Abstract
Radiation therapy (RT) and chemotherapy (CTX) following surgery are mainstays of treatment for breast cancer (BC). While multiple studies have recently revealed the significance of immune cells as mediators of CTX response in BC, less is known regarding roles for leukocytes as mediating outcomes following RT. To address this, we utilized a syngeneic orthotopic murine model of mammary carcinogenesis to investigate if response to RT could be improved when select immune cells or immune-based pathways in the mammary microenvironment were inhibited. Treatment of mammary tumor-bearing mice with either a neutralizing monoclonal antibody (mAb) to colony-stimulating factor-1 (CSF-1) or a small molecule inhibitor of the CSF-1 receptor kinase (i.e., PLX3397), resulting in efficient macrophage depletion, significantly delayed tumor regrowth following RT. Delayed tumor growth in this setting was associated with increased presence of CD8+ T cells, and reduced presence of CD4+ T cells, the main source of the Th2 cytokine interleukin (IL)4 in mammary tumors. Selective depletion of CD4+ T cells or neutralization of IL4 in combination with RT, phenocopied results following macrophage depletion, whereas depletion of CD8+ T cells abrogated improved response to RT following these therapies. Analogously, therapeutic neutralization of IL4 or IL13, or IL4 receptor alpha deficiency, in combination with the CTX paclitaxel resulted in slowed primary mammary tumor growth by CD8+ T cell-dependent mechanisms. These findings indicate that clinical responses to cytotoxic therapy in general can be improved by neutralizing dominant Th2-based programs driving protumorigenic and immune suppressive pathways in mammary (breast) tumors to improve outcomes.
Keywords: radiation therapy, breast cancer, Th2-polarization, macrophages, interleukin-4
INTRODUCTION
Breast cancer is the most prevalent malignant disease of women in the developed world, and remains one of the leading causes of death (1). Radiation and chemotherapy (CTX) form the mainstay of treatment for patients with breast cancer (BC), and more than half of all patients diagnosed with BC will receive some form of radiation therapy (RT) (2). Although the antitumor properties of RT have long been considered a neoplastic cell-intrinsic process, evidence from multiple murine tumor models have demonstrated a critical role for immune cells in mediating RT response (3–6). While much of the immune response against tumors has been attributed to T cells, myeloid cell bioactivity is also altered by RT and can regulate response to therapy (5,6).
The presence of macrophages in multiple types of human cancer, including breast, ovarian, non-small cell lung cancer, mesothelioma and Hodgkin’s lymphoma, correlates with poor clinical outcome; and it was associated with the ability of macrophages to enhance angiogenesis and promote the invasive and metastatic potential of malignant cells (7–11). The cytokine colony stimulating factor (CSF)-1 plays a key role in recruitment and activation of macrophages following interaction with the CSF-1 receptor (CSF-1R), a high-affinity transmembrane receptor tyrosine kinase (12). Accordingly, in human breast cancer, colon cancer and leiomyosarcoma, a CSF-1-response gene signature has been identified that predicts the risk of recurrence and metastasis (13–15). The ability to preferentially target macrophages via inhibiting the CSF-1/CSF-1R signaling pathway has led to several preclinical studies demonstrating therapeutic efficacy in glioblastoma, melanoma, mammary, prostate, pancreas and cervical carcinoma, either in combination with CTX and RT, or as monotherapy (16–20).
We previously described a transgenic mouse model of aggressive mammary adenocarcinoma development in which late-stage carcinogenesis and pulmonary metastasis are regulated by CSF-1, tissue macrophages and interleukin (IL) 4-producing CD4+ T cells (21–23). Using this model, we recently reported that when macrophage infiltration into tumors was minimized via blockade of the CSF-1/CSF-1R pathway, platinum and taxol-based CTX responses were improved in a CD8+ T cell-dependent manner (16) regulated by interleukin (IL) 12-expressing CD103+ dendritic cells (24). Based on these studies, we hypothesized that response to RT, like CTX, could be improved by either reducing the presence of macrophages in tumors, or by blocking induction of their Th2-polarization. Herein we report that regrowth of mammary tumors following RT correlates with influx of Th2-polarized macrophages; inhibiting macrophage recruitment after RT by neutralizing CSF-1 or blocking CSF-1R kinase activity significantly slows tumor regrowth. Moreover, our data indicate that RT promotes Th2-polarization of infiltrating IL4-expressing CD4+ T cells, and that either depleting these or neutralizing IL4 slows tumor regrowth following RT, thus demonstrating that therapeutic targeting of Th2 cytokines and the myeloid cells they regulate can enhance response to cytotoxic therapy.
MATERIALS AND METHODS
Preclinical Mouse Models and Animal Husbandry
FVB/n strain background mice harboring the polyoma middle T (PyMT) transgene under the control of the mouse mammary tumor virus (MMTV) promoter has been described previously (23,25). To generate a syngeneic orthotopic implantable tumor model, we prepared single-cell suspensions of tumor cell pools isolated from mammary tumors of 100-day old MMTV-PyMT mice, and injected 1.0 million (total) tumor cells diluted in medium and basement membrane extract (Matrigel, BD Pharmingen) orthotopically into uncleared mammary fat pads (4th gland) of 10-week old virgin FVB/n female mice (16). Following implantation, tumors were allowed to grow to an average diameter of 1.0 cm before randomization and enrollment into studies. Mice were randomized into treatment groups, e.g., PLX3397, αCSF-1 mAb (5A1), αCD4 mAb (GK1.5), αCD8 (YTS169.4), αIL4 mAb (11B11), based on tumor size and subsequently irradiated with single fraction of 5 Gy focal gamma irradiation (Mark III Cesium Irradiator) generated from a cesium source with mice mounted in a custom shielding apparatus (Fig. 1A). PLX3397 (16) was formulated in mouse chow so that the average dose per animal per day was 40 mg/kg, and chow containing vehicle alone was used as a control. Antibodies were injected i.p. at 1.0 mg to start, followed by 500 μg every 5 days. Depletion of CD4+ and CD8+ cells were confirmed by flow cytometry of peripheral blood prior to initiating therapy. Pharmaceutical grade paclitaxel (Hospira) was administered intravenously every 5 days at 10 mg/kg as described (16,24). Neutralization of IL13 was achieved via administration of a rat anti-mouse IL13 mAb (54D1) injected i.p. at 1.0 mg to start, followed by 500 μg every 5 days. Tumor burden was evaluated by caliper measurement on anesthetized mice every 2 to 5 days. Prior to tissue collection, mice were cardiac-perfused with PBS to clear peripheral blood. As described previously (23), homozygous null IL4Rα mice were obtained from Jackson Laboratories. To generate PyMT mice on the IL4Rα−/− backgrounds, IL4Rα+/− mice were backcrossed into the FVB/n strain to N6 and then intercrossed with PyMT mice to generate breeding colonies. Mice were maintained either within the UCSF Laboratory for Animal Care barrier facility or the OHSU Department of Comparative Medicine barrier facility. Experiments involving animals were approved by the Institutional Animal Care and Use Committees at the respective institutions.
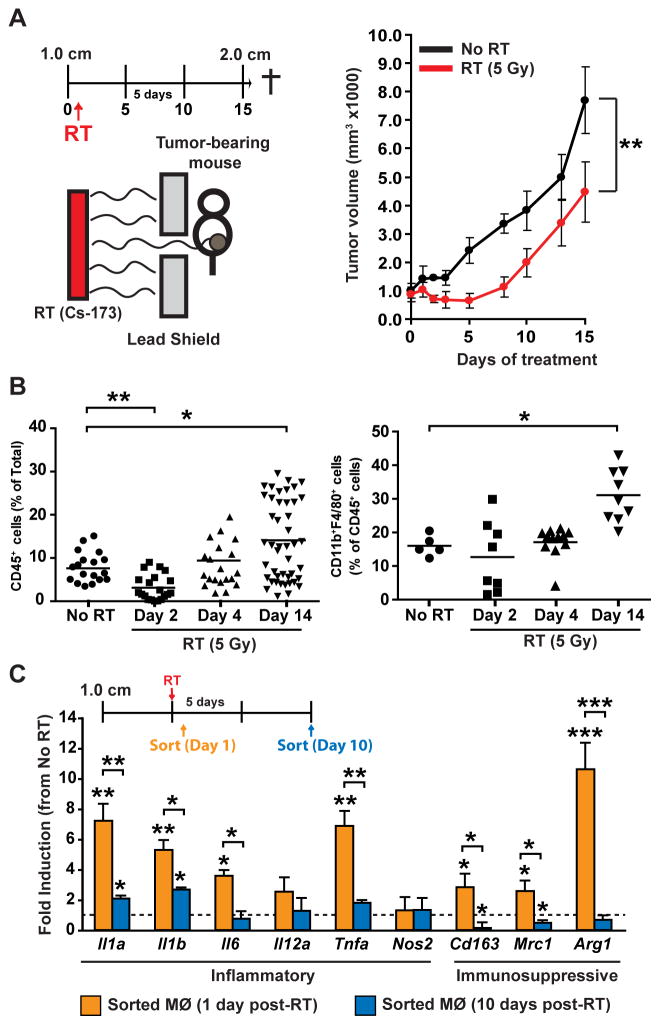
Figure 1. Macrophage recruitment and polarization following radiation therapy.
A) Orthotopic MMTV-PyMT-derived explant tumors were grown to a median diameter of 1.0 cm, at which time tumor-bearing mice were enrolled in the experiment. One day later they received localized gamma irradiation (5 Gy), and total tumor burden/animal was then assessed every 3 days until endpoint. Treatment schematic is depicted at top and data are displayed as mean tumor burden ± SEM (>8 mice/group). Statistical significance was determined by two-way ANOVA. One of two experiments is shown. B) Quantification of CD45+ (left) and CD11b+F4/80+ (right) cells in mammary tumors on day 2, 4, and 14 following RT (5 Gy) compared to unirradiated tumors harvested on Day 14. Data are depicted as mean number of CD45+ cells as a % of total cells ± SEM as analyzed by flow cytometry (>5 mice/group). Statistical significance was determined by an unpaired t-test. C) CD45+CD11b+F4/80+ macrophages (MØ) were FACS sorted from orthotopic PyMT-derived tumors at Days 1 and 10 following treatment with RT (5 Gy). mRNA expression from sorted cells was analyzed using quantitative real-time PCR for the indicated genes. Treatment schematic is depicted at top and data are expressed as mean fold-change ± SD compared to untreated tumors (4 mice/group). Statistical significance was determined by an unpaired t-test relative to untreated controls, or between Day 1 and Day 10 as indicated. For all panels, statistical significance is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
Flow cytometry analysis
Single-cell suspensions were prepared from mammary tumors disassociated by manual mincing and enzymatic digestion for 40 min at 37°C using collagenase A (3.0 mg/ml; Roche) and DNase I (Roche) dissolved in DMEM (Invitrogen) under stirring conditions. Digestion mixtures were quenched by adding DMEM containing 10% FBS and then filtered through 0.7 μm nylon strainers (Falcon). Cells were then incubated for 10 min at 4°C with rat anti-mouse CD16/CD32 mAb (BD Biosciences) at a 1:100 dilution in PBS containing 1.0% of BSA (Sigma) to prevent nonspecific antibody binding. Subsequently, cells were washed twice in PBS/BSA and incubated for 20 min with 100 μl of fluorophore-conjugated anti-mouse antibodies: B220 (RA3-6B2), CD3ε (145-2C11), CD4 (6K1.5), CD8α (53-6.7), CD11b (M1/70), CD11c (N418), CD14 (Sa2-8), CD19 (MB19-1), Ly6C (HK1.4), Ly6G (1A8), CD44 (IM7), CD45 (30-F11), CD80 (16-10A1), CD86 (GL1), CD115 (AFS98), F4/80 (BM8) and/or MHCII (M5/114.15.2) (all from eBioscience) followed by two washes with PBS/BSA. 7-AAD (BD Biosciences) was added (1:10) to discriminate between viable and dead cells, or alternatively live/dead aqua was used (Invitrogen). Data acquisition and analysis were performed on a LSRII (BD Biosciences) using the FlowJo version 8.8 software (Tree Star).
Immune cell isolation
Immune cells were isolated from tumors using a dual purification strategy including magnetic purification followed by flow sorting. Single-cell suspensions from tumors were generated as described above. Cells were incubated for 10 min at 4°C with rat anti-mouse CD16/CD32 mAb (BD Biosciences) at a 1:100 dilution in PBS/BSA then washed twice in PBS/BSA and incubated for 20 min with appropriate fluorescent primary antibodies which included CD45-APC (30-F11), in addition to CD4 (GK1.1), CD3 (145-2C11), Gr-1 (RB6-8G5), CD11b (93) and/or F4/80 (BM8) (all from eBiosciences) at 1:100 dilution depending on the population to be isolated. Total leukocytes were isolated using magnetic bead selection for CD45-APC+ cells according to manufactures specifications (Miltenyi Biotec). Magnetically selected cells were then flow sorted on a FACSAria using FACSDiva software (BD Biosciences). Gating strategies for these populations has been described previously (16,24)
Quantitative RT-PCR
Total tissue RNA was extracted from cells using an RNeasy Mini Kit (QIAGEN). cDNAs were synthesized using Superscript III first-strand synthesis (Invitrogen). Primers specific for β-actin, GAPDH, 18S, IFNγ, IL2, IL4, IL12p35, IFNα, ARG1 and IL34 (Superarray) were used and relative gene expression was determined using RT2 Real-Time SYBR Green/ROX PCR master mix (Superarray) on an ABI 7900HT quantitative PCR machine (ABI biosystems). Data were normalized to β-actin and GAPDH and/or 18S as reference genes. Alternatively, real-time PCR for gene expression was performed using individual TaqMan Assays following a preamplification step (Life Technologies). Data were normalized to (Tbp). The comparative threshold cycle method was used to calculate fold-change in gene expression for all experiments.
RESULTS
CSF-1/CSF-1R blockade delays primary tumor regrowth following RT
Using a syngeneic orthotopic murine explant model of mammary carcinoma growth, we first evaluated varying doses of radiation for effects on tumor growth and immune composition. Similar regression kinetics were observed following a single dose of 2, 5 or 8 Gray (Gy) radiation (Fig. 1A, Supplementary Fig. S1A); however only 5 Gy provided a sufficient dose to induce a period of tumor stasis, followed by tumor regrowth approximately 8 to 12 days later (Fig. 1A). Analysis of immune infiltrates in mammary tumors at distinct time points following RT revealed reduced presence of CD45+ cells immediately following RT (day 2), with infiltration restored shortly thereafter (day 4), and markedly elevated two weeks later (day 14) (Fig. 1B). Although CD11b+F4/80+ macrophage infiltration was not altered during tumor regression (day 2) or stasis (day 4), macrophages were significantly increased during the tumor regrowth phase (day 14), even as compared to untreated tumors growing at a similar rate (Fig. 1B). Macrophages were also significantly increased on day 14 by 8 Gy, but not by 2 Gy radiation (Supplementary Fig. S1B). Gene expression analysis of infiltrating macrophages revealed increased acute expression of biomarkers representing both classically and alternatively activated macrophages post RT, that returned to baseline as tumors initiated regrowth around day 10 (Fig. 1C) thus indicating that one consequence of acute RT is altered gene expression programs in resident leukocytes.
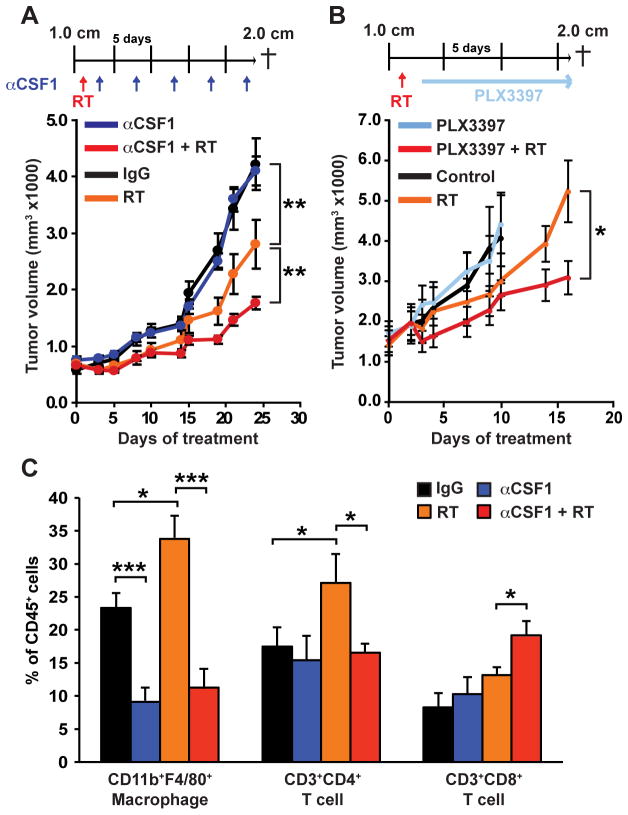
In a previous study, we reported that RT promotes CSF-1 expression by MMTV-PyMT-derived mammary epithelial cells in vitro (16); herein, we sought to evaluate whether delivery of immunologic or pharmacologic agents neutralizing the CSF-1/CSF-1R pathway could provide a therapeutic benefit to RT. To address this, mice bearing syngeneic orthotopic mammary explant tumors were treated with either a neutralizing monoclonal antibody (mAb) against CSF-1 (clone 5A1), or a competitive ATP inhibitor selective for CSF-1R and cKIT receptor tyrosine kinases (PLX3397) currently being evaluated clinically (16,20,26,27), 2 days following RT. Importantly, as low dose RT has been reported to promote T-cell responses via macrophage repolarization (28), we initiated treatment 2 days following RT, with full depletion of macrophages anticipated approximately three days later (17). Treatment of mice with either αCSF-1 mAb (Fig. 2A) or PLX3397 (Fig. 2B) resulted in a significantly delayed/slowed tumor regrowth following RT, with no effect observed with either agent as monotherapy. Similar to our previous findings with these agents when delivered in combination with CTX (16), the majority of tumor-infiltrating macrophages were efficiently eliminated by αCSF-1 mAb or PLX3397, and this was associated with an approximate 2-fold increase in CD8+ T-cell presence (Fig. 2C, Supplementary Fig. S2).
Figure 2. Macrophage depletion following radiation therapy slows tumor regrowth.
A–B) Orthotopic PyMT-derived explant tumors were grown to a median diameter of 1.0 cm and then the mice were enrolled in the study. On Day 1 mice were subjected to localized gamma irradiation (5 Gy), and 2 days later treated with either αCSF-1 neutralizing mAb (A) or PLX3397 (B). mAb or PLX3397 was administered for a further 14 or 21 days and total tumor burden/animal assessed every 2–3 days. Treatment regimens are depicted for all cohorts and data are displayed as mean tumor burden ± SEM (>5 mice/group). Statistical significance was determined by two-way ANOVA. One of two experiments is shown. C) Number of CD3+CD4+, CD3+CD8+, and CD11b+F4/80+ cells within tumors 17 days following RT in groups that were untreated, treated with αCSF-1 mAb alone, RT (5 Gy) alone or treated with a combination of RT and αCSF-1 mAb. Data are depicted as mean number of cells ± SEM as a % of CD45+ cells as analyzed by flow cytometry (>5 mice/group). For all figures statistical significance is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
Th2-polarized CD4+ T cells promote tumor regrowth after RT
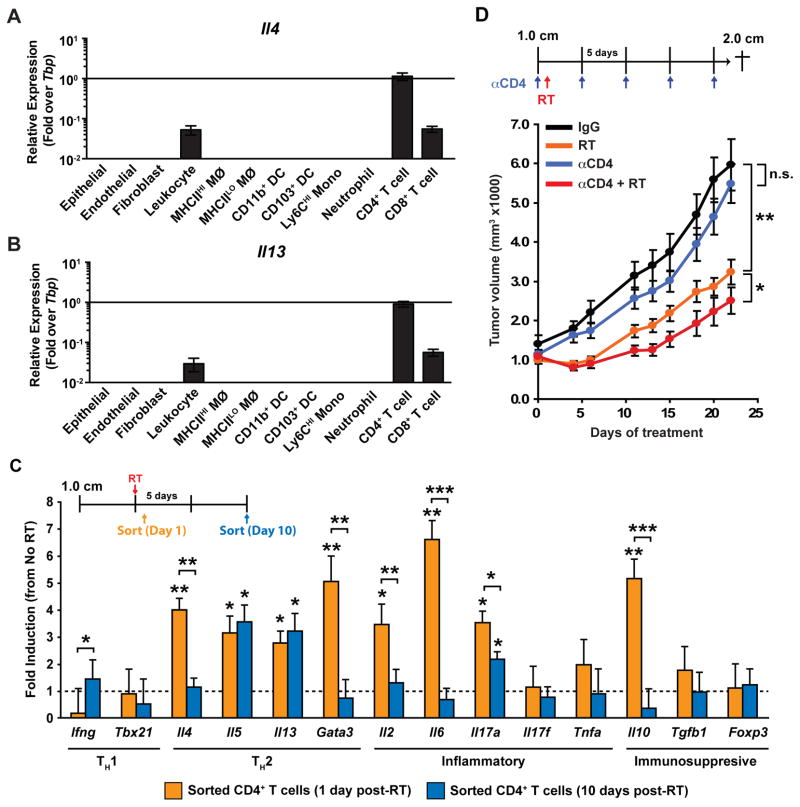
Macrophage depletion, in combination with RT, was also associated with a substantial reduction in the presence of CD4+ T cells (Fig. 2C, Supplementary Fig. S2). This result was in contrast to our previous findings in which CD4+ T-cell infiltration was unaffected by CSF-1/CSF-1R blockade as monotherapy, or in combination with CTX (16,24). That said, others have reported that a consequence of RT is altered expression of macrophage-expressed chemokines regulating T-cell infiltration in radiation-induced pneumonitis (29). In agreement with our previous results (16), CD4+ T cells in mammary tumors of MMTV-PyMT mice were main sources of IL4 (Fig. 3A) and IL13 (Fig. 3B), both are significant cytokines regulating macrophage polarization. We therefore examined gene expression profiles of CD4+ T cells from mammary tumors by FACS-sorting cells 1-day and 10-days post RT. We found that mRNA for Th2 cytokines including IL4, IL5, IL13 and the Th2 transcription factor GATA-3 were elevated at least 3-fold on day-1 post RT, with levels of IL5 and IL13 persisting to day-10 during tumor regrowth (Fig. 3C). Levels of IL2, IL6 and IL10 were also significantly induced although these were not sustained (Fig. 3C); thus, RT induces significant gene expression changes in CD4+ T cells, in addition to macrophages (Fig. 1C).
Figure 3. Th2-polarized CD4+ T cells promote tumor regrowth after radiation therapy.
A–B) mRNA levels of Il4 (B) and Il13 (C) in FACS-sorted populations of epithelial and stromal populations from untreated MMTV-PyMT mammary tumors, as determined by real-time PCR. Data are normalized to Tbp expression and displayed as mean ± SEM with n=8 per cell type. MØ, macrophage; mono, monocyte; DC, dendritic cell. C) CD45+CD3+CD4+ T cells were FACS sorted from orthotopic PyMT-derived explant tumors at Days 1 and 10 following treatment with RT (5 Gy). mRNA expression from sorted cells was then analyzed using quantitative real-time PCR for the indicated genes. Treatment schematic is depicted at the top and data are expressed as mean fold-change ± SD compared to untreated tumors (4 mice/group). Statistical significance was determined by an unpaired t-test relative to untreated controls, or between Day 1 and Day 10 as indicated. D) Orthotopic PyMT-derived explant tumors were grown to a median diameter of 1.0 cm and then the tumor-bearing mice were enrolled in the study. On Day 1 mice were then subjected to localized gamma irradiation (5 Gy) and total tumor burden/animal assessed every 3 days until endpoint. αCD4-depleting antibody was administered 2 days prior to RT and then every 5 days for the duration of the study. Treatment schematic is depicted at top and data are displayed as mean tumor burden ± SEM (>8 mice/group). Statistical significance was determined by two-way ANOVA. One of two experiments is shown. For all figures statistical significance is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
To evaluate the functional significance of these findings and to determine if CD4+ T cells might also play a critical role in promoting tumor growth post RT, we administered anαCD4-depleting mAb to deplete CD4+ T cells prior to RT and then evaluated tumor regrowth; whereas CD4-depletion had no significant influence on tumor growth in untreated animals, tumor regrowth following RT was significantly diminished when CD4+ T cells were absent (Fig. 3D).
IL4 neutralization enhances antitumor immunity in response to RT
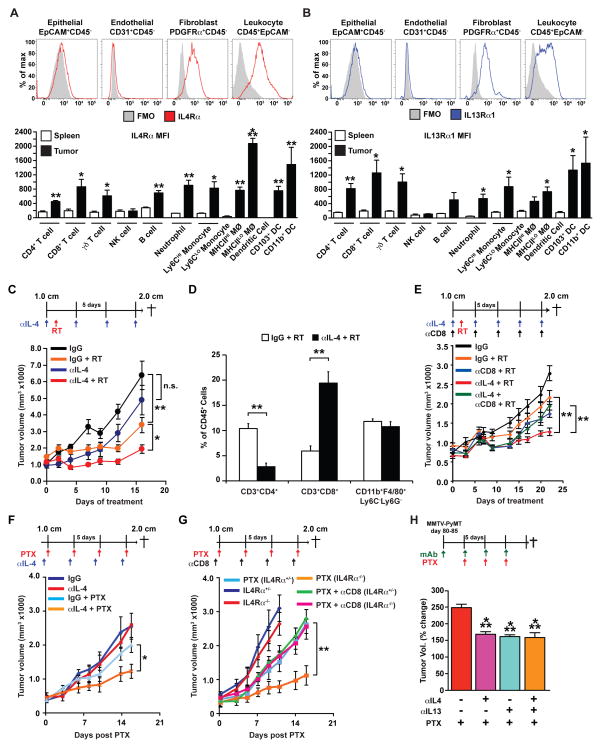
Based on these data, we next sought to evaluate the efficacy of neutralizing IL4 to determine if suppression of its Th2-polarizing effects on immune and/or non-immune cells influenced tumor growth and/or response to RT. IL4 and IL13 bind multimeric scaffold receptors composed of IL4Rα, γc (CD132), IL13Rα1 or IL13Rα2 subunits; type I receptors (IL4Rα/γc) are activated exclusively by IL4, whereas both IL4 and IL13 bind type II receptors (IL4Rα/IL13Rα1) (30). In mammary tumors of MMTV-PyMT mice, both receptor types are broadly expressed by immune cells infiltrating tumors, as well as by fibroblasts (Fig. 4A–B). Increased IL4Rα was particularly pronounced in the MHCIILO subset of macrophages (Fig. 4A), which are known to exhibit enhanced tumor-promoting properties (24,31).
Figure 4. IL4 blockade slows tumor regrowth following RT in a CD8+ T cell-dependent manner.
A–B) Surface expression of IL4Rα (A) and IL13Rα1 (B) as measured by mean fluorescence intensity (MFI) minus background in cells isolated from MMTV-PyMT mammary tumors as indicated, and as compared to equivalent populations in the spleens of non-tumor-bearing animals (lower graphs). Data are displayed as mean ± SEM with n=3 mice per group. Statistical significance determined by an unpaired t-test compared to the spleen. MØ, macrophage; mono, monocyte; DC, dendritic cell. C–D) Orthotopic PyMT-derived tumors were grown to a median diameter of 1.0 cm and then the tumor-bearing mice were enrolled in the study. On Day 1 mice were treated with localized gamma irradiation (5 Gy) and total tumor burden/animal assessed every 3 days until endpoint. αIL4 neutralizing antibody and/or αCD8-depleting antibody were administered 2 days prior to RT and then every 5 days for the duration of the experiment. Treatment schematic is depicted at top and data are displayed as mean tumor burden ± SEM (>5 mice/group). Statistical significance was determined by two-way ANOVA. E) Number of CD3+CD4+, CD3+CD8+, and CD11b+F4/80+ cells within tumors 2 days following treatment with RT. Data are depicted as mean number of cells ± SEM as a % of CD45+ cells as analyzed by flow cytometry (>5 mice/group). F) Orthotopic PyMT-derived tumors were grown to a median diameter of 1.0 cm and tumor-bearing mice were administered intravenous paclitaxel (PTX; 10 mg/kg) and total tumor burden/animal assessed until endpoint. αIL4 neutralizing antibody was administered 1 day prior to CTX and then every 5 days for the duration of the study. Treatment schematic is depicted at top and data are displayed as mean tumor burden ± SEM (>5 mice/group). Statistical significance was determined by two-way ANOVA. G) Orthotopic PyMT-derived tumors were implanted into syngeneic ILR4α-proficient (+/−) and –deficient (−/−) mice and grown to a median diameter of 1.0 cm. Mice were then treated with intravenous paclitaxel (PTX; 10 mg/kg) and total tumor burden/animal assessed until endpoint. αCD8-depleting antibody was administered 1 day prior to CTX and then every 5 days for the duration of the study. Treatment schematic is depicted at top and data are displayed as mean tumor burden ± SEM (>5 mice/group). Statistical significance was determined by two-way ANOVA. (H) MMTV-PyMT transgenic mice were treated with αIL4 and/or αIL13 mAbs between 80 to 85 days of age, and 5 days later were treated with 10 mg/kg PTX as indicated. Tumor volume is shown as a relative change between the first dose of PTX and 15 days later. Treatment schematic is depicted at top and data are displayed as the mean ± SEM (>6 mice/group). For all figures statistical significance is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
Neutralization of IL4 following RT significantly slowed tumor regrowth following RT as compared to either RT or αIL4 mAb alone (Fig. 4C), similar to CSF-1/CSF-1R blockade and CD4-depletion (Fig. 2–3). Analysis of the immune composition of tumors followingαIL4 mAb/RT similarly revealed a significantly decreased presence CD4+ T cells and significantly increased CD8+ T cells in the tumor parenchyma (Fig. 4D), that was significant as CD8+ T-cell depletion prior to RT abrogated improved responses (Fig. 4E), similar to the responses following CSF-1/CSF-1R blockade (16,24). To determine if this improved response extended to CTX, we evaluated mammary tumor growth in syngeneic IL4Rα-deficient mice and wildtype mice treated with αIL4 mAb, with and without paclitaxel (PTX), and found significantly reduced primary tumor growth as compared to mice treated with paclitaxel or αIL4 mAb alone (Fig. 4F, G). Improved responses to paclitaxel were similarly dependent on induced CD8+ T-cell infiltration (Fig. 4G). As IL4Rα acts as a receptor for both IL4 and IL13, we also evaluated the effects of IL13 neutralization and found that αIL4 and αIL13 mAb produced equivalent effects on de novo mammary adenocarcinoma growth in combination with PTX in transgenic MMTV-PyMT mice, with no additive effects observed when αIL4/αIL13 were used in combination (Fig. 4H).
DISCUSSION
Using a murine orthotopic explant model of primary mammary carcinoma, results reported herein reveal that a consequence of RT is induced recruitment of immunosuppressive F4/80+ macrophages that limit RT (and CTX) efficacy. Recruitment of these pro-tumorigenic immune cells is in part due to increased expression of CSF-1 and IL34 by mammary epithelial cells following cellular “damage” induced by RT (and CTX) observed in both murine mammary models and clinical breast tumors following therapy (16,32). Selective macrophage depletion resulting from CSF-1/CSF-1R-blockade improved responses to RT and was associated with decreased presence of type 2 cytokine-expressing CD4+ T cells. Whether macrophages are directly responsible for CD4+ T-cell recruitment is unclear; that said, in other studies, we reported that therapeutically blocking humoral-based pathways regulating protumoral macrophage programming, in combination with CTX, induced Th1-type programing of macrophages that recruit CD8+ T cells via increased expression of macrophage-derived chemokines (33), similar to results reported for radiation-induced pneumonitis implicating macrophage-derived CCL22 and CCL17 (ligands for CCR4) (29). Depletion of CD4+ T cells or neutralization of the type 2 cytokines e.g. IL4 or IL13 that they express, phenocopied CSF-1/CSF-1R-blockade without depletion of macrophages. Improved responses to RT resulting from these therapies were dependent on the increased presence of CD8+ T cells in irradiated tumors as their selective depletion prior to RT abrogated the improved outcomes.
Using subcutaneous xenograft models of head and neck cancer (34), and B16 melanoma (35), others reported that high dose RT leads to increased presence of CD11b+ myeloid cells that significantly impact outcome, as therapeutic use of CD11b blocking mAbs or administration of clodronate liposomes restricted tumor regrowth associated with reduced myeloid-dependent angiogenesis (34,36). Other studies in melanoma reported that RT as monotherapy promotes CD8+ T-cell infiltration (35), and that CSF-1R-blockade improves efficacy of adoptive T-cell transfer (18,36). These studies are in agreement with findings herein and elsewhere (16,24,33) that macrophages in tumor parenchyma limit CD8+ T-cell recruitment and effector function, and thereby restricts efficacy of cytotoxic therapies.
In mouse models of pancreatic neuroendocrine tumor growth, low dose (2 Gy) RT improved efficacy of adoptive T-cell transfer by mechanisms involving repolarization of macrophages from expressing high levels of Ym-1 and Arginase-1 to instead expressing elevated inducible nitric oxide synthase, changes that correlated with enhanced recruitment of cytotoxic CD8+ T cells (28). Using 60 Gy, others have reported that focal RT induces expression of genes associated with Th2-type macrophage activation (37,38). In the mammary carcinoma model used herein, low dose (2 Gy) RT alone was without consequence (data not shown), whereas 5 Gy focal RT led to enhanced expression of genes associated with both Th1-type (Il1a, Il1b, Tnfa) and Th2-type (Cd163, Mrc1, Arg1) macrophage activation. The polarization state of macrophages and other infiltrating cells is significant as blockade of IL4Rα signaling similarly improved RT (and CTX) response by CD8+ T cell-dependent mechanisms. In human breast cancer, CD4+ T cells are biased towards production of IL4 and IL13 (39), a phenotype that is recapitulated in MMTV-PyMT transgenic mice (23). While our results revealed that type 1 and 2 IL4/13 receptors were both broadly expressed by tumor-infiltrating stromal cells, it remains unclear whether IL4 directly or indirectly suppresses CD8+ T-cell recruitment/function. We favor the hypothesis that IL4 suppresses CD8+ T-cell activity indirectly through regulation of macrophage polarization, as we and others have reported (23,40), and consistent with a role for macrophages in mediating resistance to RT and CTX. However, it is possible that IL4 directly suppresses CD8+ T-cell cytotoxic effector function (41), and in other tumor types, malignant cells also express IL4Rα (42,43).
Based on compelling preclinical data revealing improved responses to CTX and delayed/slowed solid tumor growth following CSF-1R-blockade (16,17,44,45), clinical trials evaluating efficacy of CSF-1R antagonists in several types of solid tumors are ongoing (NCT01596751, NCT01349036, NCT01004861, NCT01346358). Salient for translation to the clinic regarding macrophage repolarization versus depletion is the fact that whereas macrophage depletion prior to RT was without consequence, IL4 neutralization prior to RT yielded an improved outcome. Based on data herein that IL4 neutralization also improved response to CTX, we assert that IL4 and/or IL13 neutralization represents a compelling immune-based strategy for improving responses in patients receiving either CTX or RT.
Supplementary Material
Acknowledgments
PLX3397 and the neutralizing IL13 mAb were generously provided by Plexxikon Inc. and Gillian Kingsbury at Abbott Bioresearch Center, respectively. The authors acknowledge technical and administrative support from Lidiya Korets, Kerry Fujikawa, Justin Tibbitts, Teresa Beechwood, Jo Hill and Jane Wiesen, and helpful discussions from David M. Underhill. The authors acknowledge funding from the UCLA Clinical and Translational Science Institute (CTSI) and the American Society of Radiation Oncology (ASTRO) to SS, a grant from the NCI/NIH to BR, and grants from the NCI/NIH, the Department of Defense Breast Cancer Research Program (W81XWH-11-1-0702), the Susan G Komen Foundation (KG110560 and KG111084), and the Breast Cancer Research Foundation to LMC.
References
- 1.Jemal A. Global burden of cancer: opportunities for prevention. Lancet. 2012;380:1797–9. doi: 10.1016/S0140-6736(12)61688-2. [DOI] [PubMed] [Google Scholar]
- 2.Fleming ST, Kimmick GG, Sabatino SA, Cress RD, Wu XC, Trentham-Dietz A, et al. Defining care provided for breast cancer based on medical record review or Medicare claims: information from the Centers for Disease Control and Prevention Patterns of Care Study. Ann Epidemiol. 2012;22:807–13. doi: 10.1016/j.annepidem.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia. 2010;15:411–21. doi: 10.1007/s10911-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiao SL, Ganessan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;15:2559–72. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–25. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 9.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, Sugarbaker DJ. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117:5234–44. doi: 10.1002/cncr.26143. [DOI] [PubMed] [Google Scholar]
- 12.Tang R, Beuvon F, Ojeda M, Mosseri V, Pouillart P, Scholl S. M-CSF (monocyte colony stimulating factor) and M-CSF receptor expression by breast tumour cells: M-CSF mediated recruitment of tumour infiltrating monocytes? J Cell Biochem. 1992;50:350–6. doi: 10.1002/jcb.240500403. [DOI] [PubMed] [Google Scholar]
- 13.Beck AH, Espinosa I, Edris B, Li R, Montgomery K, Zhu S, et al. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res. 2009;15:778–87. doi: 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–11. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma M, Beck AH, Webster JA, Espinosa I, Montgomery K, Varma S, et al. Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Res Treat. 2010;123:397–404. doi: 10.1007/s10549-009-0654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–61. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–41. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, et al. Macrophage IL-10 Blocks CD8(+) T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell. 2014;26:623–37. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West B, Denardo D, Tsai J, Hann B, Nguyen H, Wong B, et al. Efficacy of the selective CSF-1R kinase inhibitor PLX3397 in mouse models of tumor growth and bone metastasis (Abstract nr 3850) Washington, DC: AACR; 2010. p. 7864. [Google Scholar]
- 27.Anthony SP, Puzanov I, Lin PS, Nolop KB, West B, Von Hof DD. Pharmacodynamic activity demonstrated in phase I for PLX3397, a selective inhibitor of FMS and Kit. J Clin Oncol. 2011;29(suppl):abstr 3093. [Google Scholar]
- 28.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T, Fujishima S, Ikeda E, Yoshie O, Tsukamoto N, Aiso S, et al. CCL22 and CCL17 in rat radiation pneumonitis and in human idiopathic pulmonary fibrosis. Eur Respir J. 2004;24:49–56. doi: 10.1183/09031936.04.00110203. [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 32.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, et al. B cells Regulate Macrophage Phenotype and Response to Chemotherapy in Squamous Carcinomas. Cancer Cell. 2014;25:809–21. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010;107:8363–8. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 36.Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70:1534–43. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crittenden MR, Savage T, Cottam B, Baird J, Rodriguez PC, Newell P, et al. Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat Res. 2014;182:182–90. doi: 10.1667/RR13493.1. [DOI] [PubMed] [Google Scholar]
- 38.Crittenden MR, Cottam B, Savage T, Nguyen C, Newell P, Gough MJ. Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS One. 2012;7:e39295. doi: 10.1371/journal.pone.0039295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167:6497–502. doi: 10.4049/jimmunol.167.11.6497. [DOI] [PubMed] [Google Scholar]
- 42.Burt BM, Bader A, Winter D, Rodig SJ, Bueno R, Sugarbaker DJ. Expression of interleukin-4 receptor alpha in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin Cancer Res. 2012;18:1568–77. doi: 10.1158/1078-0432.CCR-11-1808. [DOI] [PubMed] [Google Scholar]
- 43.Venmar KT, Carter KJ, Hwang DG, Dozier EA, Fingleton B. IL4 receptor ILR4alpha regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res. 2014;74:4329–40. doi: 10.1158/0008-5472.CAN-14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.