Abstract
Background
Infections are an important concern in patients using immunosuppressive therapy for their inflammatory bowel disease (IBD). Diabetes affects nearly 10% of Americans. Whether it confers an additional risk with immunosuppression in IBD has not been examined previously.
Aim
To examine the association between diabetes and infections with immunomodulator use in IBD
Methods
Using a validated, multi-institutional IBD cohort, we identified all patients who received at least one prescription for immunomodulators (thiopurines, methotrexate). Our primary outcome was infection within 1 year of the prescription of the immunomodulator. Multivariable logistic regression adjusting for relevant confounders was used to estimate the independent association with diabetes.
Results
Our study included 2,766 patients receiving at least one prescription for immunomodulators among whom 210(8%) developed an infection within 1 year. Patients who developed an infection were likely to be older, have more comorbidities, more likely to have received a prescription for steroids but similar in initiation of anti-TNF therapy within that year. Only 8% of those without an infection had diabetes compared to 19% of those who developed an infection within 1 year (Odds ratio (OR) 2.74, 95% confidence interval (CI) 1.88 – 3.98, p < 0.001). On multivariate analysis, diabetes was independently associated with a nearly two-fold increase in risk of infections (OR 1.80, 95% CI 1.20 – 2.68). There was no increase in risk of infections with addition of anti-TNF therapy (OR 1.14, 95% CI 0.80 – 1.63).
Conclusions
Diabetes is an independent risk factor for infection in IBD patients using immunomodulator therapy.
Keywords: Crohn’s disease, ulcerative colitis, diabetes, infection, immunosuppression
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) (Inflammatory bowel diseases (IBD)) are chronic immunologically mediated diseases associated with significant morbidity. Though their peak incidence is during young adulthood, a second peak of incidence in seen between the ages of 50-80 years.1 This older population accounts of 15% of all IBD diagnoses and 25% of hospitalizations.1-5 Inherent in the challenge of managing IBD in older patients is the influence of non-IBD comorbidity. Advances in therapy have improved our ability to achieve remission, reduce surgery and hospitalizations, and improve health-related quality of life.6-8 However, immunosuppression, the mainstay of treating IBD, is associated with serious risks including infections and cancers.9-11 Treatment-related cancers are of concern to patients, but are very rare.10 In contrast, infections occur in over 10% of patients,9 accounting for a quarter of the hospitalizations.12 The influence of specific comorbidities on the risk of infections has not been examined previously in those with IBD. This is a particularly pertinent question in the face of the changing demographic epidemiology of IBD.
Diabetes mellitus (DM) affects an estimated 29.1 million people in the United States (9.3% of the population). Apart from macrovascular and microvascular complications, diabetes is itself associated with an increased risk for common, serious, and opportunistic infections.13-16 This increase in risk is mediated through several mechanisms including impaired inflammatory cytokine production , phagocytosis, and neutrophilic response to infectious triggers.14, 17, 18 Given the risk of infection with immunosuppression, it is particularly important to examine whether underlying diabetes confers additional risk in patients. We performed this study to define the risk of infection in a large IBD cohort initiating immunomodulator therapy and to specifically examine the impact of co-existing diabetes on this risk.
METHODS
Study Cohort
The population for our study consisted of patients with CD or UC at one of two tertiary referral centers, Massachusetts General Hospital or Brigham and Women’s Hospital or affiliated practices within the Partners Healthcare network. The development of our cohort from electronic health record (EHR) data has been described in previous publications.19-21 We identified all patients with at least one International Classification of Diseases, 9th edition, clinical modification (ICD-9-CM) code for CD or UC. Using a combination of codified data comprising disease-related complications, procedures and treatments, as well as free text data identified using natural language processing, we developed and validated a regression algorithm that classified patients as having CD or UC with a positive predictive value (PPV) of 97%, yielding a final cohort of 5,522 patients with UC and 5,506 with CD.21
For this study, we identified an incident user cohort of patients receiving their first prescription for an immunomodulator for IBD (azathioprine, 6-mercaptopurine, and methotrexate). We did not include a parallel cohort initiating anti-tumor necrosis factor α (anti-TNF) biologic therapies owing to small numbers with diabetes, but adjusted for use of such therapy as a covariate. We examined the occurrence of our outcome (infections) within one year of this first prescription for an immunomodulator. Patients who died or did not continue to follow-up within our healthcare system ceased contributing follow-up time beyond their last contact. The follow-up period for patients with an interval of less than 1 year between the first and last prescription for immunomodulator use ended at 90 days after the date of the last prescription.
Outcomes, predictors and covariates
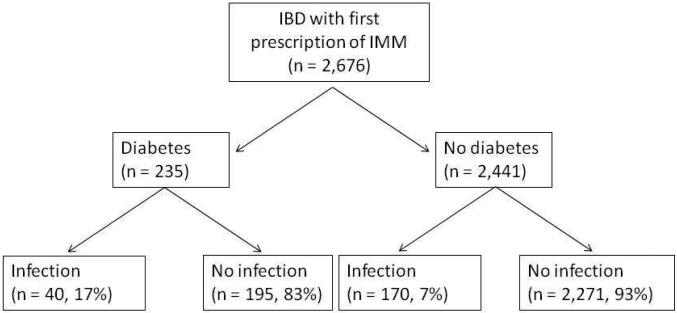
The main predictor of interest was a diagnosis of diabetes, defined as the presence of ≥ 1 relevant ICD-9-CM codes (250.0-250.7) (Figure 1). Only those with diabetes prior to immunomodulator initiation were included. A review of charts from 50 random patients meeting this criterion demonstrated a PPV of 74%. Nearly two-thirds of patients with ≥ 1 ICD-9 code for diabetes had a prescription anti-diabetic medication in our system. The PPV of the combination of ≥ 1 ICD-9 code and ≥ 1 anti-diabetic medication was 85%. Other covariates included age at immunomodulator initiation, age at first diagnosis code for IBD, gender and race. Comorbidity was assessed using the Charlson comorbidity index;22 however, we calculated a modified Charlson index that excluded diabetes and associated complications. The use of systemic corticosteroids and anti-tumor necrosis factor α biologics (infliximab, adalimumab, certolizumab pegol) was also assessed in the year following the prescription for immunomodulator therapy. For those who developed an infectious complication, only prescriptions prior to the outcome were included.
Figure 1.
Flowchart describing study design
Our primary outcome of interest was the occurrence of an infection within 365 days of initiation of immunomodulators. Relevant infections were identified using the appropriate ICD-9-CM codes (supplementary table 1). The accuracy of the codes for infections was assessed by randomly reviewing charts of 30 patients each with pneumonia, urinary tract infection, cellulitis, and bacteremia/sepsis (which were the most common infections noted) for clinical evidence of infection and treatment with antibiotics. The PPV ranged from 75% for urinary tract infection to 90% for bacteremia/sepsis.
Statistical Analysis
All statistical analysis was carried out with Stata 13.0 (StataCorp, College Station, TX). Continuous variables were summarized using medians and interquartile ranges if skewed, and means with standard deviations otherwise. Categorical variables were expressed in proportions and compared using the chi-square test while continuous variables were compared using the t-test. Univariate logistic regression with infection as the outcome of interest was performed. All variables significant at p < 0.10 were carried forward to a multivariable logistic regression adjusting for potential confounders. A p-value < 0.05 indicated independent statistical significance in the adjusted model. Internal validation was performed by bootstrapping with 500 replications.
We performed a number of sensitivity analyses. As patients with diabetes may be more closely followed contributing to ascertainment bias, we adjusted for intensity of healthcare utilization measured as a ‘fact density’. Each encounter with the healthcare system – laboratory test, radiology, procedures, hospitalizations, office visits – contributed to an individual fact, and dividing the sum total of facts by follow-up time yielded fact density. We repeated our analysis by varying the definition of diabetes to either an ICD-9 code for diabetes OR a prescription anti-diabetic agent or presence of both ≥ 1 ICD-9 code AND a prescription anti-diabetic. We also repeated our analysis including only infections that required hospitalization. Our study was approved by the Institutional Review Board of Partners Healthcare.
RESULTS
Study Population
Our study included 2,676 patients with IBD (62% CD, 38% UC) initiating n immunomodulator therapy (90% thiopurines, 10% methotrexate). The mean interval between the first and most recent prescription for immunomodulators was 23 months. The median age of the cohort was 38 years (interquartile range (IQR) 27-54 years) and the median age at first diagnosis code for IBD was 31 years (IQR 22-46 years). The cohort was predominantly white, just over half were women (51%) and over two-thirds (70%) had one or more comorbidities. Just under 9% of cohort had diabetes (n=235). Diabetic patients were older at immunomodulator initiation (57 vs. 39 years), more likely to be Black (11% vs. 7%), have ulcerative colitis (55% vs. 49%) and more comorbidities. They were also more likely to receive systemic steroids (43% vs. 32%, p=0.01) but not anti-TNF biologics (16% vs. 19%, p=0.21).
Within the first year of immunomodulator initiation, 210 patients developed an infection (7.8%). Patients who developed infections were older and had more comorbidities than those who did not develop an infection (Table 1). While there was no difference in anti-TNF biologic use between the two groups, patients who developed infections were more likely to have received systemic steroids (62% vs. 31%). Among those with diabetes, 17% developed infections within the first year compared to 7% of those without diabetes (unadjusted odds ratio (OR) 2.74, 95% confidence interval (CI) 1.88 – 3.98) (Figure 1). Table 2 presents the frequency of various infections. The most common infections in diabetics were pneumonia, urinary tract infection, sepsis and cellulitis. These were also the most common infections in non-diabetics, but cellulitis formed a larger proportion among those without diabetes than those with.
Table 1.
Characteristics of the study population
| Characteristics | No infection (n = 2,466) % |
Infection (n = 210) % |
p-value |
|---|---|---|---|
| Age (in years) | 40.5 (17.3) | 45.8 (18.9) | < 0.001 |
| Age at first IBD ICD9 code (in years) | 34.0 (16.6) | 39.7 (19.6) | < 0.001 |
| Sex | 0.086 | ||
| Female | 52 | 58 | |
| Male | 48 | 42 | |
| Race | 0.18 | ||
| White | 89 | 87 | |
| Black | 7 | 10 | |
| Other | 4 | 3 | |
| Charlson comorbidity† | < 0.001 | ||
| 0 | 30 | 5 | |
| 1 or more | 70 | 95 | |
| Type of IBD | 0.30 | ||
| Crohn’s disease | 63 | 59 | |
| Ulcerative colitis | 37 | 41 | |
| Diabetes | < 0.001 | ||
| No | 92 | 81 | |
| Yes | 8 | 19 | |
| Medication use | |||
| Anti-TNF | 19 | 22 | 0.23 |
| Steroid | 31 | 62 | < 0.001 |
IBD – inflammatory bowel disease; Anti-TNF – monoclonal antibodies to tumor necrosis factor α (infliximab, adalimumab, certolizumab pegol)
Charlson index was modified to exclude diabetes and related complications
Table 2.
Types of infections within 1 year of immunomodulator use, stratified by concomitant diabetes
| Type of infection | IBD with concomitant diabetes (n = 40) |
IBD without diabetes (n = 170) |
|---|---|---|
| Pneumonia | 15 | 42 |
| Urinary tract infection | 13 | 45 |
| Sepsis | 10 | 27 |
| Cellulitis | 9 | 94 |
| Herpes Simplex | 3 | 17 |
| Herpes zoster | 3 | 12 |
| Bacteremia | 3 | 23 |
| CMV | 2 | 10 |
| Tuberculosis | 1 | 4 |
| Streptococcal | 1 | 0 |
| Aspergillosis | 1 | 3 |
| Meningitis | 1 | 2 |
| Meningitis | 1 | 2 |
| Streptococcal | 1 | 0 |
| Influenza | 0 | 5 |
| EBV | 0 | 4 |
| Lung abscess | 0 | 1 |
IBD – inflammatory bowel disease; CMV – cytomegalovirus, EBV – Epstein Barr Virus
Predictors of infection
Variables selected for the final regression model (p < 0.10 on univariate analysis) were age, gender, comorbidity, diabetes, corticosteroid use in addition to anti-TNF biologic therapy which included as a covariate a priori due to its potential association with infections in previous literature23, 24. In the final adjusted model, diabetes was independently associated with a nearly two-fold increase in risk of infections (adjusted OR 1.80, 95% CI 1.20 – 2.68) (Table 3). The increase in risk of infection with diabetes was similar in those older than 65 years as in younger patients. The risk of infection was greater among insulin users (OR 2.01, 95% CI 1.22 – 3.31) than among users of oral anti-diabetic agents (OR 1.54, 95% CI 0.83 – 2.84), possibly reflecting the effect of more difficult to control diabetes.
Table 3.
Multivariable logistic regression for predictors of infection within 1 year of immunomodulator use
| Characteristic | Odds Ratio | 95% confidence interval |
p-value |
|---|---|---|---|
| Age (for each1 year) | 1.01 | 1.00 – 1.01 | 0.16 |
| Sex | |||
| Male | 1.0 | Reference | |
| Female | 1.24 | 0.93 – 1.66 | 0.15 |
| Charlson comorbidity* | |||
| 0 | 1.0 | Reference | |
| ≥ 1 | 5.85 | 3.13 – 10.94 | < 0.0001 |
| Type of IBD | |||
| Ulcerative colitis | 1.0 | Reference | |
| Crohn’s disease | 0.96 | 0.71 – 1.29 | 0.78 |
| Concomitant medications | |||
| Anti-TNF therapy | |||
| No | 1.0 | Reference | |
| Yes | 1.14 | 0.80 – 1.63 | 0.46 |
| Corticosteroids | |||
| No | 1.0 | Reference | |
| Yes | 1.98 | 1.35 – 2.90 | < 0.001 |
| Diabetes | |||
| No | 1.0 | Reference | |
| Yes | 1.80 | 1.20 – 2.68 | 0.004 |
IBD – inflammatory bowel disease; Anti-TNF – monoclonal antibodies to tumor necrosis factor α (infliximab, adalimumab, certolizumab pegol)
Charlson index modified to exclude diabetes
Corticosteroid use (OR 1.98, 95% CI 1.35 – 2.90) and the modified Charlson comorbidity index (OR 5.85, 95% CI 3.13 – 10.94) were also associated independently with an increased risk of infection. Concomitant anti-TNF therapy was not associated with an elevated risk of infection (OR 1.14, 95% CI 0.80 – 1.63). Only a small number of patients had a hemoglobin A1C available during this time period; consequently we were not able to address whether the risk of infection demonstrated a dose-response relationship with tightness of glycemic control.
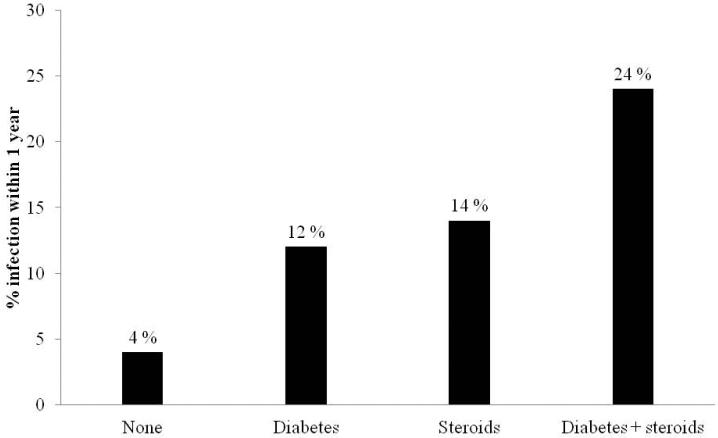
To compare the effect of concomitant diabetes to corticosteroid use, we stratified patients by whether they were diabetic, had been prescribed systemic steroids, or had both exposures during the first year. The proportion of patients with diabetes (12%) who developed infections in the year following immunomodulator initiation was similar to the rate among corticosteroid users in non-diabetics (14%) (Figure 2). Patients with both exposures had the greatest increase in frequency and likelihood of infections (adjusted OR 5.13, 95% 2.98 – 8.82) (Table 4).
Figure 2.
Frequency of infections within 1 year of initiating immunomodulator therapy, stratified by diabetes and corticosteroid use
Table 4.
Multivariable analysis of risk of infection, stratified by diabetes and concomitant steroid use
| Characteristic | Odds Ratio | 95% confidence interval | p-value |
|---|---|---|---|
| None | 1.0 | Reference | |
| Only diabetes | 2.24 | 1.23 – 4.07 | 0.008 |
| Only corticosteroids use | 3.46 | 2.47 – 4.83 | < 0.0001 |
| Corticosteroid use and diabetes | 5.13 | 2.98 – 8.82 | < 0.0001 |
Adjusted for age, gender, modified Charlson co-morbidity index, anti-TNF use, and type of IBD P-value for interaction between corticosteroid use and diabetes > 0.05
Sensitivity Analyses
Adjusted for healthcare utilization (OR 1.78, 95% CI 1.19 – 2.67) or for the presence of a primary care provider within our hospitals (OR 1.81, 95% CI 1.21 – 2.70) did not significantly influence our estimates. We also examined the robustness of association with varying our definitions of diabetes. The association with infection persisted when defining diabetes as either one ICD-9-CM code or an anti-diabetic medication (OR 2.49, 95% CI 1.78 – 3.50) or requiring both a code for diabetes and a prescription for anti-diabetic therapy (OR 1.84, 95% CI 1.16 – 2.93). Internal validation using bootstrapping with 500 replications yielded similar association with diabetes (OR 1.89, 95% CI 1.24 – 2.90). Restricting the analysis to infections that resulted in hospitalizations also yielded a similar magnitude of association with diabetes (OR 1.97, 95% CI 1.24 – 3.12).
DISCUSSION
With the aging of the population and rising incidence in IBD, the intersection of IBD and non- disease related comorbidity is increasingly important, particularly with reference to safety of immunosuppressive therapies which form the cornerstone of management of these complex diseases. In the present study from a large cohort of 2,676 patients with IBD initiating immunomodulator therapy, we demonstrate that co-existing diabetes is associated independently with a nearly two-fold increase in risk of infections.
Despite the growing prevalence of diabetes among those with IBD in addition to the effect of corticosteroids in precipitating diabetes or hyperglycemia in those with a predisposition, there is a remarkable paucity of data on the effect of co-existing diabetes (or indeed, significant other comorbidity) on risk for infections associated with immunosuppressive therapy in IBD. Supporting our findings of increased susceptibility to infections with diabetes among those using immunosuppressive therapy are complementary studies in those with rheumatoid arthritis (RA). In a study of 16,788 RA patients followed for over 3 years, co-existing diabetes was associated with a 50% increase in hospitalizations for pneumonia and similar in magnitude to the excess risk conferred by prednisone.25 The North American CORRONA registry of patients with RA including nearly 8,000 patients also similarly demonstrated an increased risk of infection associated with diabetes.26 Among RA patients using anti-TNF biologics, diabetes was associated with a nearly two-fold independent increased in risk of hospitalization with a definite bacterial infection.27 However, infection risks in RA patients cannot be directly extrapolated to those with IBD owing to a different natural history of disease, age and comorbidity burden, and differences in use of concomitant medications including steroids, doses of immunomodulators and biologics, as well as differences in intrinsic susceptibility to infections.
An increase in risk of infections with diabetes in the general population has been debated,15 but several large studies suggest a modest increase in risk. Analysis of the National Health and Nutrition Examination Survey (NHANES) mortality study demonstrated that infection-related deaths were twice as common in those with diabetes compared to those without13 while a prospective Dutch cohort showed both type 1 and type 2 diabetes to be associated with an increased risk for lower respiratory infections, urinary infections, bacterial skin and mucous membrane infections.16 There are several mechanisms how diabetes may predispose to infections.14 Diabetes is associated with reduced secretion of interleukin-1 (IL-1) and IL-6 in response to lipopolysaccharide stimulation (LPS) and glycation inhibits production of IL-10 by myeloid cells.14, 15, 17, 18, 28 Other studies have suggested a deficiency of C4 component of the complement system in patients with diabetes, decreased mobilization of leucocytes via chemotaxis and reduced phagocytic activity during hyperglycemia.14
The association between older age and infections in IBD patients has been well replicated in the literature1, 23 and our findings in this largely ambulatory IBD cohort confirmed previous studies among hospitalized patients demonstrating an association between age, comorbidity and infections.12 Our findings of lack of an additional risk of infection associated with biologic therapy in immunomodulator users is consistent with a growing body of literature that suggests anti-TNF biologics themselves do not significantly elevate infection risk over that conferred by immunomodulators, or indeed if they do so, the effect is very modest. In a large, pooled analysis by Grijalva et al. that included patients with various autoimmune diseases, the rates of hospitalization for infection were similar among patients initiating anti-TNF biologics (10.9 per 100 person-years) and comparators (9.6 per 100 person years).29 Pooled analysis of randomized controlled trials as well as large observational cohorts revealed a modest increase in risk of infections with immunomodulators but not biologics.11, 24 In contrast, five year analysis from the Crohn’s Therapy, Resource, Evaluation, and Assessment Tool (TREAT) registry did suggest a small increase in risk of infections with infliximab use, but a stronger effect was observed for severity of disease, use of narcotic analgesics, and prednisone.23 The excess infection risk conferred with prednisone is widely supported in the literature as evident from several studies universally demonstrating a moderate increase in risk of serious infections and opportunistic infections among users compared to non-users.23, 30 In a case control study of 100 consecutive IBD patients with opportunistic infections, corticosteroid use was associated with a three-fold increase in risk of infections on univariate analysis, and further numerically increased risk of opportunistic infections even among those who were on concomitant immunomodulator or infliximab therapy.30
There are several implications to our findings. To our knowledge, ours is the first study to specifically quantify the absolute and relative risk of infections associated with diabetes among patients initiating immunomodulator therapy for their IBD. The magnitude of this excess risk was similar to that associated with systemic corticosteroid use which is widely recognized by treating providers and patients as risk factor. Consequently, with an expected growing intersection between diabetes and IBD owing to the aging of the population, it is important for providers involved in treating IBD to recognize this excess risk posed by diabetes, and furthermore the added risk imposed by use of systemic corticosteroids in such patients. Steroid sparing strategies may need to play a particularly important role among older IBD patients with diabetes. Secondly, pneumonia was the most common infection in such patients, but yet is one that lends itself, at least in part, to prevention with appropriate vaccination.31, 32 Recognizing the excess risk associated with diabetes, such patients should be targeted for appropriate health maintenance. While we were not able to directly examine the effect of glycemic control on the risk of infection, other studies have suggested that hyperglycemia is associated with impaired immune function.15, 18, 28 Consequently, it is important to facilitate tight glycemic control in IBD patients with diabetes on immunosuppressive therapy. Future studies are also essential to determine if the increase in risk of infections associated with diabetes is specific to immunomodulator therapy or of similar magnitude in those with chronic low-dose steroid use or biologics. As agents with likely different mechanisms of actions and safety profile become available, it is increasingly important to compare relative safety of therapies in population with specific high-risk comorbidities such as diabetes to inform treatment choice.
We acknowledge several limitations to our study. First, our EHR cohort was primarily composed of patients receiving care at two tertiary referral centers and consequently may be skewed towards more severe disease. Analysis of population-based cohorts are essential to complement our findings; yet given the 10% prevalence of both diabetes and infections among those using immunomodulators, large sample sizes would be required for robust analyses. In addition, the rate of infections in our study is consistent with other reported cohorts of thiopurine users (7– 14%), suggesting generalizability of our findings.33, 34 Second, we were not able to specifically examine the effect of diabetes on patients who are on anti-TNF biologic monotherapy as the numbers of diabetics among this group was too small. This is an important question to examine in future studies. Third, ascertainment of infections was using administrative codes; however, chart review confirmed high positive predictive value for such codes within our cohort. It is possible that patients with diabetes are under closer surveillance within the healthcare system, consequently leading to an ascertainment bias in the detection of infections. However, our findings were robust on adjusting for intensity of healthcare utilization. Additionally, the odds ratio was numerically higher and remained statistically significant if our outcome was defined as infections that required hospitalizations, arguably an outcome which is less likely to be influenced by such bias. Fourth, we were not able to differentiate between type 1 and type 2 diabetes mellitus, or that precipitated by long-term use of corticosteroids. However, prior studies suggest that the risk of infection associated with diabetes is similar in magnitude across all types of diabetes.16 Chart review also confirmed good positive predictive value for ICD-9 codes in defining diabetes and our findings were robust on a number of sensitivity analyses with varying assumptions for this exposure of interest. Finally, we did not have sufficient data to examine the impact of both short- and long-term glycemic control.
In conclusion, utilizing a large cohort of patients with IBD initiating immunomodulator therapy, we demonstrate that diabetes is associated independently with an increased risk for infections within the first year of therapy. Appropriate physician and patient education strategies on this excess risk are essential and preventive healthcare maintenance emphasized to reduce risk of preventable infections and improve patient outcomes.
Supplementary Material
Acknowledgments
Sources of Funding: The study was supported by NIH U54-LM008748. A.N.A is supported by funding from the US National Institutes of Health (K23 DK097142). K.P.L. is supported by NIH K08 AR060257 and the Harold and Duval Bowen Fund. E.W.K is supported by grants from the NIH (K24 AR052403, P60 AR047782, R01 AR049880).
Footnotes
Conflicts of interest: Ananthakrishnan has served on the scientific advisory boards for Cubist and Abbvie, and has received research funding from Cubist and Amgen. None of hte other authors have any conflicts to declare.
REFERENCES
- 1.Ha CY, Katz S. Clinical implications of ageing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2014;11:128–38. doi: 10.1038/nrgastro.2013.241. [DOI] [PubMed] [Google Scholar]
- 2.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–77. doi: 10.1111/apt.12616. [DOI] [PubMed] [Google Scholar]
- 3.Ha CY. Risks of Infection among the Older Inflammatory Bowel Disease Patients. Curr Treat Options Gastroenterol. 2014;12:283–91. doi: 10.1007/s11938-014-0023-x. [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–9. doi: 10.1002/ibd.20628. [DOI] [PubMed] [Google Scholar]
- 5.Shung DL, Abraham B, Sellin J, et al. Medical and Surgical Complications of Inflammatory Bowel Disease in the Elderly: A Systematic Review. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3462-2. [DOI] [PubMed] [Google Scholar]
- 6.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 7.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–19. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 8.Peyrin-Biroulet L, Lemann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870–9. doi: 10.1111/j.1365-2036.2011.04599.x. [DOI] [PubMed] [Google Scholar]
- 9.Afif W, Loftus EV., Jr Safety profile of IBD therapeutics: infectious risks. Gastroenterol Clin North Am. 2009;38:691–709. doi: 10.1016/j.gtc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Bewtra M, Lewis JD. Safety profile of IBD: lymphoma risks. Gastroenterol Clin North Am. 2009;38:669–89. doi: 10.1016/j.gtc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–8. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7:107–12. doi: 10.1016/j.crohns.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care. 2001;24:1044–9. doi: 10.2337/diacare.24.6.1044. [DOI] [PubMed] [Google Scholar]
- 14.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi N, Caputo GM, Weitekamp MR, et al. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 16.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–8. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 17.Geerlings SE, Brouwer EC, Van Kessel KC, et al. Cytokine secretion is impaired in women with diabetes mellitus. Eur J Clin Invest. 2000;30:995–1001. doi: 10.1046/j.1365-2362.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 19.Ananthakrishnan AN, Cagan A, Cai T, et al. Colonoscopy Is Associated With a Reduced Risk for Colon Cancer and Mortality in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflamm Bowel Dis. 2013;19:1921–7. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananthakrishnan AN, Cai T, Savova G, et al. Improving case definition of Crohn's disease and ulcerative colitis in electronic medical records using natural language processing: a novel informatics approach. Inflamm Bowel Dis. 2013;19:1411–20. doi: 10.1097/MIB.0b013e31828133fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–22. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1051–63. doi: 10.1038/ajg.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–34. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg JD, Reed G, Kremer JM, et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis. 2010;69:380–6. doi: 10.1136/ard.2008.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125–33. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 28.Peleg AY, Weerarathna T, McCarthy JS, et al. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 29.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toruner M, Loftus EV, Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Rahier JF. Prevention and management of infectious complications in IBD. Dig Dis. 2012;30:408–14. doi: 10.1159/000338143. [DOI] [PubMed] [Google Scholar]
- 32.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–68. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Warman JI, Korelitz BI, Fleisher MR, et al. Cumulative experience with short- and long-term toxicity to 6-mercaptopurine in the treatment of Crohn's disease and ulcerative colitis. J Clin Gastroenterol. 2003;37:220–5. doi: 10.1097/00004836-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–9. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.