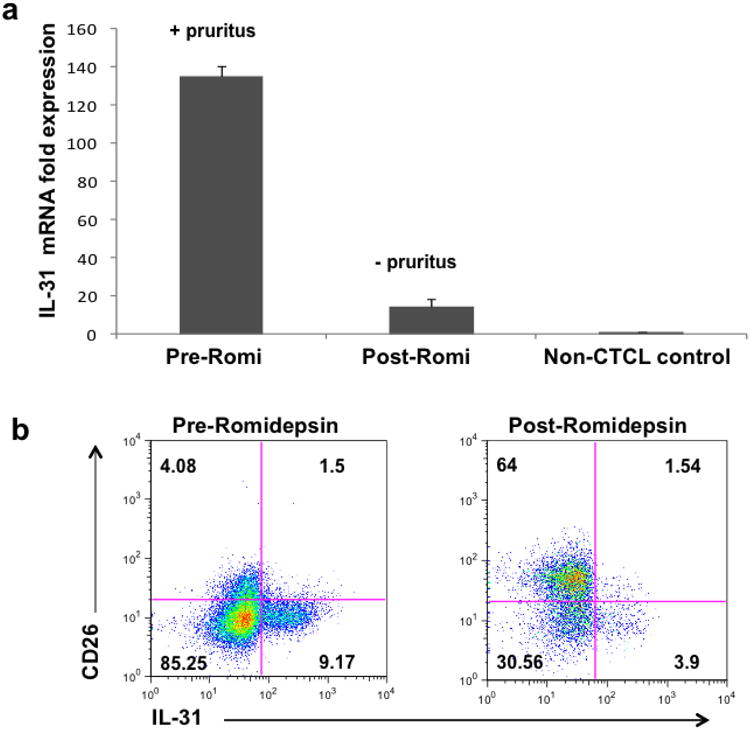
PBMCs from pruritic advanced CTCL patients were obtained before and after their first infusion with IV romidepsin. A representative sample was analyzed for IL-31 expression by quantitative RT-PCR (a) or flow cytometry (b), as depicted in Figure 2.
3.3.The anti-CCR4 monoclonal antibody, mogamulizumab, is a promising therapeutic option to control IL-31 expression in CTCL
In leukemic stages of CTCL, the circulating malignant T cell population exhibits either central (CD45RO+/CCR7+) or effector (CD45RO+/CCR7-) memory phenotypes with high capacity to recirculate between the peripheral blood and the skin through homing mechanisms mediated by the expression of cell surface selectin ligands (cutaneous lymphocyte antigen-CLA) and more importantly, the chemokine receptor-4 (CCR4) [14]. As depicted in Figure 3a and in accordance to previous reports, PBMCs of a typical leukemic pruritic CTCL patient exhibit a discrete subset of the malignant T cell population (as defined by expression of CD3/CD4 and lack of CD26) which produces IL-31. This population is almost negligible in non-pruritic CTCL patients. Moreover, while almost half of the malignant T cell population in both pruritic and non-pruritic patients express surface-bound CCR4, it is evident that the great majority of the IL-31+ T cells express this important receptor, thereby facilitating their skin-trafficking capability.
Figure 3. Reduction in the number of CCR4+ T cells correlates with decreased numbers of IL-31-expressing T cells.
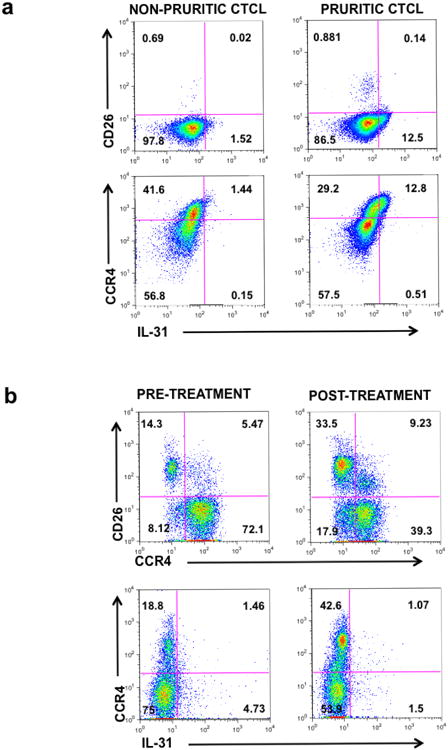
(a) PBMCs from pruritic and non-pruritic advanced CTCL samples were stained with fluorophore-conjugated monoclonal antibodies against surface-bound CD3, CD8, CD26, CCR4 and intracellular IL-31 and further analyzed by flow cytometry. Representative plots show pre-gated cells on CD3+/CD8- (upper row) and CD3+/CD8-/CD26- (lower row).
(b) PBMCs from pruritic advanced stage CTCL samples were obtained before and after the first treatment with mogamulizumab. PBMCs were then stained with fluorophore-conjugated monoclonal antibodies against surface-bound CD3, CD8, CD26, CCR4 and intracellular IL-31 and further analyzed by flow cytometry. Representative plots show pre-gated cells on CD3+/CD8-.
Mogamulizumab is a novel fully humanized monoclonal antibody against CCR4 that was recently approved in Japan for the treatment of refractory CCR4-positive adult T cell leukemia/lymphoma (ATLL), peripheral T cell lymphoma (PTCL) and CTCL [15]. In the US, there is an ongoing phase III clinical trial currently investigating its efficacy in comparison to the oral HDACi, vorinostat. Given that the majority of IL-31-producing malignant T cells express CCR4, we hypothesized that abrogating the CCR4+ population may improve pruritus and concomitantly decrease IL-31 expression. We therefore collected PBMCs from a pruritic, leukemic CTCL patient with high expression of CCR4 on the malignant T cell repertoire (91%) both before and after the first infusion with mogamulizumab and then analyzed IL-31 expression. As hypothesized, a single mogamulizumab infusion significantly decreased the patient's pruritus and dramatically restored the CD4+/CD26+/CCR4- population (Figure 3b). Similarly, reduced numbers of IL-31+ T cells are observed after mogamulizumab treatment, suggesting the potential efficacy of this monoclonal antibody in the symptomatic control of pruritus and overall treatment of CTCL resistant to other conventional therapies (Figure 3b).
4. Discussion
Recalcitrant chronic pruritus is a debilitating disorder present in a number of skin conditions associated with an immunologic Th2 imbalance including atopic dermatitis. The cytokine milieu in patients with atopic dermatitis has long been known to include abnormally high expression of IL-4, IL-5 and IL-13 and based on more recent evidence, also increased IL-31 [16].
Both skin-restricted and systemic forms of CTCL display a similar cytokine signature to that of atopic dermatitis [17]. Moreover, recent reports demonstrate that even the non-malignant circulating cells in patients with CTCL display a Th2 phenotype in which high levels of IL-4 disturb an effective anti-tumor immune response [3]. Furthermore, IL-4 promotes class switch recombination to IgE and facilitates the expression of the eosinophil colony-stimulating factor IL-5, which synergistically facilitates a pruritogenic environment.
A recent article demonstrated that IL-31 is the end product of a complex cascade seen in polarized Th2 cells through phosphorylation of STAT-6 and activation of the transcription factor NK-κB [6]. The same study shows that in contrast to IL-9 producing T helper cells (Th9), which are also dependent on the influence of IL-4, Th9 cells produce transforming growth factor (TGF-β), which seems to suppress the transcription of IL-31 [6].
While the therapeutic use of TGF-β1 to suppress pruritus may be detrimental in the context of anti-tumor CTCL responses, the use of interferon gamma (IFN-γ), the canonical Th1 cytokine, may hypothetically provide reversal of the Th2 phenotype through suppression of the GATA3 transcription factor. Similarly, dampening of the IL-4 pathway through the use of monoclonal antibodies against its alpha-receptor, and the use of anti-IL-4 neutralizing antibodies or the use of STAT6 inhibitors may represent useful therapeutic alternatives that suppress upstream the production of IL-31.
In addition, blockage of the stimuli upstream of IL-4 could antagonize the Th2 polarization observed in CTCL. Recent reports have demonstrated the role of galectins, a novel family of β-galactoside-binding proteins as facilitators of the Th2 imbalance seen in CTCL patients, compromising efficacious anti-tumor immune responses [18]. A neutralizing humanized monoclonal antibody is currently under development.
5. Conclusion
In this report, we demonstrate how pro-apoptotic agents and epigenetic modifiers of the malignant CTCL cells can mitigate pruritus in advanced CTCL patients through suppression of IL-31 production. As shown by our flow cytometry data, in vitro treatment of PBMCs from severely pruritic CTCL patients either with dexamethasone or vorinostat induce a reduction in the number of IL-31-producing T cells, without inducing a reduction in the percentage of malignant T cells. In vivo, there was a clear effect of romidepsin in the viability of circulating malignant T cells and a marked reduction in the expression of IL-31 by mRNA analysis. In addition, we provide novel insights into the phenotype of the IL-31-producing cell in CTCL, while showing that the use of monoclonal antibodies targeting homing-molecules like CCR4 will be of significant symptomatic and therapeutic benefit.
Highlights.
+ Corticosteroids and histone deacetylase inhibitors decrease expression of IL-31.
+ Treated Sezary patients show lower levels of IL-31 coinciding with less pruritus.
+ IL-31-producing malignant T cells express the chemokine receptor type-4 (CCR4).
Acknowledgments
This work was supported by NCI grant R01CA122569 and a Translation Research Grant from the Leukemia and Lymphoma Society (to AHR).
Footnotes
Authorship Contributions: F.C.-L and E.M.S. designed the research, performed the experiments, analyzed the data, and wrote the manuscript; M.W. and B.M.B. performed the experiments and analyzed the data; C.V., E.J.K. and G.Y provided samples and analyzed the data and A.H.R conceived the study, designed the research, provided the reagents, supervised all experimentation, analyzed the data, and wrote the manuscript.
Conflict of Interest: The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim EJ, et al. Mycosis fungoides and sezary syndrome: an update. Curr Oncol Rep. 2006;8(5):376–86. doi: 10.1007/s11912-006-0061-1. [DOI] [PubMed] [Google Scholar]
- 2.Ahern K, Gilmore ES, Poligone B. Pruritus in cutaneous T-cell lymphoma: a review. J Am Acad Dermatol. 2012;67(4):760–8. doi: 10.1016/j.jaad.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guenova E, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19(14):3755–63. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer EM, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133(12):2783–5. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 5.Sonkoly E, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–7. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Maier E, et al. Human Th2 but Not Th9 Cells Release IL-31 in a STAT6/NF-kappaB-Dependent Way. J Immunol. 2014;193(2):645–54. doi: 10.4049/jimmunol.1301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy GF. Cutaneous T-cell Lymphoma. Adv Pathol. 1988;1:131–156. [Google Scholar]
- 8.Lubowe II. Use of hydrocortisone and 9-alpha-fluorohydrocortisone derivatives; evaluation in the treatment of the pruritic dermatoses. AMA Arch Derm. 1955;72(2):164–70. doi: 10.1001/archderm.1955.03730320066010. [DOI] [PubMed] [Google Scholar]
- 9.Olsen EA, et al. Sezary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC) J Am Acad Dermatol. 2011;64(2):352–404. doi: 10.1016/j.jaad.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Rangwala S, Zhang C, Duvic M. HDAC inhibitors for the treatment of cutaneous T-cell lymphomas. Future Med Chem. 2012;4(4):471–86. doi: 10.4155/fmc.12.6. [DOI] [PubMed] [Google Scholar]
- 11.Mann BS, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13(8):2318–22. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, et al. Clinically meaningful reduction in pruritus in patients with cutaneous T-cell lymphoma treated with romidepsin. Leuk Lymphoma. 2013;54(2):284–9. doi: 10.3109/10428194.2012.711829. [DOI] [PubMed] [Google Scholar]
- 13.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 14.Ferenczi K, et al. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119(6):1405–10. doi: 10.1046/j.1523-1747.2002.19610.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogura M, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–63. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 16.Raap U, et al. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012;23(3):285–8. doi: 10.1111/j.1399-3038.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 17.Vowels BR, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103(5):669–73. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 18.Cedeno-Laurent F, et al. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood. 2012;119(15):3534–8. doi: 10.1182/blood-2011-12-396457. [DOI] [PMC free article] [PubMed] [Google Scholar]


