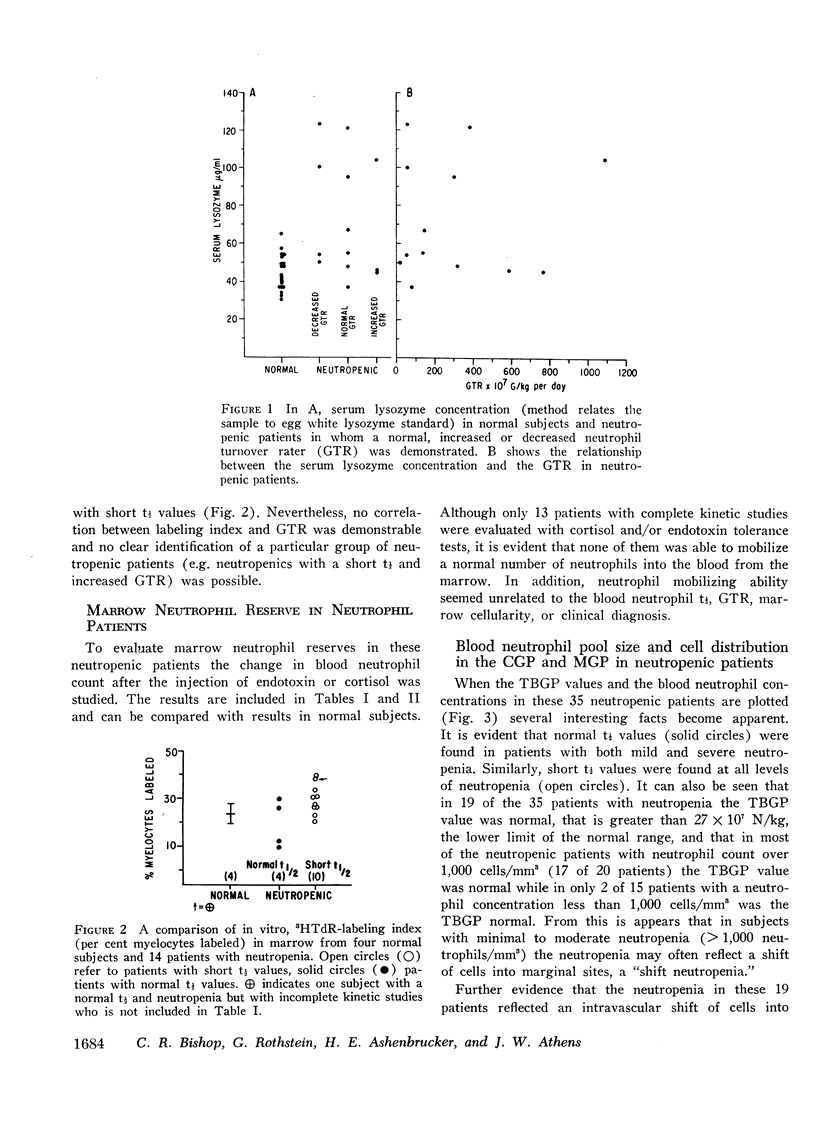
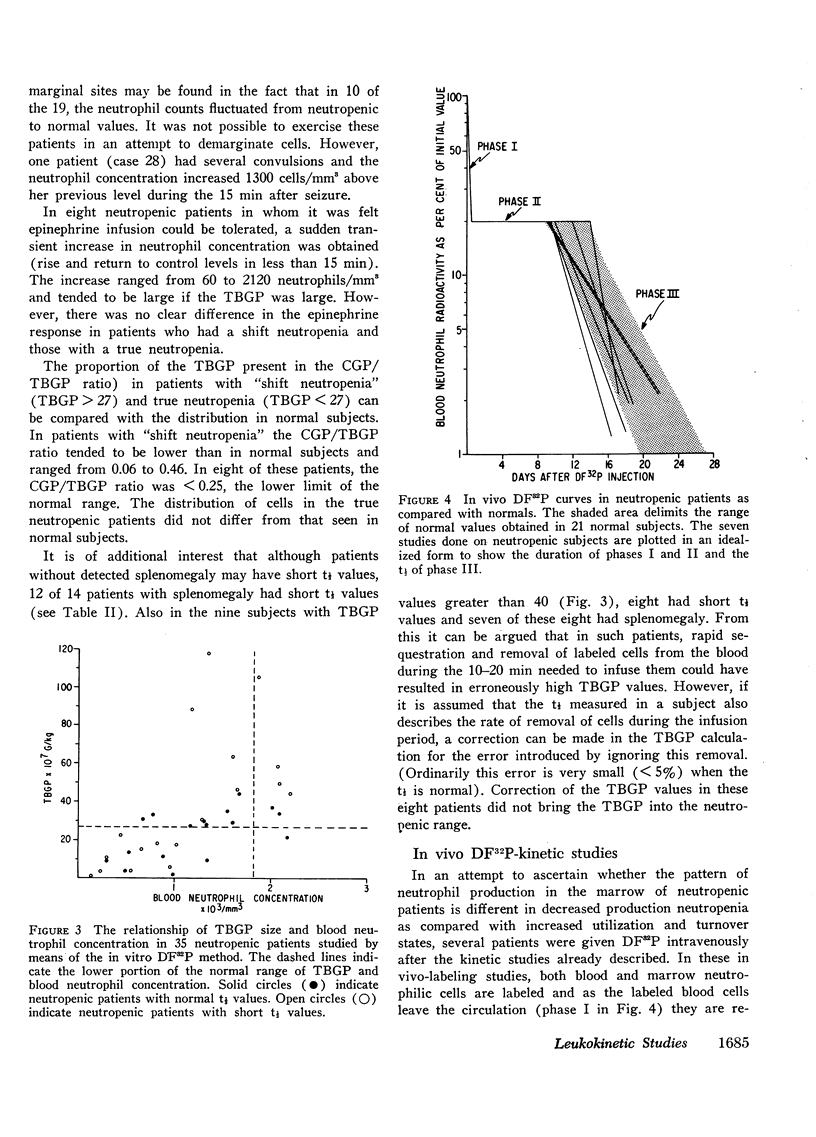
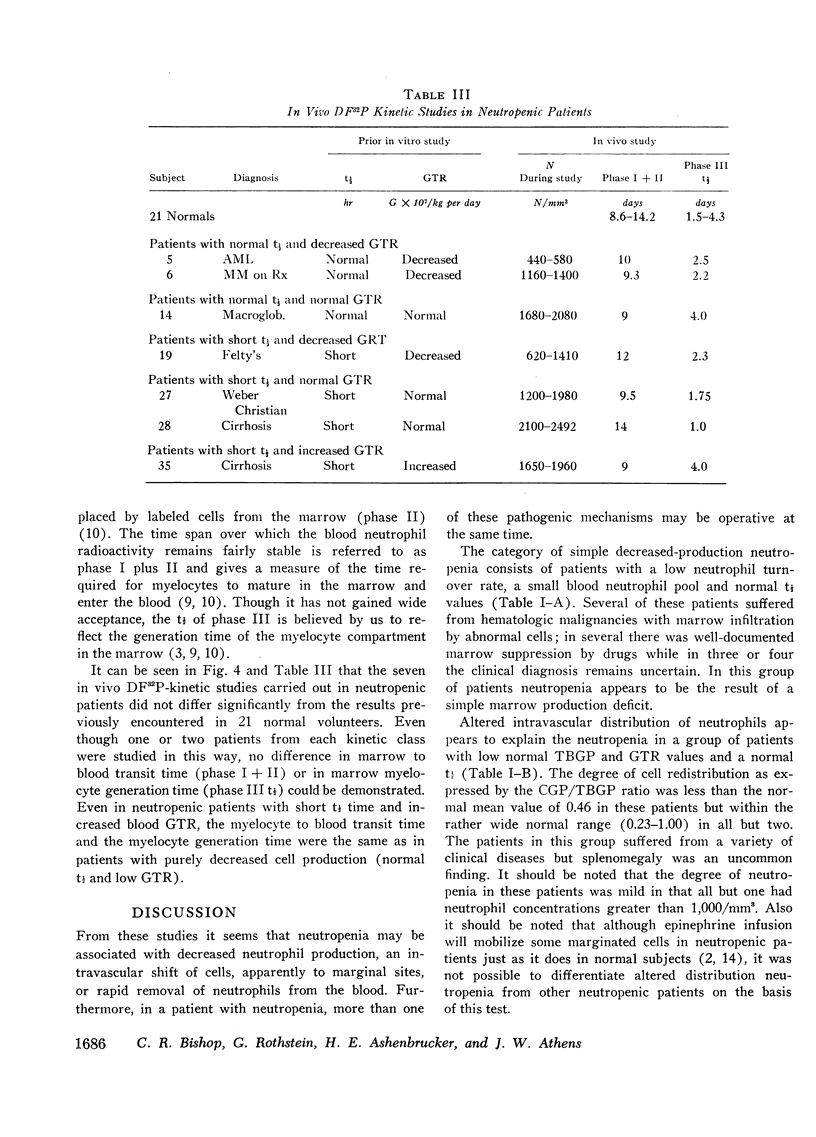
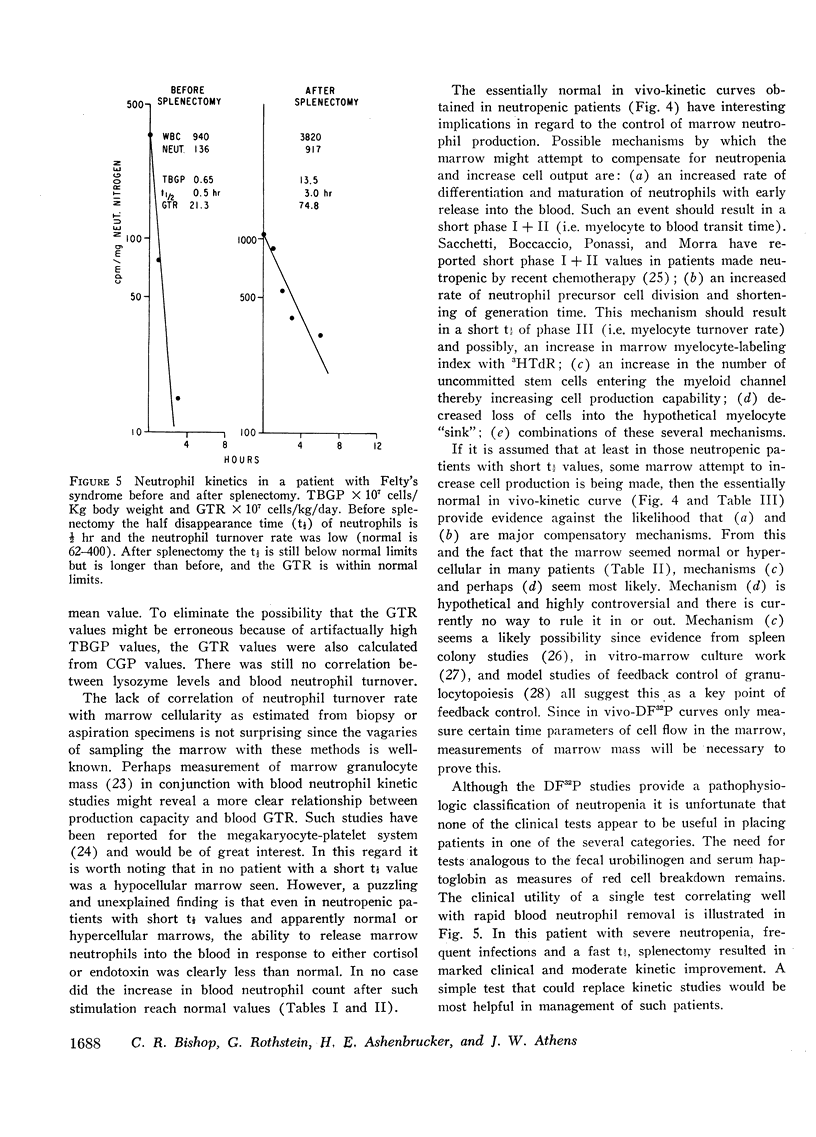
Abstract
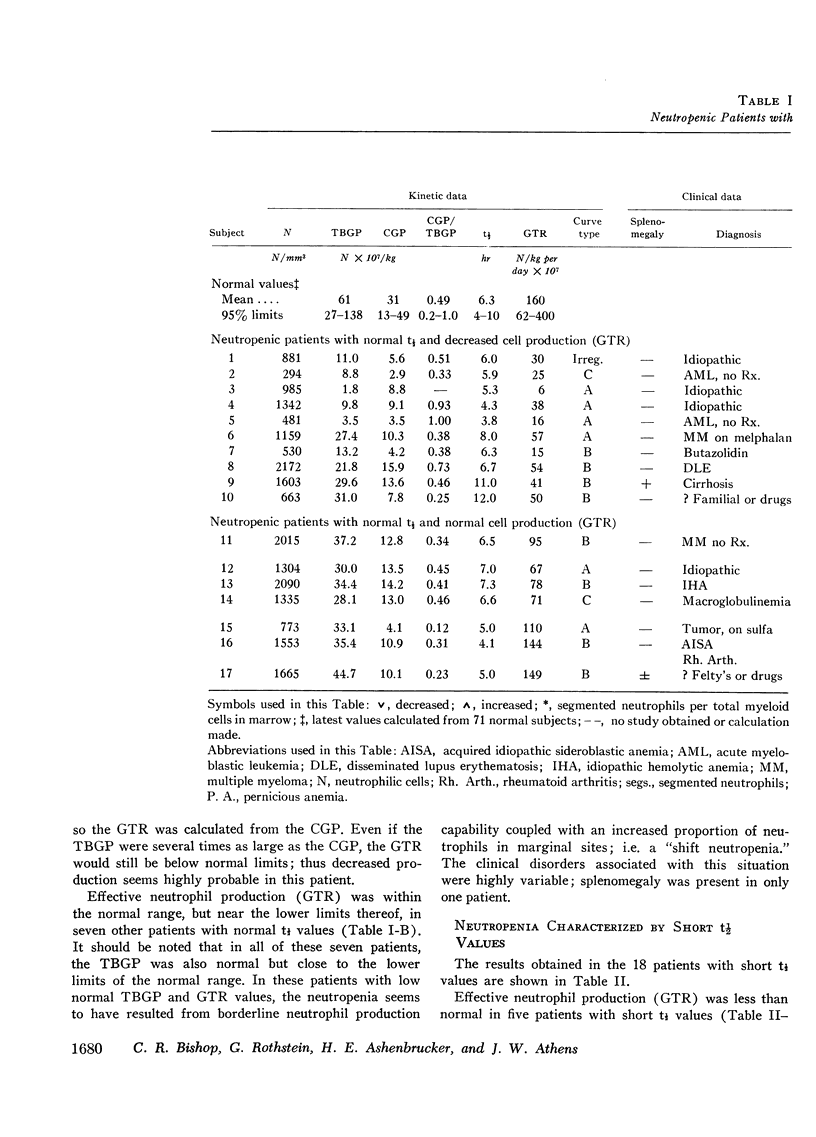
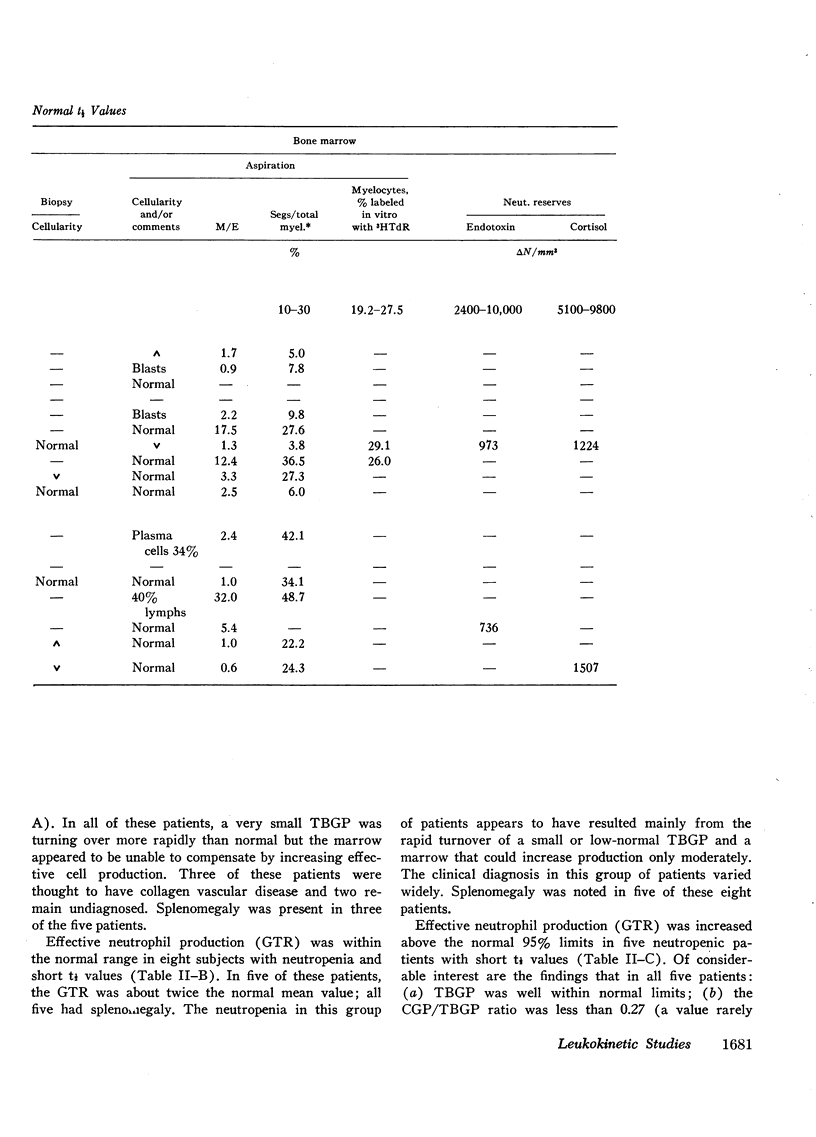
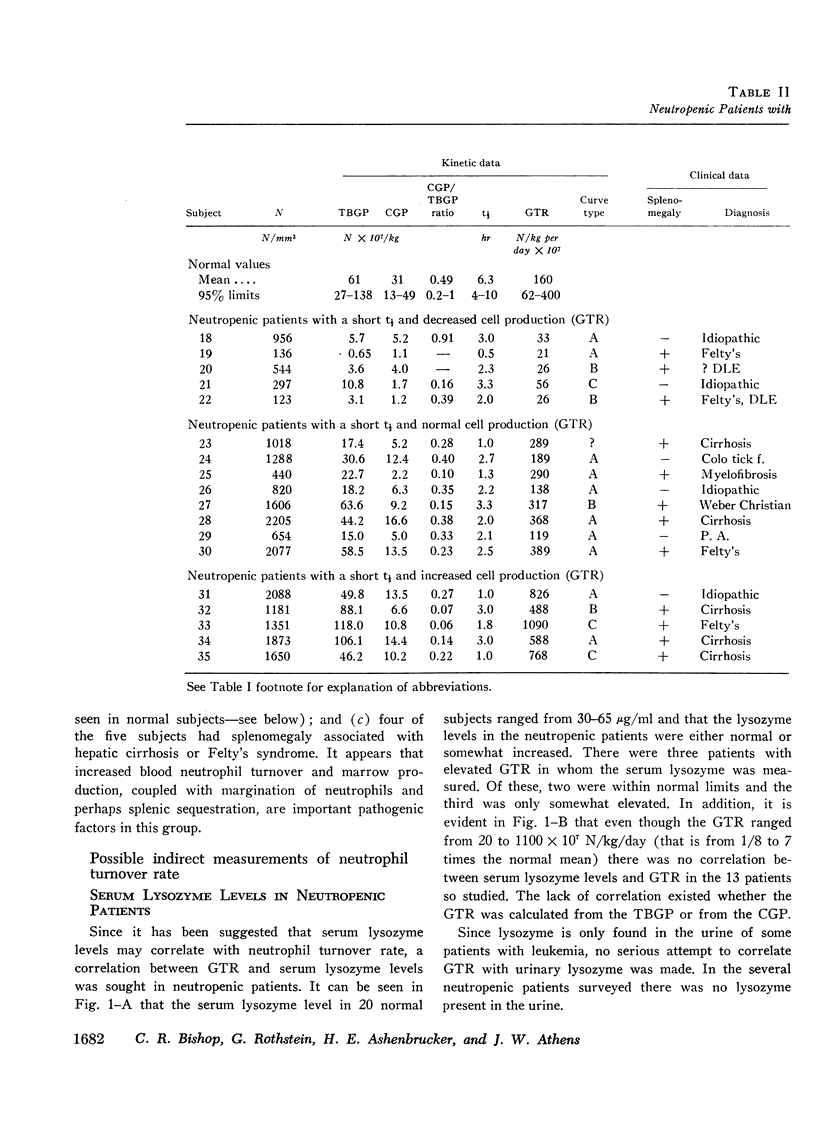
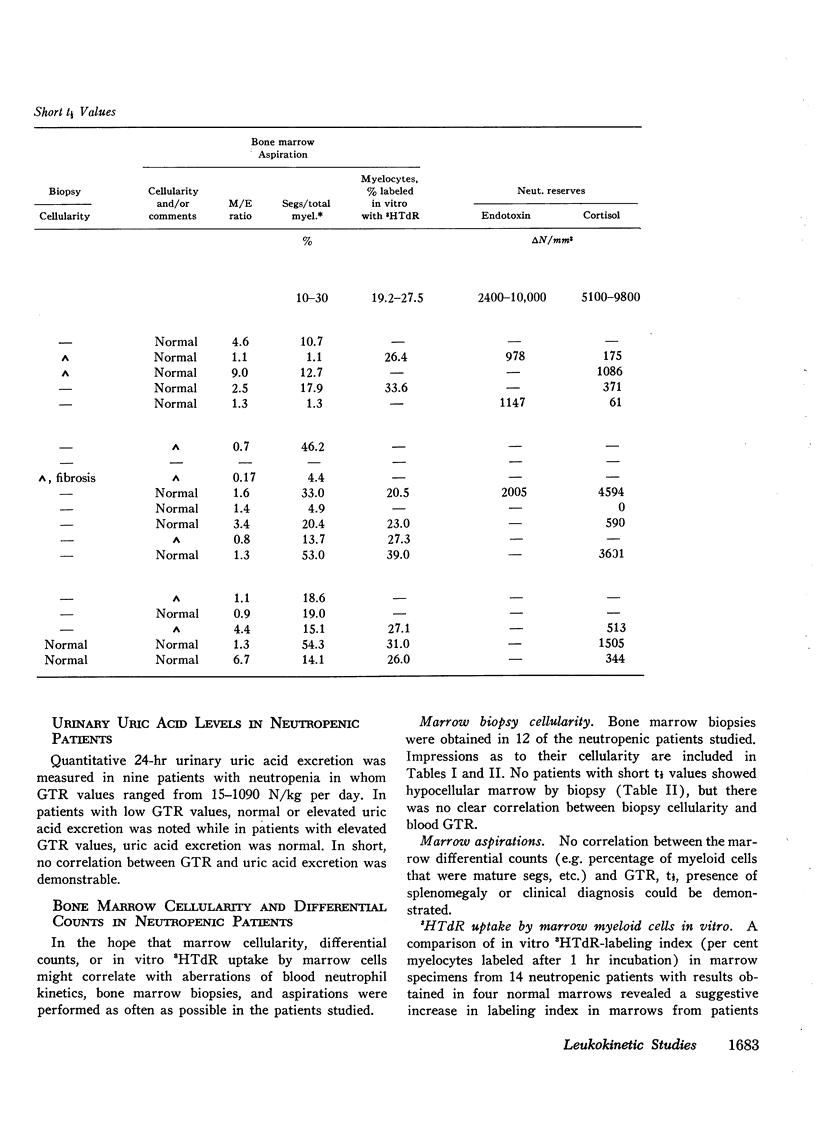
The kinetics of blood neutrophils was investigated by means of the in vitro radioactive diisopropyl fluorophosphate method in 35 patients with a chronic, steady-state neutropenia. There were 17 patients in whom the half disappearance time of neutrophils was normal. In 10 of these patients, the production of neutrophils was low and in 7, production was normal. In 18 patients the half disappearance time of neutrophilic granulocytes was shorter than normal. The production of neutrophilic granulocytes was low in five of these patients, normal in eight patients, and increased in five. An attempt was made to correlate other laboratory measurements with the kinetic picture, but no relationship was found; the marrow neutrophil reserve as measured by endotoxin or cortisol injection; marrow cellularity on aspiration or biopsy; in vitro-labeling index with 3HTdR; or serum lysozyme concentration proved of no value in identifying the various kinetic groups. The only finding that seemed to correlate with the kinetic picture was the presence or absence of splenomegaly. In 12 of the 18 patients with a short half disappearance time, splenomegaly was present whereas in 15 of 17 patients with a normal half disappearance time, there was no splenomegaly. Of 20 patients with greater than 1000 neutrophils per mm3, 17 were found to have a normal total-blood neutrophil pool. Thus these patients, with many of their cells marginated, agree to have a “shift neutropenia.”
Myelocyte to blood transit time and myelocyte generation time, as measured in seven patients by in vivo labeling with diisopropy fluorophosphate, proved to be essentially normal. Thus, it appears that in chronic neutropenia, increased or decreased production of neutrophils is accomplished by increasing or decreasing early precursor input into the system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATHENS J. W., HAAB O. P., RAAB S. O., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961 Jun;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., RAAB S. O., HAAB O. P., BOGGS D. R., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. LEUKOKINETIC STUDIES. X. BLOOD GRANULOCYTE KINETICS IN CHRONIC MYELOCYTIC LEUKEMIA. J Clin Invest. 1965 May;44:765–777. doi: 10.1172/JCI105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., RAAB S. O., HAAB O. P., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J Clin Invest. 1961 Jan;40:159–164. doi: 10.1172/JCI104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athens J. W. Granulocyte kinetics in health and disease. Natl Cancer Inst Monogr. 1969 May;30:135–155. [PubMed] [Google Scholar]
- Briggs R. S., Perillie P. E., Finch S. C. Lysozyme in bone marrow and peripheral blood cells. J Histochem Cytochem. 1966 Feb;14(2):167–170. doi: 10.1177/14.2.167. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., ATHENS J. W., WINTROBE M. M. THE KINETICS OF GRANULOPOIESIS IN NORMAL MAN. Blood. 1964 Dec;24:780–803. [PubMed] [Google Scholar]
- DONOHUE D. M., GABRIO B. W., FINCH C. A. Quantitative measurement of hematopoietic cells of the marrow. J Clin Invest. 1958 Nov;37(11):1564–1570. doi: 10.1172/JCI103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A. Magakaryocyte quantitation. J Clin Invest. 1968 Mar;47(3):452–457. doi: 10.1172/JCI105741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer A. M., Athens J. W., Ashenbrucker H., Cartwright G. E., Wintrobe M. M. LEUKOKINETIC STUDIES. II. A METHOD FOR LABELING GRANULOCYTES IN VITRO WITH RADIOACTIVE DIISOPROPYLFLUOROPHOSPHATE (DFP). J Clin Invest. 1960 Sep;39(9):1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch E. A., Thompson M. W., Siminovitch L., Till J. E. Effects of bacterial endotoxin on hemopoietic colony-forming cells in the spleens of normal mice and mice of genotype S1-S1 d . Cell Tissue Kinet. 1970 Jan;3(1):47–54. doi: 10.1111/j.1365-2184.1970.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Orfanakis N. G., Ostlund R. E., Bishop C. R., Athens J. W. Normal blood leukocyte concentration values. Am J Clin Pathol. 1970 May;53(5):647–651. doi: 10.1093/ajcp/53.5.647. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACCHETTI C., BOCCACCIO P., PONASSI A., MORRA L. NEUTROPHILIC LEUKOCYTE RESERVE OF THE BONE MARROW. 1. "IN VIVO" DETECTION AND QUANTITATION WITH DFP32. Minerva Nucl. 1965 Jan-Feb;9:55–62. [PubMed] [Google Scholar]
- WARNER H. R., ATHENS J. W. AN ANALYSIS OF GRANULOCYTE KINETICS IN BLOOD AND BONE MARROW. Ann N Y Acad Sci. 1964 Feb 28;113:523–536. doi: 10.1111/j.1749-6632.1964.tb40689.x. [DOI] [PubMed] [Google Scholar]
- Wiernik P. H., Serpick A. A. Clinical significance of serum and urinary muramidase activity in leukemia and other hematologic malignancies. Am J Med. 1969 Mar;46(3):330–343. doi: 10.1016/0002-9343(69)90036-9. [DOI] [PubMed] [Google Scholar]



