Abstract
Background
Chronic infection with hepatitis C virus (HCV) genotype 2 or 3 can be treated with sofosbuvir without interferon. Because sofosbuvir is costly, its benefits should be compared with the additional resources used.
Objective
To estimate the cost-effectiveness of sofosbuvir-based treatments for HCV genotype 2 or 3 infection in the United States.
Design
Monte Carlo simulation, including deterministic and probabilistic sensitivity analyses.
Data Sources
Randomized trials, observational cohorts, and national health care spending surveys.
Target Population
8 patient types defined by HCV genotype (2 vs. 3), treatment history (naive vs. experienced), and cirrhosis status (noncirrhotic vs. cirrhotic).
Time Horizon
Lifetime.
Perspective
Payer.
Intervention
Sofosbuvir-based therapies, pegylated interferon–ribavirin, and no therapy.
Outcome Measures
Discounted quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs).
Results of Base-Case Analysis
The ICER of sofosbuvir-based treatment was less than $100 000 per QALY in cirrhotic patients (genotype 2 or 3 and treatment-naive or treatment-experienced) and in treatment-experienced noncirrhotic patients but was greater than $200 000 per QALY in treatment-naive noncirrhotic patients.
Results of Sensitivity Analysis
The ICER of sofosbuvir-based therapy for treatment-naive noncirrhotic patients with genotype 2 or 3 infection was less than $100 000 per QALY when the cost of sofosbuvir was reduced by approximately 40% and 60%, respectively. In probabilistic sensitivity analyses, cost-effectiveness conclusions were robust to uncertainty in treatment efficacy.
Limitation
The analysis did not consider possible benefits of preventing HCV transmission.
Conclusion
Sofosbuvir provides good value for money for treatment-experienced patients with HCV genotype 2 or 3 infection and those with cirrhosis. At their current cost, sofosbuvir-based regimens for treatment-naive noncirrhotic patients exceed willingness-to-pay thresholds commonly cited in the United States.
Primary Funding Source
National Institute on Drug Abuse and National Institute of Allergy and Infectious Diseases.
In December 2013, the U.S. Food and Drug Administration approved sofosbuvir, a nucleotide analogue inhibitor of hepatitis C virus (HCV) NS5B polymerase with activity against all HCV genotypes (1). In combination with ribavirin, sofosbuvir can be used to treat patients with chronic HCV genotype 2 or 3 infection without interferon, yielding cure rates greater than with the previous standard of care (2, 3). Cure, also known as sustained virologic response (SVR), is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality and short-term survival improvements for patients with advanced liver disease (4).
Sofosbuvir changed the landscape of HCV therapy given that concerns about interferon-related toxicity historically limited provider and patient enthusiasm for HCV treatment (5). However, sofosbuvir currently costs approximately $1000 per tablet, or $28 000 for 4 weeks (6). Many HCV-infected persons rely on publicly funded health insurance, and these programs do not guarantee access to such costly drugs (7). Several state Medicaid programs recently announced that sofosbuvir will be available only to patients with advanced liver disease (8).
Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments. Our goal was to evaluate the cost-effectiveness of sofosbuvir-based treatment strategies for patients with HCV genotype 2 or 3 infection to identify approaches that would maximize the number of patients who achieve HCV cure given competing demands on resources.
Methods
Analytic Overview
We used the Hepatitis C Cost-Effectiveness model, a Monte Carlo simulation of screening and treatment for HCV, to estimate the effectiveness and cost-effectiveness of strategies for treating chronic HCV genotype 2 or 3 infection. The model is summarized in this section, and details are available in the Supplement (available at www.annals.org) and elsewhere (9, 10). We considered 8 patient types defined by HCV genotype (2 vs. 3), treatment history (naive vs. experienced), and fibrosis stage (noncirrhotic vs. cirrhotic) (Table 1). We did not consider treatment of decompensated cirrhosis because management of end-stage liver disease is beyond the scope of this article.
Table 1.
Treatment Strategies Considered in Cost-Effectiveness Analysis of Therapies for HCV Genotype 2 or 3 Infection
| Treatment History | Strategies |
|---|---|
| Genotype 2 | |
|
| |
| Naive | No treatment |
| 24 wk of PEG–RBV | |
| 12 wk of SOF–RBV* | |
|
| |
| Experienced | No treatment |
| 12 wk of SOF–RBV* | |
| 16 wk of SOF–RBV | |
| Genotype 3 | |
|
| |
| Naive | No treatment |
| 24 wk of PEG–RBV | |
| 12 wk of SOF–RBV | |
| 24 wk of SOF–RBV* | |
| 12 wk of PEG–RBV–SOF | |
|
| |
| Experienced | No treatment |
| 12 wk of SOF–RBV | |
| 16 wk of SOF–RBV | |
| 24 wk of SOF–RBV* | |
| 12 wk of PEG–RBV–SOF | |
HCV = hepatitis C virus; PEG = pegylated interferon; RBV = ribavirin; SOF = sofosbuvir.
Recommended by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
For each patient type and treatment strategy, we used the model to simulate clinical outcomes and costs. Outcomes included quality-adjusted life expectancy (QALE) (measured in quality-adjusted life-years [QALYs]) and lifetime medical costs, both discounted at 3% annually (11). We calculated the incremental cost-effectiveness ratio (ICER) of each treatment strategy as the additional cost divided by the additional QALYs gained compared with the next less expensive strategy (11, 12). Strategies that resulted in higher costs but fewer QALYs gained and those with a higher ICER compared with a more effective strategy were considered inefficient (“dominated”) and were excluded from the final incremental comparisons (11, 13).
We used reports from published clinical trials and observational cohorts to inform base-case parameters for treatment efficacy, disease progression, toxicity, quality of life (QoL), and cost (2–4, 6, 14–34). We conducted 1-way deterministic sensitivity analyses for plausible ranges (Table 2) and also modeled 2 specific scenarios. In the first scenario, we modeled a cohort of patients with fibrosis (METAVIR score F3) to determine the cost-effectiveness of treating patients with advanced fibrosis but not cirrhosis. In the second scenario, which would favor HCV treatment, costs attributable to all stages of HCV infection were doubled and were then assumed to return to zero after attainment of SVR.
Table 2.
Model Inputs for Analysis of Cost-Effectiveness of SOF-Based Therapy for Treatment of HCV Genotype 2 or 3 Infection
| Variable | Base-Case Value | Range Evaluated in Sensitivity Analyses* | Reference |
|---|---|---|---|
| Cohort characteristics | |||
|
| |||
| Mean age of treatment-naive patients (SD), y | 48 (7) | 38 (7)–58 (7) | 2 |
|
| |||
| Mean age of treatment-experienced patients (SD), y | 54 (7) | 44 (7)–64 (7) | 3 |
|
| |||
| Proportion of treatment-naive men | 0.66 | 0–1 | 2 |
|
| |||
| Proportion of treatment-experienced men | 0.71 | 0–1 | 3 |
|
| |||
| Mean age at HCV infection, y | 26 | 16–36 | 34 |
|
| |||
| HCV disease progression | |||
| Median time to cirrhosis from age of infection (10th, 90th percentiles) y | 25 (23–27) | 10–40 | 32, 33 |
|
| |||
| Median time to first liver-related event after development of cirrhosis (10th, 90th percentiles), y | 11 (9–14) | 6–19 | 31 |
|
| |||
| Liver-related mortality with compensated cirrhosis, deaths per 100 person-years | 1.39 | 0.96–1.82 | 31 |
|
| |||
| Liver-related mortality with decompensated cirrhosis, deaths per 100 person-years | 12.00 | 8.28–15.72 | 31 |
|
| |||
| Reduction in liver-related mortality after SVR, %† | 94 | 81–98 | 4 |
| HCV therapy efficacy (SVR probabilities) in treatment-naive patients‡ | |||
|
| |||
| 24 wk of PEG–RBV | 2 | ||
|
| |||
| Genotype 2 without cirrhosis | 0.82 | 0.82 (0.06) | |
|
| |||
| Genotype 2 with cirrhosis | 0.62 | 0.62 (0.13) | |
|
| |||
| Genotype 3 without cirrhosis | 0.71 | 0.71 (0.04) | |
|
| |||
| Genotype 3 with cirrhosis | 0.29 | 0.29 (0.08) | |
|
| |||
| 12 wk of SOF–RBV | 2 | ||
|
| |||
| Genotype 2 without cirrhosis | 0.98 | 0.98 (0.02) | |
|
| |||
| Genotype 2 with cirrhosis | 0.90 | 0.90 (0.09) | |
|
| |||
| Genotype 3 without cirrhosis | 0.61 | 0.61 (0.04) | |
|
| |||
| Genotype 3 with cirrhosis | 0.34 | 0.34 (0.08) | |
|
| |||
| 24 wk of SOF–RBV | 30 | ||
|
| |||
| Genotype 3 without cirrhosis | 0.93 | 0.93 (0.03) | |
|
| |||
| Genotype 3 with cirrhosis | 0.90 | 0.90 (0.08) | |
|
| |||
| 12 wk of PEG–RBV–SOF | 29 | ||
|
| |||
| Genotype 3 without cirrhosis | 0.82 | 0.82 (0.12) | |
|
| |||
| Genotype 3 with cirrhosis | 0.82 | 0.82 (0.12) | |
| HCV therapy efficacy (SVR probabilities) in treatment-experienced patients‡ | |||
|
| |||
| 12 wk of SOF–RBV | 3 | ||
|
| |||
| Genotype 2 without cirrhosis | 0.96 | 0.96 (0.04) | |
|
| |||
| Genotype 2 with cirrhosis | 0.58 | 0.58 (0.15) | |
|
| |||
| Genotype 3 without cirrhosis | 0.37 | 0.37 (0.08) | |
|
| |||
| Genotype 3 with cirrhosis | 0.19 | 0.19 (0.01) | |
|
| |||
| 16 wk of SOF–RBV | 3 | ||
|
| |||
| Genotype 2 without cirrhosis | 0.99 | 0.99 (0.002) | |
|
| |||
| Genotype 2 with cirrhosis | 0.77 | 0.77 (0.13) | |
|
| |||
| Genotype 3 without cirrhosis | 0.59 | 0.59 (0.08) | |
|
| |||
| Genotype 3 with cirrhosis | 0.57 | 0.57 (0.02) | |
|
| |||
| 24 wk of SOF–RBV | 30 | ||
|
| |||
| Genotype 3 without cirrhosis | 0.86 | 0.86 (0.04) | |
|
| |||
| Genotype 3 with cirrhosis | 0.59 | 0.59 (0.02) | |
|
| |||
| 12 wk of PEG–RBV–SOF | 29 | ||
|
| |||
| Genotype 3 without cirrhosis | 0.83 | 0.83 (0.11) | |
|
| |||
| Genotype 3 with cirrhosis | 0.82 | 0.82 (0.01) | |
| Costs§ | |||
| Non–HCV-related medical costs, $ per mo | |||
|
| |||
| Background medical costs (without HCV infection)|| | 135–1035 | 70–1550 | 28 |
|
| |||
| HCV-related medical costs, $ per mo | |||
| No cirrhosis (SD) | 240 (60) | 180 (45)–300 (75) | 27 |
|
| |||
| Mild to moderate cirrhosis (SD) | 430 (120) | 310 (87)–540 (146) | 27 |
| Decompensated cirrhosis (SD) | 820 (210) | 610 (156)–1030 (258) | 27 |
|
| |||
| Cost multiplier after achievement of SVR | 0.50 | 0.00–0.70 | 27 |
|
| |||
| HCV therapy costs, $ per 4 wk | |||
| Provider visits¶ | 120 | 60–180 | 25, 26 |
|
| |||
| PEG** | 2500 | 1300–3800 | 6 |
|
| |||
| RBV†† | |||
| 1200 mg/d | 2500 | 1300–3800 | 6 |
|
| |||
| 800 mg/d | 1700 | 900–2600 | 6 |
|
| |||
| SOF | 28 000 | 5000–30 000 | 6 |
|
| |||
| Filgrastim‡‡ | 2000 | 1000–3000 | 6 |
|
| |||
| Total HCV therapy costs, $ | 6, 25, 26 | ||
| 24 wk of PEG–RBV | 25 300 | 12 800–37 800 | |
|
| |||
| 12 wk of SOF–RBV | 91 500 | 22 000–97 500 | |
|
| |||
| 16 wk of SOF–RBV | 121 900 | 30 000–129 900 | |
|
| |||
| 24 wk of SOF–RBV | 182 900 | 44 900–194 900 | |
|
| |||
| 12 wk of PEG–RBV–SOF | 99 000 | 30 000–105 000 | |
| 1-time costs, $ | |||
|
| |||
| Managing treatment-ending toxicity during interferon-containing therapy | 750 | 380–1100 | 2, 6, 23–26 |
|
| |||
| Managing treatment-ending toxicity during interferon-free therapy | 600 | 300–900 | 2, 6, 23–26 |
| Quality of life | |||
|
| |||
| After achievement of SVR§§ | 0.74–0.90 | 0.60–1.00 | 20–22 |
|
| |||
| With HCV infection | |||
| No to moderate fibrosis | 0.89 | 0.75–1.00 | 16, 18, 19 |
|
| |||
| Cirrhosis | 0.62 | 0.55–0.75 | 16, 18, 19 |
|
| |||
| Decompensated cirrhosis | 0.48 | 0.40–0.60 | 16, 18, 19 |
|
| |||
| Receiving interferon-containing therapy|||| | 0.88 | 0.50–0.96 | 15, 17, 20 |
|
| |||
| Receiving interferon-free therapy|||| | 0.99 | 0.95–1.00 | 15 |
|
| |||
| Major toxicity decrement¶¶ | 0.16 | 0.09–0.25 | 14 |
HCV = hepatitis C virus; PEG = pegylated interferon; RBV = ribavirin; SOF = sofosbuvir; SVR = sustained virologic response.
Efficacy estimates for patient subgroups (e.g., treatment-naive patients with HCV genotype 2 infection and cirrhosis) were informed by clinical trials. The exact estimates presented reflect parameters derived from the published manuscript, supplemental materials, and communication with the investigator team when necessary.
Applied only to patients who had cirrhosis before initiating treatment and subsequently attained SVR because HCV-attributable mortality was only applied in the model once patients developed cirrhosis.
Sensitivity analyses were probabilistic as opposed to deterministic (see Methods). Data are approximate means (SDs) of the β distributions developed to reflect uncertainty in efficacy estimates.
2013 U.S. dollars.
Varied as a function of age and sex.
Higher in the first month ($750).
17% of patients receiving PEG–RBV and PEG–RBV–SOF received a reduced weekly dose of 135 mcg because of non–treatment-ending neutropenia (absolute neutrophil count <750 cells/mL) in addition to twice-weekly filgrastim, 300 mcg (35).
A dosage of 800 mg/d was used for PEG–RBV, whereas a dosage of 1200 mg/d was used for all other RBV-containing regimens. 23% of patients receiving PEG–RBV and PEG–RBV–SOF and 10% of those receiving SOF–RBV were treated with a reduced dose of daily RBV, 600 mg, in response to non–treatment-ending anemia (grade 3 or 4 adverse event of hemoglobin level <100 g/L) (2, 29, 30, 35).
Cost of a nursing visit ($20.41) is also included for anemia management (26).
Utility without HCV infection is a function of age and sex. To estimate utility in a given month, the model used a multiplicative assumption to combine HCV-related utility with age- and sex-stratified utility without HCV. For example, the utility estimate of being alive without HCV infection for a 55-year-old man is 0.84. The utility estimate for having compensated cirrhosis is 0.62. A 55-year-old man with compensated cirrhosis would have a modeled utility of 0.84 × 0.62 = 0.52.
Multiplied by a patient’s health state utility during the months that he or she received HCV therapy without major toxicity.
Subtracted from a patient’s health state utility during the month of a major toxicity event.
We also performed probabilistic sensitivity analyses on treatment efficacy parameters. We defined distributions for each parameter based on outcomes from clinical trials. We conducted multiple iterations of the analyses by drawing parameter values from the distributions, and we displayed results by using cost-effectiveness acceptability curves (36).
We constructed the model and performed analyses using TreeAge Pro 2012 software (TreeAge Software).
Model Structure
The model functioned on 2 levels: disease progression and clinical decision making.
Disease Progression–Level Simulation
The disease progression–level simulation modeled 3 stages of liver disease: mild to moderate fibrosis, cirrhosis, and decompensated cirrhosis. Each of 1 million hypothetical persons in the patient-level simulation was assigned a unique time from HCV infection to development of cirrhosis such that some progressed rapidly while others did not develop cirrhosis before they died of non–HCV-related causes (32, 33). Consistent with previous studies, all disease stages of HCV infection were associated with increased resource use and decreased QoL compared with no infection, with progressively higher resource use and lower QoL for each successive stage of disease (Table 2) (18, 19, 37–41). When persons became cirrhotic, they were subject to increased mortality attributable to liver disease, which further increased with decompensated cirrhosis (31, 42, 43).
Clinical Decision-Making–Level Simulation: HCV Therapy
Although the model included 3 stages of HCV disease progression for the disease progression–level simulation, for the purpose of HCV treatment, all patients were categorized as either “cirrhotic” or “noncirrhotic.”
Pegylated Interferon–Ribavirin (Treatment-Naive Patients Only)
Patients initiated a planned 24-week course of weekly pegylated interferon-α2a (180 mcg subcutaneously) in combination with twice-daily oral ribavirin (400 mg). Patients in the simulation had routine HCV RNA testing at the end of treatment week 4. To maximize the benefit while minimizing exposure to interferon, those with detectable viremia stopped therapy, whereas those with suppressed HCV RNA (rapid virologic response) continued a planned 24-week treatment course (2). In sensitivity analyses, we also modeled a scenario with no response-guided therapy using pegylated interferon–ribavirin.
In each treatment month, patients were at risk for premature treatment discontinuation due to nonadherence or major toxicity (Table 1 of the Supplement) (2, 30). Major toxicity resulted in immediate cessation of therapy and was associated with additional costs and a QoL decrement (14, 23–26, 44), whereas nonadherence resulted in withdrawal without additional costs or QoL decrements (Table 2 and Table 2 of the Supplement). Patients who withdrew from therapy were not eligible to attain SVR and continued to progress according to their assigned disease progression parameters.
All patients receiving HCV medications experienced a monthly QoL decrement related to adverse symptoms (14, 15). A proportion of patients experienced non–treatment-ending toxicity, including moderate anemia managed by ribavirin dose reduction and moderate neutropenia managed by pegylated interferon dose reduction and twice-weekly filgrastim (35). Because toxicity management varies, sensitivity analyses considered management of anemia without colony-stimulating factors. Patients with non–treatment-ending toxicity accrued cost adjustments related to dosing changes and additional therapies, but they continued to receive HCV treatment and were eligible to attain SVR.
Sofosbuvir–Ribavirin for 12, 16, or 24 Weeks
Patients initiated a 12-, 16-, or 24-week course of once-daily oral sofosbuvir (400 mg) and twice-daily oral ribavirin (600 mg). Similar to pegylated interferon–ribavirin, all patients had decreased QoL while taking medications, some experienced non–treatment-ending toxicity, and some withdrew from therapy due to nonadherence or toxicity (2, 3). Compared with pegylated interferon–ribavirin, however, patients receiving sofosbuvir–ribavirin had higher QoL during therapy, less toxicity, and a lower probability of treatment withdrawal (Table 2 and Table 1 of the Supplement).
Pegylated Interferon–Ribavirin–Sofosbuvir (Patients With Genotype 3 Infection Only)
Patients initiated a 12-week course of weekly pegylated interferon (180 mcg subcutaneously) in combination with twice-daily ribavirin (600 mg) and once-daily sofosbuvir (400 mg) (29). The approach to modeling adherence, toxicity, and QoL during therapy was the same as for pegylated interferon–ribavirin.
HCV Therapy Efficacy
The model had 3 treatment-related parameters that affected SVR: probability of withdrawal due to nonadherence or treatment-ending toxicity, probability of virologic failure (for response-guided pegylated interferon–ribavirin therapy only), and probability of achieving SVR conditional on treatment completion.
We used a Bayesian approach and the mathematical properties of the β distribution to develop probability density functions for each parameter based on data from clinical trials (Supplement) (2, 3, 29, 30).
Costs
We assessed costs from the payer perspective in 2013 U.S. dollars. During each simulation month, persons accrued age- and sex-stratified “background costs” attributable to non–HCV-related health care (20). They also accrued monthly HCV-specific costs, which varied by disease stage, with increasing resource use for each stage of advancing disease (Table 2) (27, 37). Costs of HCV therapy included drugs, clinic visits, laboratory tests, and toxicity management (Table 2 and Tables 3 and 4 of the Supplement). Monthly costs related to liver disease (including physician visits, emergency department visits, and hospitalizations) were estimated using γ distributions, from which each simulated person was assigned a randomly drawn value.
Quality of Life
Quality of life reflected the combination of 3 independent utility functions, which we combined multiplicatively. First, we considered utility related to non-HCV comorbid conditions; all simulated persons had a “background” QoL that was a function of age and sex. Second, we considered HCV-specific utility; HCV-related QoL was a function of fibrosis stage, with progressively lower utility with advancing disease. Third, we considered treatment-related utility. All patients had decreased QoL while receiving HCV therapy, which reflected the disutility of treatment adverse effects. Quality of life during interferon-free treatment was higher (multiplier, 0.99) than during therapy involving interferon (multiplier, 0.88) (15). Moreover, when patients had a treatment-ending major toxicity event, they experienced a temporary disutility related to the event (14).
Benefits of SVR
With successful treatment (SVR), fibrosis progression halted; HCV-attributable costs were reduced to 50% of the person’s disease stage–specific cost before initiation of therapy (27, 45); QoL reverted to that of HCV-uninfected persons of the same age and sex (35); and, in patients who were cirrhotic before attaining SVR, liver-related mortality decreased by 94% (4).
Role of the Funding Source
This study was funded by the National Institute on Drug Abuse and the National Institute of Allergy and Infectious Diseases, which had no role in the conception, design, conduct, or analysis or the decision to submit the manuscript for publication.
Results
Genotype 2
Noncirrhotic Patients
Without therapy, discounted QALE among treatment-naive noncirrhotic patients with HCV genotype 2 infection was 13.9 QALYs, with a mean discounted lifetime medical cost of $169 000 (Table 3). Treatment with 24 weeks of pegylated interferon–ribavirin resulted in SVR in 82% of patients, leading to an incremental QALE gain of 1.5 QALYs, an incremental cost of $4300, and an ICER of $3000 per QALY gained compared with no treatment. Compared with pegylated interferon–ribavirin, 12 weeks of sofosbuvir–ribavirin resulted in SVR in 98% of patients, an incremental QALE gain of 0.4 QALY, and an incremental cost of $87 900, corresponding to an ICER of $238 000 per QALY gained.
Table 3.
Cost-Effectiveness of Treatment of Patients With HCV Infection: Genotype 2*
| Treatment Strategy | SVR, % | Cost, $ | Incremental Cost, $ | QALYs | Incremental QALYs | ICER, $/QALY |
|---|---|---|---|---|---|---|
| No cirrhosis | ||||||
| Naive | ||||||
|
| ||||||
| No treatment | – | 169 000 | – | 13.9 | – | – |
|
| ||||||
| 24 wk of PEG–RBV | 82 | 173 000 | 4300 | 15.5 | 1.5 | 3000 |
|
| ||||||
| 12 wk of SOF–RBV | 98 | 261 000 | 87 900 | 15.8 | 0.4 | 238 000 |
|
| ||||||
| Experienced | ||||||
| No treatment | – | 163 000 | – | 12.3 | – | – |
|
| ||||||
| 12 wk of SOF–RBV | 96 | 258 000 | 95 700 | 13.8 | 1.5 | 63 700 |
|
| ||||||
| 16 wk of SOF–RBV | 99 | 288 000 | 29 500 | 13.9 | 0.1 | 468 000 |
| Cirrhosis | ||||||
| Naive | ||||||
|
| ||||||
| No treatment | – | 94 000 | – | 5.1 | – | – |
|
| ||||||
| 24 wk of PEG–RBV | 62 | 150 000 | 54 000 | 11.3 | 6.2 | 8700 |
|
| ||||||
| 12 wk of SOF–RBV | 90 | 253 000 | 103 000 | 14.2 | 2.9 | 35 500 |
|
| ||||||
| Experienced | ||||||
| No treatment | – | 85 000 | – | 4.1 | – | – |
|
| ||||||
| 12 wk of SOF–RBV | 58 | 230 000 | 145 000 | 9.3 | 5.2 | Dominated† |
|
| ||||||
| 16 wk of SOF–RBV | 77 | 268 000 | 183 000 | 10.8 | 6.7 | 27 300 |
HCV = hepatitis C virus; ICER = incremental cost-effectiveness ratio; PEG = pegylated interferon; QALY = quality-adjusted life-year; RBV = ribavirin; SOF = sofosbuvir; SVR = sustained virologic response.
All costs are in 2013 U.S. dollars.
More costly and less effective than a competing strategy or had an ICER greater than that of a more effective strategy.
In treatment-experienced patients, sofosbuvir–ribavirin was compared with no treatment because pegylated interferon–ribavirin was not a treatment option. Twelve weeks of sofosbuvir–ribavirin resulted in SVR in 96% of patients, with an ICER of $63 700 per QALY gained. Extending therapy to 16 weeks resulted in SVR in 99% of patients and an ICER of $468 000 per QALY compared with 12 weeks of therapy (Table 3).
Cirrhotic Patients
Treatment with 24 weeks of pegylated interferon–ribavirin resulted in SVR in 62% of treatment-naive cirrhotic patients. Compared with no treatment, the incremental QALE gain was 6.2 QALYs and the incremental cost was $54 000, resulting in an ICER of $8700 per QALY gained. Treatment with sofosbuvir–ribavirin for 12 weeks resulted in 90% attaining SVR, with an ICER of $35 500 per QALY compared with 24 weeks of pegylated interferon–ribavirin (Table 3).
In treatment-experienced cirrhotic patients, 16 weeks of sofosbuvir–ribavirin resulted in longer QALE than 12 weeks of the same therapy at a lower cost per QALY gained. Consequently, the latter regimen was dominated by the former. Sixteen weeks of sofosbuvir–ribavirin resulted in SVR in 77% of patients, with an additional 6.7 QALYs, an incremental cost of $183 000, and an ICER of $27 300 per QALY gained compared with no treatment (Table 3).
Genotype 3
Noncirrhotic Patients
In treatment-naive noncirrhotic patients with genotype 3 infection, pegylated interferon–ribavirin resulted in SVR in 71%, a QALE gain of 1.9 QALYs compared with no treatment, and an incremental cost of $9300, resulting in an ICER of $4800 per QALY (Table 4). Only 61% achieved SVR with 12 weeks of sofosbuvir–ribavirin, resulting in lower QALE than for pegylated interferon–ribavirin at a higher cost, thus making it dominated. Treatment with 12 weeks of pegylated interferon–ribavirin–sofosbuvir resulted in SVR in 82% of patients, a QALE gain of 0.4 QALY compared with pegylated interferon–ribavirin, an incremental cost of $95 400, and an ICER of $263 000 per QALY. Treatment with 24 weeks of sofosbuvir–ribavirin resulted in SVR in 93% of patients and extended QALE by 0.3 QALY compared with 12 weeks of pegylated interferon–ribavirin–sofosbuvir at an incremental cost of $80 200, resulting in an ICER of $266 000 per QALY.
Table 4.
Cost-Effectiveness of Treatment of Patients With HCV Infection: Genotype 3*
| Treatment Strategy | SVR, % | Cost, $ | Incremental Cost, $ | QALYs | Incremental QALYs | ICER, $/QALY |
|---|---|---|---|---|---|---|
| No cirrhosis | ||||||
| Naive | ||||||
|
| ||||||
| No treatment | – | 165 000 | – | 13.0 | – | – |
|
| ||||||
| 24 wk of PEG–RBV | 71 | 175 000 | 9300 | 15.0 | 1.9 | 4800 |
|
| ||||||
| 12 wk of SOF–RBV | 61 | 269 000 | 94 000 | 14.8 | −0.2 | Dominated† |
|
| ||||||
| 12 wk of PEG–RBV–SOF | 82 | 270 000 | 95 400 | 15.4 | 0.4 | 263 000 |
|
| ||||||
| 24 wk of SOF–RBV | 93 | 350 000 | 80 200 | 15.7 | 0.3 | 266 000 |
|
| ||||||
| Experienced | ||||||
| No treatment | – | 163 000 | – | 12.3 | – | – |
|
| ||||||
| 12 wk of PEG–RBV–SOF | 83 | 267 000 | 105 000 | 13.6 | 1.3 | 82 000 |
|
| ||||||
| 12 wk of SOF–RBV | 36 | 273 000 | 5700 | 12.9 | −0.7 | Dominated† |
|
| ||||||
| 16 wk of SOF–RBV | 59 | 294 000 | 26 900 | 13.2 | −0.3 | Dominated† |
|
| ||||||
| 24 wk of SOF–RBV | 86 | 349 000 | 81 500 | 13.7 | 0.1 | 1 100 000 |
| Cirrhosis | ||||||
| Naive | ||||||
|
| ||||||
| No treatment | – | 95 000 | – | 5.1 | – | – |
|
| ||||||
| 24 wk of PEG–RBV | 30 | 135 000 | 39 600 | 8.0 | 2.9 | 13 600 |
|
| ||||||
| 12 wk of SOF–RBV | 34 | 228 000 | 92 500 | 8.5 | 0.5 | Dominated† |
|
| ||||||
| 12 wk of PEG–RBV–SOF | 81 | 256 000 | 120 700 | 13.3 | 5.3 | 22 600 |
|
| ||||||
| 24 wk of SOF–RBV | 90 | 341 000 | 85 600 | 14.1 | 0.8 | 107 000 |
|
| ||||||
| Experienced | ||||||
| No treatment | – | 85 000 | – | 4.1 | – | – |
|
| ||||||
| 12 wk of SOF–RBV | 19 | 211 000 | 126 000 | 5.8 | 1.7 | Dominated† |
|
| ||||||
| 12 wk of PEG–RBV–SOF | 80 | 247 000 | 162 000 | 11.4 | 7.2 | 22 300 |
|
| ||||||
| 16 wk of SOF–RBV | 57 | 256 000 | 8700 | 9.1 | −2.2 | Dominated† |
|
| ||||||
| 24 wk of SOF–RBV | 59 | 314 000 | 67 100 | 9.2 | −2.2 | Dominated† |
HCV = hepatitis C virus; ICER = incremental cost-effectiveness ratio; PEG = pegylated interferon; QALY = quality-adjusted life-year; RBV = ribavirin; SOF = sofosbuvir; SVR = sustained virologic response.
All costs are in 2013 U.S. dollars.
More costly and less effective than a competing strategy or had an ICER greater than that of a more effective strategy.
In treatment-experienced noncirrhotic patients, pegylated interferon–ribavirin–sofosbuvir resulted in a higher percentage attaining SVR, longer QALE, and lower incremental lifetime medical costs compared with both 12 and 16 weeks of sofosbuvir–ribavirin, making both strategies dominated. Compared with no treatment, pegylated interferon–ribavirin–sofosbuvir resulted in an additional 1.3 QALYs at an incremental cost of $105 000, resulting in an ICER of $82 000 per QALY. Extending therapy to 24 weeks of sofosbuvir–ribavirin resulted in 86% achieving SVR, a QALE gain of 0.1 QALY compared with pegylated interferon–ribavirin–sofosbuvir, an incremental cost of $81 500, and an ICER of $1 100 000 per QALY (Table 4).
Cirrhotic Patients
Among treatment-naive cirrhotic patients with genotype 3 infection, treatment with 24 weeks of pegylated interferon–ribavirin resulted in 30% achieving SVR, an incremental QALE gain of 2.9 QALYs, an incremental cost of $39 600, and an ICER of $13 600 per QALY compared with no treatment. Twelve weeks of sofosbuvir–ribavirin was dominated by pegylated interferon–ribavirin–sofosbuvir. Treatment with 12 weeks of pegylated interferon–ribavirin–sofosbuvir had an ICER of $22 600 per QALY compared with pegylated interferon–ribavirin. Twenty-four weeks of sofosbuvir–ribavirin resulted in SVR in 90% of patients, with an ICER of $107 000 per QALY compared with pegylated interferon–ribavirin–sofosbuvir (Table 4).
Among treatment-experienced patients, pegylated interferon–ribavirin–sofosbuvir resulted in a higher percentage attaining SVR, longer QALE, and lower lifetime medical costs than 12, 16, or 24 weeks of sofosbuvir–ribavirin, making these 3 strategies dominated. Compared with no treatment, pegylated interferon–ribavirin–sofosbuvir led to an additional 7.2 QALYs at an incremental cost of $162 000, corresponding to an ICER of $22 300 per QALY (Table 4).
Deterministic Sensitivity Analysis
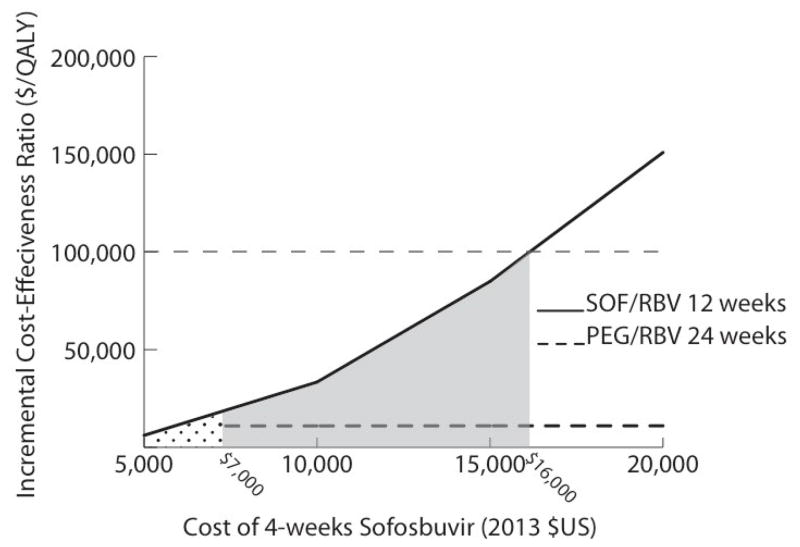
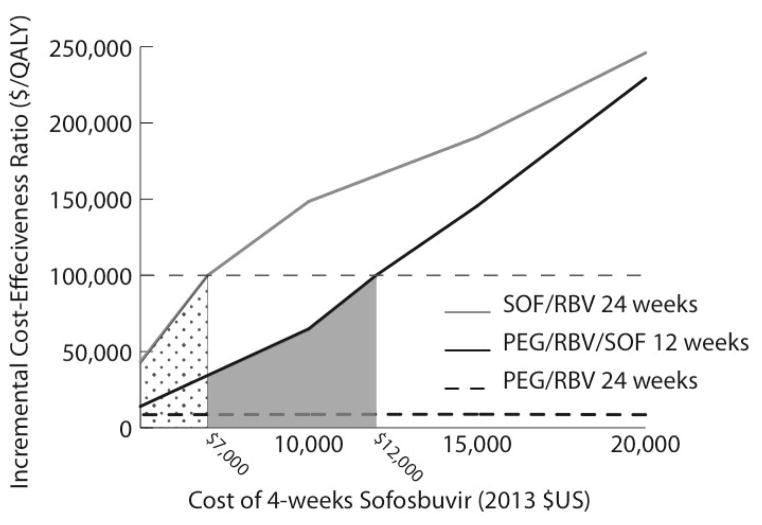
The cost-effectiveness of sofosbuvir-based therapy was most sensitive to the cost of sofosbuvir. In treatment-naive noncirrhotic patients with genotype 2 infection, the ICER of 12 weeks of sofosbuvir–ribavirin compared with 24 weeks of pegylated interferon–ribavirin was less than $100 000 per QALY gained when the cost of 4 weeks of sofosbuvir was less than $16 000, and sofosbuvir became cost-saving when it cost less than $7000 for 4 weeks (Figure 1). In treatment-naive noncirrhotic patients with genotype 3 infection, the ICER for 12 weeks of pegylated interferon–ribavirin–sofosbuvir compared with 24 weeks of pegylated interferon–ribavirin was less than $100 000 per QALY when 4 weeks of sofosbuvir cost less than $12 000. The higher ICERs for genotype 3 infection compared with genotype 2 infection reflect the smaller incremental benefit of sofosbuvir-based regimens for genotype 3 infection. The ICER for 24 weeks of sofosbuvir–ribavirin compared with 12 weeks of pegylated interferon–ribavirin–sofosbuvir was less than $100 000 per QALY only when the cost of 4 weeks of sofosbuvir was less than $7000 (Figure 2). If pegylated interferon–ribavirin–sofosbuvir was not considered as a treatment option, the ICER of 24 weeks of sofosbuvir–ribavirin compared with 24 weeks of pegylated interferon–ribavirin was less than $100 000 per QALY when 4 weeks of sofosbuvir cost less than $11 000. Among treatment-naive noncirrhotic patients, we also investigated cost-effectiveness by disease stage and found that in those with METAVIR stage F3 disease, the ICERs of 12 weeks of sofosbuvir–ribavirin for genotype 2 treatment and 12 weeks of pegylated interferon–ribavirin–sofosbuvir for genotype 3 treatment were less than $100 000 per QALY gained compared with pegylated interferon–ribavirin (Table 5 of the Supplement). However, in patients with stage F0 to F2 disease, the ICER of sofosbuvir-based regimens was more than $100 000 per QALY gained compared with pegylated interferon–ribavirin. Similarly, when we decreased the median time from infection to development of cirrhosis (consequently increasing the fibrosis stage of patients who were not yet cirrhotic), the ICERs of sofosbuvir-based therapy for treatment-naive noncirrhotic patients were less than $100 000 per QALY gained compared with pegylated interferon–ribavirin (Table 6 of the Supplement).
Figure 1. Results of 1-way sensitivity analyses of the effect of the cost of SOF on the ICER of SOF-based therapy for treatment of HCV genotype 2 infection in treatment-naive non-cirrhotic patients.
The dotted area illustrates the range of costs at which SOF–RBV is cost-saving compared with PEG–RBV. The gray shaded area depicts the range of costs at which the ICER of SOF–RBV is <$100 000 per QALY gained compared with PEG–RBV. HCV = hepatitis C virus; ICER = incremental cost-effectiveness ratio; PEG = pegylated interferon; QALY = quality-adjusted life-year; RBV = ribavirin; SOF = sofosbuvir.
Figure 2. Results of 1-way sensitivity analyses of the effect of the cost of SOF on the ICER of SOF-based therapy for treatment of HCV genotype 3 infection in treatment-naive non-cirrhotic patients.
The dotted area illustrates the range of costs at which the ICER of SOF–RBV is <$100 000 per QALY gained compared with PEG–RBV–SOF. The gray shaded area depicts the range of costs at which the ICER of PEG–RBV–SOF is <$100 000 per QALY compared with PEG–RBV. HCV = hepatitis C virus; ICER = incremental cost-effectiveness ratio; PEG = pegylated interferon; QALY = quality-adjusted life-year; RBV = ribavirin; SOF = sofosbuvir.
Other than treatment costs, uncertainty in base-case parameter estimates had no effect on the conclusions from the base-case analysis. When we changed base-case model parameters to reflect higher HCV-related mortality, successful treatment was associated with greater QALE gains and lower ICERs for sofosbuvir-based therapy. However, none of the ICERs crossed the $100 000-per-QALY threshold (Table 7 of the Supplement). Likewise, when we assumed both younger and older mean age, cost-effectiveness conclusions did not change (Tables 8 and 9 of the Supplement), and when we assumed a lower utility with HCV infection or varied the utility during interferon-based therapy, none of the base-case conclusions changed (Tables 10 to 12 of the Supplement). Among noncirrhotic patients with genotype 2 infection, we found no utility loss value during interferon therapy that resulted in an ICER less than $100 000 per QALY for sofosbuvir–ribavirin compared with pegylated interferon–ribavirin. Similarly, costs of toxicity management, including use of colony-stimulating factors, did not affect cost-effectiveness conclusions (Tables 13 and 14 of the Supplement). In a scenario with high costs in all stages of chronic infection and no HCV-related costs after attainment of SVR, the ICERs of sofosbuvir-based therapy in treatment-naive noncirrhotic patients remained above $200 000 per QALY (Table 15 of the Supplement). A scenario considering the effect of removing rules for discontinuation of therapy based on rapid virologic response (thus exposing viremic patients to the full treatment course) yielded no overall changes (Table 16 of the Supplement).
Probabilistic Sensitivity Analysis
For all patient types, the base-case findings were robust to uncertainty in treatment efficacy parameters. Among treatment-naive noncirrhotic patients with genotype 2 or 3 infection, the probability that sofosbuvir-based therapy would be preferred to pegylated interferon–ribavirin was greater than 50% only when the societal willingness-to-pay threshold was greater than $250 000 per QALY (Figures 3 and 4 of the Supplement). In contrast, among treatment-experienced patients and those with cirrhosis, sofosbuvir-based therapy was cost-effective in more than 90% of simulations when the societal willingness-to-pay threshold was less than $100 000 per QALY.
Discussion
We used evidence-based simulation modeling to investigate the cost-effectiveness of treating patients with HCV genotype 2 or 3 infection with interferon-free therapy. We found that at its current cost, sofosbuvir-based HCV therapy improves outcomes and provides good economic value in patients with cirrhosis and genotype 2 or 3 infection and in those who were previously treated with interferon. However, in treatment-naive noncirrhotic patients, for whom pegylated interferon–ribavirin remains a treatment option, the ICER of sofosbuvir-based therapy for genotype 2 or 3 infection is well over $100 000 per QALY. These conclusions were robust to sensitivity analyses that incorporated uncertainty around treatment efficacy, mortality, QoL, and costs.
There are 2 reasons that sofosbuvir-based therapy is not a cost-effective option for treatment-naive noncirrhotic patients with genotype 2 or 3 infection. First, the relative benefits of interferon-free treatment are reduced because pegylated interferon–ribavirin remains an effective treatment option in treatment-naive patients (approximately 80% efficacy). In contrast, pegylated interferon–ribavirin is not an effective option for treatment-experienced patients, and the incremental benefits of sofosbuvir are larger than for no treatment, resulting in ICERs of less than $100 000 per QALY.
Second, HCV-infected patients are at low risk for death from HCV until they develop cirrhosis, and not all develop cirrhosis. As a result, the SVR benefits of interferon-free therapy do not translate directly into substantial increases in life expectancy or QALE in noncirrhotic patients. This finding was consistent even when we increased mortality in cirrhotic patients, assumed lower QoL with early-stage HCV infection, and assumed higher costs attributable to HCV infection.
Translating these results into clinical practice raises 2 key questions about affordability. First, from the societal perspective, what is a reasonable price for sofosbuvir (or any new HCV medication)? In our analyses, the price of sofosbuvir was the primary driver of cost-effectiveness. At a current U.S. cost of $28 000 for 4 weeks of therapy, no reasonable estimates of HCV mortality or QoL effects led to sofosbuvir having an ICER less than $100 000 per QALY gained in treatment-naive noncirrhotic patients.
Second, should providers avoid administering interferon, regardless of the cost? Recent HCV guidelines identified pegylated interferon–ribavirin as a regimen “to avoid” for treating HCV (24). Patients with insurance have little motivation to initiate older therapies associated with longer treatment and substantial toxicity. From a societal perspective, however, is it acceptable to limit the total number of people treated for HCV to avoid using interferon at all costs? If not, the best approach would be for payers to negotiate price discounts for sofosbuvir consistent with its value because such negotiations could play a major role in improving access to HCV therapy.
If such negotiations are unsuccessful, however, our analysis supports an approach where providers offer pegylated interferon–ribavirin to treatment-naive noncirrhotic patients and reserve sofosbuvir for cirrhotic patients and those who cannot tolerate or have previously not benefitted from interferon-based therapy. Such a strategy would prioritize the sickest patients for costly new therapies while offering a less costly alternative to patients with early disease. However, this may raise provider concerns because before sofosbuvir was approved, many providers deferred treatment for patients with early-stage HCV in anticipation of interferon-free regimens (46). Now that such therapy is available, can providers feasibly defer therapy until these patients have more advanced disease?
Our approach has several limitations. First, our analysis did not consider costs and benefits of preventing HCV transmission. Epidemiologic modeling suggests that aggressive screening and treatment for HCV could decrease its incidence in injection drug users (47). Treating HCV genotype 2 and 3 infection may be particularly important for prevention given that up to 45% of injection drug–using youths in some cohorts are infected (48). Including the benefits of prevention in a cost-effectiveness analysis of HCV therapy would be premature, however, because the concept is unproven.
Second, our efficacy inputs were based on results from clinical trials. To the extent that real-world effectiveness of sofosbuvir-based regimens is lower than the efficacy observed in clinical trials, the ICERs of sofosbuvir-based therapy may be higher than those reported.
Third, we included 12 weeks of pegylated interferon–ribavirin–sofosbuvir as a treatment option for patients with genotype 3 infection and found that it was cost-effective for certain subgroups (29). However, 24 weeks of sofosbuvir–ribavirin is the only sofosbuvir-based regimen that is approved by the U.S. Food and Drug Administration for genotype 3 infection (1). Assuming that pegylated interferon–ribavirin–sofosbuvir is not a feasible treatment strategy, we calculated ICERs comparing 24 weeks of sofosbuvir–ribavirin with 12 weeks of pegylated interferon–ribavirin and found that sofosbuvir was more economically attractive but still had a cost-effectiveness ratio greater than $100 000 in treatment-naive noncirrhotic patients (Table 17 of the Supplement).
Fourth, we did not consider sofosbuvir–ledipasvir–ribavirin for treatment of patients with genotype 3 infection. Findings from the ELECTRON-2 study suggest that this regimen may be effective for such patients (49). However, the generalizability of those results is not certain, and the most recent treatment guidelines, which are based on ELECTRON-2 data, do not recommend this regimen for genotype 3 infection (24).
Finally, we performed an analysis of sofosbuvir for HCV genotypes 2 and 3, but most HCV infection in the United States is genotype 1 (50). Because approval of regimens treating genotypes 2 and 3 is not imminent, we expect therapeutic options to be more static in the near future compared with those for genotype 1. The cost-effectiveness and budgetary impact of recently approved agents for genotype 1 are important questions that will be best answered when those regimens are approved and it becomes clearer how they will be used in practice. The conclusions of this analysis of treatment of genotype 2 or 3 infection should not be used to infer the likely cost-effectiveness of new regimens for HCV genotype 1.
In summary, this analysis shows that at its current U.S. cost of $28 000 for 4 weeks of therapy, sofosbuvir-based treatment for HCV genotype 2 or 3 infection extends QALE and provides good value for money in cirrhotic patients and in treatment-experienced patients. Among treatment-naive noncirrhotic patients, however, the ICER of sofosbuvir-based treatments exceeds the commonly cited U.S. willingness-to-pay threshold of $100 000 per QALY gained. One option for using sofosbuvir is to treat only cirrhotic patients and those who have previously not benefitted from interferon. However, this approach would mean requiring persons with early-stage HCV infection, who have been waiting for the availability of interferon-free regimens before initiating treatment, to continue waiting until they develop more advanced liver disease or, alternatively, treating patients with early-stage infection with interferon followed by a sofosbuvir-based regimen if interferon is not effective. Options that would expand access to curative therapy include negotiating a reduced cost of sofosbuvir or waiting for approval of new medications with the expectation that market forces could decrease costs. At its current cost, access to sofosbuvir will likely be unequal and restricted and will limit the number of people ultimately cured of HCV infection in the United States.
Supplementary Material
Acknowledgments
Grant Support: By the National Institute on Drug Abuse (R01DA031059 and R01DA027379) and the National Institute of Allergy and Infectious Diseases (R37AI042006).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available for review upon discussion with the authors and as resources are available. Contact Dr. Linas (Benjamin.Linas@bmc.org). Data set: Not available.
References
- 1.U.S. Food and Drug Administration. Approval of Sovaldi (sofosbuvir) tablets for the treatment of chronic hepatitis C. Silver Spring, MD: U.S. Food and Drug Administration; 2013. [Accessed at on 7 February 2014.]. www.fda.gov/ForPatients/Illness/HepatitisBC/ucm377920.htm. [Google Scholar]
- 2.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. POSITRON Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micromedex 2.0. Drug Topics Red Book Online. [Accessed at on 25 June 2013.];Truven Health Analytics. 2013 www.micromedexsolutions.com.
- 7.Reau NS, Jensen DM. Sticker shock and the price of new therapies for hepatitis C: is it worth it? [Editorial] Hepatology. 2014;59:1246–9. doi: 10.1002/hep.27039. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D. Hepatitis C Drug Price Limiting State Medicaid Approvals. [Accessed at on 3 April 2014.];Bloomberg Business. 2014 Mar 5; www.bloomberg.com/news/2014-03-05/hepatitis-c-drug-price-limiting-state-medicaid-approvals.html.
- 9.Linas BP, Barter DM, Leff JA, DiLorenzo M, Schackman BR, Horsburgh CR, et al. The cost-effectiveness of improved hepatitis C virus therapies in HIV/hepatitis C virus coinfected patients. AIDS. 2014;28:365–76. doi: 10.1097/QAD.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One. 2014;9:e97317. doi: 10.1371/journal.pone.0097317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford Univ Pr; 1996. [Google Scholar]
- 12.Drummond MF, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. 3. Oxford, United Kingdom: Oxford Univ Pr; 2005. [Google Scholar]
- 13.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14:259–65. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
- 14.Schackman BR, Teixeira PA, Weitzman G, Mushlin AI, Jacobson IM. Quality-of-life tradeoffs for hepatitis C treatment: do patients and providers agree? Med Decis Making. 2008;28:233–42. doi: 10.1177/0272989X07311753. [DOI] [PubMed] [Google Scholar]
- 15.Stepanova M, Nader F, Cure S, Bourhis F, Hunt S, Younossi ZM. Patients’ preferences and health utility assessment with SF-6D and EQ-5D in patients with chronic hepatitis C treated with sofosbuvir regimens. Aliment Pharmacol Ther. 2014;40:676–85. doi: 10.1111/apt.12880. [DOI] [PubMed] [Google Scholar]
- 16.Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess. 2002;6:1–122. [PubMed] [Google Scholar]
- 17.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, et al. German Hepatitis C Model (GEHMO) Group. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425–32. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 19.Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, Rosenberg W, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55:1332–8. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman KE, Muir A, Aggarwal J, Donepudi M, Goss T, Martin M, et al. Health-related quality-of-life among genotype 1 treatment-experienced chronic hepatitis C patients: post-hoc analyses from the REALIZE Study [Abstract]. Presented at The International Liver Congress 2013, Amsterdam, the Netherlands, 24–28 April 2013. J Hepatol. 2013;58:S372. Abstract no. 904. [Google Scholar]
- 21.Yoshida H, Arakawa Y, Sata M, Nishiguchi S, Yano M, Fujiyama S, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483–91. doi: 10.1053/gast.2002.34785. [DOI] [PubMed] [Google Scholar]
- 22.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96:1267–78. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 23.Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009;110:351–60. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 24.American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. Alexandria, VA: American Association for the Study of Liver Diseases, Infectious Diseases Society of America; 2015. [Accessed at on 5 January 2015.]. www.hcvguidelines.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Medicare & Medicaid Services. Clinical Laboratory Fee Schedule. Baltimore: Centers for Medicare & Medicaid Services; 2013. [Accessed at on 20 June 2013.]. www.cms.gov/ClinicalLabFeesched. [Google Scholar]
- 26.Centers for Medicare & Medicaid Services. Physician Fee Schedule. Baltimore: Centers for Medicare & Medicaid Services; 2013. [Accessed at on 15 July 2013.]. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched. [Google Scholar]
- 27.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45:e17–24. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey Household Component Data. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [Accessed at on 15 July 2013.]. Total Health Services—Mean and Median Expenses per Person With Expense and Distribution of Expenses by Source of Payment. http://meps.ahrq.gov/mepsweb. [Google Scholar]
- 29.Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Dvory-Sobol H, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2015;61:769–75. doi: 10.1002/hep.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. VALENCE Investigators. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 31.Bruno S, Zuin M, Crosignani A, Rossi S, Zadra F, Roffi L, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104:1147–58. doi: 10.1038/ajg.2009.31. [DOI] [PubMed] [Google Scholar]
- 32.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 33.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 34.Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–16. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 35.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Solá R, et al. ACCELERATE Investigators. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–34. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 36.Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI) Value Health. 2008;11:886–97. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 37.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17:531–46. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linas BP, Wang B, Smurzynski M, Losina E, Bosch RJ, Schackman BR, et al. The impact of HIV/HCV co-infection on health care utilization and disability: results of the ACTG Longitudinal Linked Randomized Trials (ALLRT) Cohort. J Viral Hepat. 2011;18:506–12. doi: 10.1111/j.1365-2893.2010.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daltro-Oliveira R, Morais-de-Jesus M, Pettersen KM, Paraná R, Quarantini LC. Impact of sustained virologic response on quality of life in chronic HVC carriers. Ann Hepatol. 2013;12:399–407. [PubMed] [Google Scholar]
- 40.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 41.Vera-Llonch M, Martin M, Aggarwal J, Donepudi M, Bayliss M, Goss T, et al. Health-related quality of life in genotype 1 treatment-naïve chronic hepatitis C patients receiving telaprevir combination treatment in the ADVANCE study. Aliment Pharmacol Ther. 2013;38:124–33. doi: 10.1111/apt.12354. [DOI] [PubMed] [Google Scholar]
- 42.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8. 288.e1. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–10. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 44.Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 45.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 46.Kim AY. Management algorithm for genotype 1 hepatitis C virus. F1000Prime Rep. 2013;5:24. doi: 10.12703/P5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 48.Dias PT, Hahn JA, Delwart E, Edlin B, Martin J, Lum P, et al. Temporal changes in HCV genotype distribution in three different high risk populations in San Francisco, California. BMC Infect Dis. 2011;11:208. doi: 10.1186/1471-2334-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pianko S, Flamme SL, Shiffman ML, Kumar S, Strasser S, Dore GJ, et al. High efficacy of treatment with sofosbuvir+GS-5816+/−ribavirin for 12 weeks in treatment experienced patients with genotype 1 or 3 HCV infection [Abstract]. Presented at the American Association for the Study of Liver Diseases Liver Meeting; Boston, Massachusetts. 7–12 November 2014; p. Abstract no. 197. [Google Scholar]
- 50.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14:339–47. doi: 10.1002/hec.985. [DOI] [PubMed] [Google Scholar]
- 52.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—6. Value Health. 2012;15:835–42. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Degos F, Christidis C, Ganne-Carrie N, Farmachidi JP, Degott C, Guettier C, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–6. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitaker B, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 55. [Accessed at on 28 May 2014.];Physician’s Desk Reference Web site. www.pdr.net.
- 56.Quest Diagnostics. Hepatitis C Virus (HCV) FibroSURE. Madison, NJ: Quest Diagnostics; 2013. [Accessed at on 9 March 2015.]. www.questdiagnostics.com/testcenter/BUOrderInfo.action?tc=17611&labCode=MET. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.