Abstract
IMPORTANCE
Colorectal cancers are a leading cause of cancer mortality, and their primary prevention by diet is highly desirable. The relationship of vegetarian dietary patterns to colorectal cancer risk is not well established.
OBJECTIVE
To evaluate the association between vegetarian dietary patterns and incident colorectal cancers.
DESIGN, SETTING, AND PARTICIPANTS
The Adventist Health Study 2 (AHS-2) is a large, prospective, North American cohort trial including 96 354 Seventh-Day Adventist men and women recruited between January 1, 2002, and December 31, 2007. Follow-up varied by state and was indicated by the cancer registry linkage dates. Of these participants, an analytic sample of 77 659 remained after exclusions. Analysis was conducted using Cox proportional hazards regression, controlling for important demographic and lifestyle confounders. The analysis was conducted between June 1, 2014, and October 20, 2014.
EXPOSURES
Diet was assessed at baseline by a validated quantitative food frequency questionnaire and categorized into 4 vegetarian dietary patterns (vegan, lacto-ovo vegetarian, pescovegetarian, and semivegetarian) and a nonvegetarian dietary pattern.
MAIN OUTCOMES AND MEASURES
The relationship between dietary patterns and incident cancers of the colon and rectum; colorectal cancer cases were identified primarily by state cancer registry linkages.
RESULTS
During a mean follow-up of 7.3 years, 380 cases of colon cancer and 110 cases of rectal cancer were documented. The adjusted hazard ratios (HRs) in all vegetarians combined vs nonvegetarians were 0.78 (95% CI, 0.64–0.95) for all colorectal cancers, 0.81 (95%CI, 0.65–1.00) for colon cancer, and 0.71 (95% CI, 0.47–1.06) for rectal cancer. The adjusted HR for colorectal cancer in vegans was 0.84 (95% CI, 0.59–1.19); in lacto-ovo vegetarians, 0.82 (95% CI, 0.65–1.02); in pescovegetarians, 0.57 (95% CI, 0.40–0.82); and in semivegetarians, 0.92 (95% CI, 0.62–1.37) compared with nonvegetarians. Effect estimates were similar for men and women and for black and nonblack individuals.
CONCLUSIONS AND RELEVANCE
Vegetarian diets are associated with an overall lower incidence of colorectal cancers. Pescovegetarians in particular have a much lower risk compared with nonvegetarians. If such associations are causal, they may be important for primary prevention of colorectal cancers.
Colorectal cancer remains the second leading cause of cancer mortality in the United States.1 Although much attention has focused on improving screening for and treatment of colorectal cancer, enhancing primary prevention through risk factor reduction remains an important objective.
Dietary factors have been implicated as important sources of modifiable risk for colorectal cancer.2 Among dietary factors thought to influence risk, the evidence that red meat, especially processed meat, consumption is linked to increased risk3–6 and that foods containing dietary fiber are linked to decreased risk has been judged to be convincing.2,7 The evidence for a link to decreased risk has been judged as probable for garlic, milk, and calcium.2 Evidence for other dietary components is considered limited.2
Vegetarian dietary patterns might be expected to be associated with a lower risk of colorectal cancer given their lack of or reduced meat (including red and processed meat) content. Vegetarian diets may also be higher in fiber-containing foods.8 Such diets have also consistently been associated with lower body mass index (BMI),9–12 and evidence convincingly links increased adiposity to increased colorectal cancer risk.2,7,13 However, British vegetarian diets have not been associated with a decreased incidence.14
The Adventist Health Study 2 (AHS-2) is a large, prospective, North American cohort with a substantial proportion of vegetarians. Vegetarian dietary patterns in AHS-2 have been associated with several beneficial health outcomes, including lower mortality15; lower prevalence of obesity,10 hypertension,16,17 metabolic syndrome,18 and type 2 diabetes mellitus10; and lower incidence of type 2 diabetes mellitus.19 Preliminary investigations have demonstrated vegetarian dietary patterns to be associated with reduced incidence of all cancers combined and of cancers of the gastrointestinal tract20 but not with reduced mortality from all cancers.15 Results from a previous cohort (AHS-1)21 found meat intake to be associated with an increased risk of colon cancer and legume consumption with a decreased risk.
We hypothesized that vegetarian dietary patterns inAHS-2 would be associated with reductions in the risk for cancers of the colon and rectum. In this analysis, we examined that hypothesis.
Methods
Study Population
Study participants were recruited between January 1, 2002, and December 31, 2007, across all US states and Canadian provinces. Recruitment took place in Seventh-Day Adventist churches. A total of 96 354 persons participated in AHS-2. Butler et al22 provides a detailed description of the formation and characteristics of the cohort. The AHS-2 was approved by the institutional review board of Loma Linda University; written informed consent was obtained. Participants received financial compensation upon completion of the study questionnaire.
Of the 96 354 participants, linkage with US cancer registries was possible for 90 422 individuals in 48 states. Among these people, the following exclusion criteria were applied: age younger than 25 years or missing data for age or sex (n = 32), improbable response patterns in questionnaire data (eg, identical high-frequency responses to all questions on a page) (n = 366), more than 69 missing values in dietary data (n = 1705), estimated energy intake less than 500 kcal/d or greater than 4500 kcal/d (n = 3174), a self-reported history of cancer (except for nonmelanoma skin cancer) (n = 7403), consent form not returned (n = 17), no date of cancer diagnosis (n = 4), and medical record not available (n = 62). After all exclusions, there remained an analytic sample of 77 659 participants.
Outcome Data
Information on incident cancers was obtained primarily via computer-assisted record linkage with state cancer registries. At the time of the present analysis, linkage had been achieved for 48 states and Washington, DC. The linkage was through December 31, 2011, for 33 states; December 31, 2010, for 10 states; December 31, 2009, for 3 states; and December 31, 2008, for 2 states. The procedure for record linkage varied according to state regulations. Whenever possible, a programmer from our team (L.S.) was sent to conduct the record linkage at each registry. Potential matches were identified based on a 3-stage process, including (1) a probabilistic screen using Link Plus software (Centers for Disease Control and Prevention; http://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm), (2) a deterministic algorithm based on defined criteria if the screen was inconclusive, and (3) where necessary, a manual review. When state regulations did not allow for our programmer to conduct the linkage, we supplied the state with identifying information necessary to match participants to cancer cases.
International Statistical Classification of Diseases, Tenth Edition and International Classification of Diseases for Oncology, Third Edition coding was used to identify cases of colorectal cancer. The definitions applied were colon cancer, primary site (C18.0–C18.9 but not C18.1) and rectal cancer, primary site (C19.9 or C20.9). Carcinomas in situ were not considered to be cases, nor were tumors with histology codes 9050–9055, 9140, and 9590–9992.
In addition to the record linkages with cancer registries, each participant was sent a follow-up questionnaire biennially, which asked whether the participant had received a cancer diagnosis. These responses were compared with information from the registry linkages. When participants reported a new cancer that was not found in the registry linkage, the participant was telephoned and asked clarifying questions. When indicated, medical records were requested and reviewed by the principal investigator (G.E.F.) to ascertain whether the self-reported cancer could be verified. This secondary process yielded 5 of the 490 colorectal cancer cases.
Dietary Data
Diet was assessed at baseline by means of a detailed, quantitative food frequency questionnaire. Frequency and quantity of consumption were queried for more than200fooditems. Jaceldo-Siegel et al23,24 provide detailed descriptions of the methods of dietary measurement using the questionnaire and its validation by repeated 24-hour recalls. Validity correlations for red meat, poultry, fish, dairy, and eggswere0.76,0.76, 0.53,0.86, and0.64, respectively, in white participantsand0.72, 0.77, 0.57, 0.82, and 0.52, respectively, in black individuals.24
Five vegetarian and nonvegetarian dietary patterns were defined a priori according to the absence of intake of particular animal foods. As described by Orlich et al15(p1231): “Dietary patterns were determined according to the reported intake of foods of animal origin. Thus, vegans consumed eggs/dairy, fish, and all other meats less than 1 time/month; lacto-ovo vegetarians consumed eggs/dairy 1 or more time/month but fish and all other meats less than 1 time/month; pescovegetarians consumed fish 1 or more times/month but all other meats less than 1 time/month; semivegetarians consumed nonfish meats 1 or more times/month and all meats combined (fish included) 1 or more times/month but 1 or less time/week; lastly, nonvegetarians consumed nonfish meats 1 or more times/month and all meats combined (fish included) more than 1 time/week.” In many analyses, the 4 vegetarian groups (vegan, lacto-ovo vegetarian, pescovegetarian, and semivegetarian) were combined and compared with the nonvegetarian dietary pattern because the numbers of cases for specific vegetarian dietary patterns (other than lacto-ovo vegetarian) were relatively small.
Covariate Data
Additional information was ascertained at baseline using a questionnaire. This questionnaire included a wide variety of questions related to demographics, family history, biometrics, prior or current diseases and medications, use of tobacco and alcohol, exercise and other lifestyle factors, and reproductive and gynecologic information.
Statistical Analysis
The analysis was conducted between June 1, 2014, and October 20, 2014. Baseline descriptive statistics were calculated according to the 5 dietary pattern categories, adjusted (when appropriate) for age by direct standardization (using the entire analytic sample as the standard population). Standardized incidence ratios (SIRs) were computed using age-, sex-, and race-specific colorectal cancer incidence rates from the Surveillance, Epidemiology, and End Results 18 registries for 2007–2011.25
We used Cox proportional hazards regression models to assess the relationship between vegetarian dietary patterns and the risk of colorectal cancers, controlling for likely confounders; separate analyses were conducted for all colorectal cancers, colon cancers alone, and rectal cancers alone. Attained age was the Cox proportional hazards regression time variable, with left truncation at age of study entry. Survival plots by attained age were produced from the survival estimates of a Cox proportional hazards regression model stratified by dietary pattern, with covariates fixed at mean values. This approach was used rather than a Kaplan-Meier plot to accommodate left truncation by age at study entry.
Covariates were selected for inclusion in the analytic models in an a priori fashion as likely confounders. For each analysis, 3 models were used to show the effect of including additional covariates. The following variables (and categories) were included in the analytic models: age (attained age as time variable), sex (male or female), educational level (up to high school graduate, trade school/some college/associate degree, or bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, or >60 min/wk), smoking (never, quit ≥1 year ago, or current or quit <1 year ago), alcohol (none, <28 servings/mo, or ≥28 servings/mo), family history of colorectal cancer (yes or no), history of peptic ulcer (yes or no), history of inflammatory bowel disease (yes or no), treatment for diabetes mellitus within the past year (yes or no), aspirin use at least weekly at least 2 of the past 5 years (yes or no), statin therapy for at least 2 of the past 5 years (yes or no), supplemental calcium consumption (yes or no), supplemental vitamin D (micrograms per day), dietary energy (kilocalories per day), hormone therapy among menopausal women (yes or no), fiber consumption (<20 g/d, 20 to <30 g/d, 30 to <40 g/d, or ≥40 g/d), and BMI (<18.5, 18.5–24.9, 25.0–29.9, or ≥30.0; calculated as weight in kilograms divided by height in meters squared). Participants self-identified their race/ethnicity in 1 or more of 21 categories. Those self-identifying at least in part as black/African American, West Indian/Caribbean, African, or other black were categorized as black for this analysis and all others as nonblack. Covariates were tested for possible interaction with the diet variable and for suspected interactions between selected covariates.
A sensitivity analysis was performed to assess robustness to potential inadequate model specification since covariate category specification was limited by the number of events. A propensity score analysis was used in which covariates (often with many specified categories) were included to compute a propensity for the vegetarian dietary pattern; this propensity score was then used as a covariate in Cox proportional hazards regression models in lieu of other covariates.
The proportional hazards assumption was evaluated using Schoenfeld residuals, log(−log) plots, and attained-age interaction terms; there was a significant interaction of sex with the attained-age time variable, so the interaction term was included in the models. Residual methods were used to evaluate possible outliers and influential data points; no data points required removal. Multiple imputation of missing values was done for the small amount of missing data in the dietary variables used to calculate vegetarian status and for most covariates; a guided multiple imputation approach was used when possible26 since we have evidence that many of the missing dietary data are true zeroes.27 Analyses were performed using SAS, version 9.4 (SAS Institute Inc). Guided multiple imputation was performed using R, version 2.13.128 and the Hmisc, version 3.14-0 package.29
Results
During a mean follow-up period of 7.3 years (total, 569 714 person-years of follow-up) among 77 659 study participants, there were 490 cases of colorectal cancer (380 colon, 110 rectal). (See the Outcome Data subsection of the Methods section for follow-up dates, which varied by state.) The crude incidence rate of colorectal cancer for all participants was 86.0 cases per 100 000 person-years (95%CI, 78.7–94.0). The age-, sex-, and race-standardized SIR was 0.66 (95%CI, 0.60–0.72) for all participants (vegetarians: SIR, 0.61; 95% CI 0.53–0.68; nonvegetarians: SIR, 0.73; 95% CI, 0.64–0.83).
Table 1 compares the baseline characteristics of the 4 different groups of vegetarians and nonvegetarians. Vegetarians tended to be older than nonvegetarians. Blacks were less well represented among vegetarians (particularly lacto-ovo vegetarians) with the notable exception of pescovegetarians. Vegetarians were more likely to have higher educational levels, to exercise, and to use calcium supplements (except vegans); they were less likely to have ever smoked, to drink alcohol, to have had a colonoscopy or sigmoidoscopy (especially vegans), to use aspirin or statins, to have diabetes treated within the past year, or to have a history of peptic ulcers. Vegetarians had lower BMI and lower intakes of total fat, saturated fat, total meat, red meat, and processed meat but a higher intake of fiber. Vegans and semivegetarians had a lower dietary calcium intake. Energy intake was notably lower among semivegetarians but similar among the other dietary groups.
Table 1.
Baseline Characteristics Among 77659 Adventist Health Study 2 Participants According to Dietary Patterna
| Characteristic | Vegetarian Type | Missingb | ||||
|---|---|---|---|---|---|---|
| Vegan | Lacto-Ovo Vegetarian |
Pescovegetarian | Semivegetarian | Nonvegetarian | ||
| Categorical Variable, No. (%)c,d | ||||||
| Total | 5861 (7.6) | 22 424 (28.9) | 7811 (10.0) | 4271 (5.5) | 37 292 (48.0) | 0 |
| Female sex | 3714 (63.4) | 14 295 (63.8) | 5266 (67.6) | 2902 (67.8) | 24 203 (64.8) | 0 |
| Black race | 1208 (21.3) | 2964 (13.8) | 2910 (38.8) | 739 (18.1) | 12 910 (33.9) | 823 |
| Educational level ≤ high school | 1059 (17.7) | 3289 (14.1) | 1566 (19.8) | 952 (21.5) | 9075 (25.8) | 1021 |
| Smoking, ever | 901 (15.5) | 2545 (11.5) | 1181 (15.4) | 784 (18.6) | 9007 (25.0) | 1544 |
| Alcohol use, current | 58 (1.0) | 680 (3.3) | 486 (6.6) | 320 (8.1) | 6045 (16.1) | 1824 |
| Exercise, >60 min/wke | 2949 (52.4) | 10 182 (47.7) | 3611 (19.2) | 1735 (43.5) | 14 417 (40.2) | 3745 |
| Family history, yesf | 525 (8.6) | 2269 (9.7) | 639 (7.9) | 394 (8.8) | 3167 (8.8) | 0 |
| Endoscopy, everg | 1788 (31.3) | 8712 (38.9) | 2976 (38.9) | 1702 (39.9) | 13 968 (41.0) | 4141 |
| Aspirin use, ≥ weeklyh | 351 (5.8) | 2755 (11.8) | 1002 (12.7) | 629 (16.5) | 6071 (17.9) | 2046 |
| Statin use, yesh | 190 (3.9) | 1641 (7.1) | 706 (9.1) | 497 (11.4) | 4844 (14.2) | 2192 |
| Supplemental calcium use, yes | 2311 (38.9) | 10 466 (46.0) | 3591 (45.4) | 1974 (45.5) | 15 149 (41.3) | 0 |
| Diabetes mellitus, currenti | 164 (2.7) | 818 (3.5) | 378 (5.2) | 280 (6.2) | 3092 (8.6) | 210 |
| IBD, yes | 67 (1.2) | 272 (1.2) | 90 (1.1) | 47 (1.3) | 433 (1.2) | 210 |
| Peptic ulcer, yes | 590 (9.9) | 2540 (11.0) | 976 (12.2) | 602 (13.7) | 5186 (14.2) | 210 |
| Continuous Variable, Mean (SD)j | ||||||
| Age, y | 58.3 (14.0) | 58.6 (14.6) | 58.4 (14.6) | 58.6 (14.6) | 55.6 (13.7) | 0 |
| BMI | 24.0 (4.7) | 25.9 (5.1) | 26.2 (5.0) | 27.2 (5.6) | 28.6 (6.1) | 2122 |
| Energy, kcal/d | 1933 (732) | 1948 (722) | 1996 (793) | 1768 (722) | 1968 (798) | 0 |
| Total fat, g/d | 63.1 (31.1) | 72.3 (32.7) | 72.2 (35.6) | 66.3 (33.0) | 76.5 (37.3) | 0 |
| Saturated fat, g/d | 12.2 (6.2) | 17.4 (8.8) | 16.8 (9.2) | 17.1 (9.4) | 21.0 (11.3) | 0 |
| Total meat, g/dk | 0.0 (0.3) | 0.0 (0.7) | 16.6 (24.2) | 6.8 (3.9) | 54.2 (44.5) | 0 |
| Red meat, g/d | 0.0 (0.1) | 0.0 (0.2) | 0.0 (0.7) | 1.2 (2.5) | 16.2 (24.3) | 0 |
| Processed meat, g/d | 0.0 (0.0) | 0.0 (0.1) | 0.0 (0.2) | 0.2 (0.5) | 2.6 (5.0) | 0 |
| Fiber, g/d | 44.3 (18.5) | 35.6 (15.9) | 37.7 (18.3) | 30.4 (15.3) | 29.3 (15.2) | 0 |
| Dietary calcium, mg/d | 801 (377) | 883 (424) | 913 (460) | 821 (429) | 882 (472) | 0 |
| Supplemental vitamin D, µg/d | 3.7 (25.8) | 3.6 (18.4) | 4.1 (19.2) | 4.1 (25.4) | 3.9 (22.1) | 0 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IBD, inflammatory bowel disease.
The dietary patterns are described in the Dietary Data subsection of the Methods section.
Number with missing values. Dietary patterns and dietary variables, which were estimated from multiple questionnaire items, had missing values imputed in their calculation. Most other missing values indicated here were subsequently multiply imputed in the main analyses.
Actual counts (ie, unadjusted).
Percentages were adjusted for age by direct standardization (except for the total sample). Percentages are of nonmissing responses.
Exercise was defined as “vigorous activities, such as brisk walking, jogging, bicycling, etc, long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath.”
Family history of colorectal cancer.
Colonoscopy or flexible sigmoidoscopy.
For at least 2 of the past 5 years.
Treated within the past year.
Means and SDs were adjusted for age by direct standardization (except age).
All meat consumed (including poultry and fish).
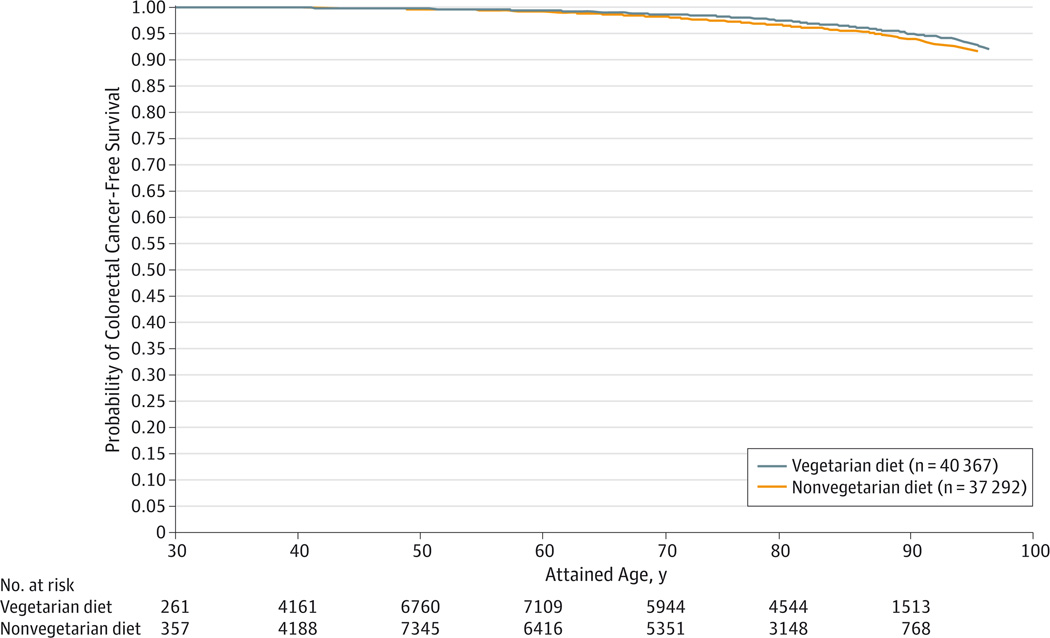
Vegetarian diets were associated with an overall reduced risk of colorectal cancer. The Figure displays curves indicating the probability of surviving to a given age without a diagnosis of colorectal cancer (with race and sex held constant) for all vegetarians compared with nonvegetarians. These findings show improved colorectal cancer–free survival among vegetarians across a spectrum of attained ages. Table 2 presents the results of Cox proportional hazards regression models for all vegetarians compared with nonvegetarians, for all colorectal cancers combined, and for colon and rectal cancers separately. In each case, 3 adjustment models are presented: model 1, with adjustment for age, sex, and race; model 2, with additional adjustment for a variety of plausible confounders; and model 3, with additional adjustment for BMI and fiber intake. Because BMI may represent a causal intermediate and fiber is a component of the dietary patterns, we consider model 2 as the likely best model for the total effect of dietary pattern on colorectal cancers; all of the results discussed below were obtained using model 2. The vegetarian dietary pattern was associated with a reduced risk of all colorectal cancers (hazard ratio [HR], 0.78; 95% CI, 0.64–0.95) and for colon cancer (HR, 0.81; 95% CI, 0.65–1.00). A similar point estimate of association for vegetarian diets and rectal cancer risk was observed but was not statistically significant (HR, 0.71; 95% CI, 0.47–1.06). Highly significant covariates (for the colorectal cancer end point) were any prior sigmoidoscopy or colonoscopy (HR, 0.69;95% CI,0.56–0.84), family history of colorectal cancer (HR, 1.41; 95% CI, 1.10–1.82), and moderate to vigorous exercise of 1 to 60 min/wk (HR, 0.71; 95% CI, 0.56–0.91) or more than 60 min/wk(HR,0.71; 95% CI,0.57–0.90). Effect estimates and 95% CIs for propensity score sensitivity analyses did not differ meaningfully from the results of the standard regression modeling strategy. This finding was true for all outcome seven when the number of events was limited.
Figure.
Comparison of the Probability of Surviving to a Given Age Without Having Received a Diagnosis of Colorectal Cancer
Colorectal cancer–free survival for all vegetarians compared with nonvegetarians generated from the survival estimates of a Cox proportional hazards regression model stratified by dietary pattern; race and sex remained constant.
Table 2.
Relative Hazard of Incident Cancers of the Colon and Rectum: Vegetarians vs Nonvegetarians
| Dietary Patterna | No. of Participants | HR (95% CI) | P Valueb | |
|---|---|---|---|---|
| Total | Cancer Cases | |||
| Colorectal cancer | ||||
| Model 1c | ||||
| Vegetarian | 40 367 | 252 | 0.80 (0.67–0.96) | .02 |
| Nonvegetarian | 37 292 | 238 | 1 [Reference] | |
| Model 2d | ||||
| Vegetarian | 40 367 | 252 | 0.78 (0.64–0.95) | .01 |
| Nonvegetarian | 37 292 | 238 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 40 367 | 252 | 0.79 (0.64–0.97) | .03 |
| Nonvegetarian | 37 292 | 238 | 1 [Reference] | |
| Colon cancer | ||||
| Model 1c | ||||
| Vegetarian | 40 367 | 197 | 0.80 (0.65–0.97) | .04 |
| Nonvegetarian | 37 292 | 183 | 1 [Reference] | |
| Model 2d | ||||
| Vegetarian | 40 367 | 197 | 0.81 (0.65–1.00) | .053 |
| Nonvegetarian | 37 292 | 183 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 40 367 | 197 | 0.83 (0.66–1.05) | .12 |
| Nonvegetarian | 37 292 | 183 | 1 [Reference] | |
| Rectal cancer | ||||
| Model 1c | ||||
| Vegetarian | 40 367 | 55 | 0.80 (0.54–1.18) | .26 |
| Nonvegetarian | 37 292 | 55 | 1 [Reference] | |
| Model 2d | ||||
| Vegetarian | 40 367 | 55 | 0.71 (0.47–1.06) | .09 |
| Nonvegetarian | 37 292 | 55 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 40 367 | 55 | 0.66 (0.43–1.02) | .06 |
| Nonvegetarian | 37 292 | 55 | 1 [Reference] | |
Abbreviation: HR, hazard ratio.
The dietary patterns are described in the Dietary Data subsection of the Methods section.
P value for Wald χ2 test of β coefficient in the Cox proportional hazards regression model.
Model 1 was adjusted by age (ie, attained age as time variable), race (black, nonblack), and sex (male, female).
Model 2 was adjusted as in model 1 and by educational level (up to high school graduate, trade school/some college/associate degree, or bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, or >60 min/wk), smoking (never, quit ≥1 year ago, or current or quit <1 year ago), alcohol use (none, <28 servings/mo, or ≥28 servings/mo), family history of colorectal cancer (yes, no), history of peptic ulcer (yes, no), history of inflammatory bowel disease (yes, no), treatment for diabetes mellitus within the past year (yes, no), used aspirin at least weekly at least 2 of the past 5 years (yes, no), used statins at least 2 of the past 5 years (yes, no), prior colonoscopy or flexible sigmoidoscopy (yes, no), supplemental calcium use (yes, no), supplemental vitamin D (micrograms per day), dietary energy (kilocalories per day), and hormone therapy among menopausal women (yes, no).
Model 3 was adjusted as in model 2 and by body mass index (calculated as weight in kilograms divided by height in meters squared) (<18.5, 18.5–24.9, 25.0–29.9, or ≥30.0) and fiber intake (<20 g/d, 20 to <30 g/d, 30 to <40 g/d, or ≥40 g/d).
Table 3 and Table 4 provide a covariate adjustment modeling strategy similar to that demonstrated in Table 2. The results reported below are based on model 2 for each Table. Table 3 presents the results of analyses comparing the adjusted hazard of all colorectal cancers for the 4 vegetarian dietary patterns separately compared with the nonvegetarian diet. Pescovegetarians had a significantly reduced adjusted hazard (HR, 0.57; 95% CI, 0.40–0.82). Lacto-ovo vegetarians had a reduced effect estimate that approached significance (HR, 0.82; 95% CI, 0.65–1.02). A post hoc comparison of pescovegetarians with other vegetarian groups produced the following results: vegans: HR, 0.68 (95% CI, 0.43–1.08; P = .10); lacto-ovo vegetarians: HR, 0.70 (95% CI, 0.48–1.01; P = .06); and semivegetarians: HR, 0.62 (95% CI, 0.37–1.01; P = .06). Table 4 presents sex-specific results for the dichotomous vegetarian variable and all colorectal cancers. Effect estimates for men and women were similar but did not reach statistical significance in men. Table 4 demonstrates results stratified by race; point estimates for blacks and nonblacks were similar but were statistically significant only in nonblacks.
Table 3.
Relative Hazard of Incident Colorectal Cancer: Several Vegetarian Patterns vs Nonvegetarians
| Dietary Patterna | No. of Participants | HR (95% CI) | P Valueb | |
|---|---|---|---|---|
| Total | Cancer Cases | |||
| Model 1c | ||||
| Vegetarian | ||||
| Vegan | 5861 | 40 | 0.89 (0.64–1.25) | .52 |
| Lacto-ovo | 22 424 | 147 | 0.83 (0.67–1.03) | .10 |
| Pesco | 7811 | 35 | 0.58 (0.40–0.83) | .003 |
| Semi | 4271 | 30 | 0.94 (0.64–1.39) | .76 |
| Nonvegetarian | 37 292 | 238 | 1 [Reference] | |
| Model 2d | ||||
| Vegetarian | ||||
| Vegan | 5861 | 40 | 0.84 (0.59–1.19) | .32 |
| Lacto-ovo | 22 424 | 147 | 0.82 (0.65–1.02) | .08 |
| Pesco | 7811 | 35 | 0.57 (0.40–0.82) | .002 |
| Semi | 4271 | 30 | 0.92 (0.62–1.37) | .69 |
| Nonvegetarian | 37 292 | 238 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | ||||
| Vegan | 5861 | 40 | 0.86 (0.59–1.24) | .42 |
| Lacto-ovo | 22 424 | 147 | 0.83 (0.66–1.05) | .11 |
| Pesco | 7811 | 35 | 0.58 (0.40–0.84) | .004 |
| Semi | 4271 | 30 | 0.93 (0.62–1.38) | .71 |
| Nonvegetarian | 37 292 | 238 | 1 [Reference] | |
Abbreviation: HR, hazard ratio.
The dietary patterns are described in the Dietary Data subsection of the Methods section.
P value for Wald χ2 test of β coefficient in the Cox proportional hazards regression model.
Model 1 was adjusted by age (ie, attained age as time variable), race (black, nonblack), and sex (male, female).
Model 2 was adjusted as in model 1 and by education (up to high school graduate, trade school/some college/associate degree, or bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, or >60 min/wk), smoking (never, quit ≥1 year ago, or current or quit <1 year ago), alcohol use (none, <28 servings/mo, or ≥28 servings/mo), family history of colorectal cancer (yes, no), history of peptic ulcer (yes, no), history of inflammatory bowel disease (yes, no), treatment for diabetes mellitus within the past year (yes, no), used aspirin at least weekly at least 2 of the past 5 years (yes, no), used statins at least 2 of the past 5 years (yes, no), prior colonoscopy or flexible sigmoidoscopy (yes, no), supplemental calcium use (yes, no), supplemental vitamin D (micrograms per day), dietary energy (kilocalories per day), and hormone therapy among menopausal women (yes, no).
Model 3 was adjusted as in model 2 and by body mass index (calculated as weight in kilograms divided by height in meters squared) (<18.5, 18.5–24.9, 25–29.9, or ≥30.0) and fiber intake (<20 g/d, 20 to <30 g/d, 30 to <40 g/d, or ≥40 g/d).
Table 4.
Relative Hazard of Incident Colorectal Cancer, Stratified by Sex and by Race: Vegetarians vs Nonvegetarians
| Dietary Patterna | No. of Participants | HR (95% CI) | P Valueb | |
|---|---|---|---|---|
| Total | Cancer Cases | |||
| Men | ||||
| Model 1c | ||||
| Vegetarian | 14 190 | 103 | 0.90 (0.67–1.20) | .46 |
| Nonvegetarian | 13 089 | 82 | 1 [Reference] | |
| Model 2d | ||||
| Vegetarian | 14 190 | 103 | 0.81 (0.59–1.02) | .18 |
| Nonvegetarian | 13 089 | 82 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 14 190 | 103 | 0.81 (0.58–1.12) | .20 |
| Nonvegetarian | 13 089 | 82 | 1 [Reference] | |
| Women | ||||
| Model 1c | ||||
| Vegetarian | 26 177 | 149 | 0.75 (0.59–0.95) | .02 |
| Nonvegetarian | 24 203 | 156 | 1 [Reference] | |
| Model 2d,f | ||||
| Vegetarian | 26 177 | 149 | 0.77 (0.60–0.99) | .04 |
| Nonvegetarian | 24 203 | 156 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 26 177 | 149 | 0.79 (0.61–1.03) | .08 |
| Nonvegetarian | 24 203 | 156 | 1 [Reference] | |
| Blacks | ||||
| Model 1g | ||||
| Vegetarian | 7919 | 37 | 0.80 (0.53–1.20) | .28 |
| Nonvegetarian | 13 115 | 69 | 1 [Reference] | |
| Model 2d,f | ||||
| Vegetarian | 7919 | 37 | 0.73 (0.48–1.12) | .15 |
| Nonvegetarian | 13 115 | 69 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 7919 | 37 | 0.73 (0.47–1.14) | .16 |
| Nonvegetarian | 13 115 | 69 | 1 [Reference] | |
| Nonblacks | ||||
| Model 1g | ||||
| Vegetarian | 32 448 | 215 | 0.80 (0.65–0.98) | .04 |
| Nonvegetarian | 24 177 | 169 | 1 [Reference] | |
| Model 2d,f | ||||
| Vegetarian | 32 448 | 215 | 0.80 (0.64–1.00) | .046 |
| Nonvegetarian | 24 177 | 169 | 1 [Reference] | |
| Model 3e | ||||
| Vegetarian | 32 448 | 215 | 0.81 (0.65–1.03) | .08 |
| Nonvegetarian | 24 177 | 169 | 1 [Reference] | |
Abbreviation: HR, hazard ratio.
The dietary patterns are described in the Dietary Data subsection of the Methods section.
P value for Wald χ2 test of β coefficient in the Cox proportional hazards regression model.
Model 1 was adjusted by age (ie, attained age as time variable) and race (black, nonblack).
Model 2 was adjusted as in model 1 and by education (up to high school graduate, trade school/some college/associate degree, or bachelor degree or higher), moderate or vigorous exercise (none, ≤60 min/wk, or >60 min/wk), smoking (never, quit ≥1 year ago, or current or quit <1 year ago), alcohol use (none, <28 servings/mo, or ≥28 servings/mo), family history of colorectal cancer (yes, no), history of peptic ulcer (yes, no), history of inflammatory bowel disease (yes, no), treatment for diabetes mellitus within the past year (yes, no), used aspirin at least weekly at least 2 of the past 5 years (yes, no), used statins at least 2 of the past 5 years (yes, no), prior colonoscopy or flexible sigmoidoscopy (yes, no), supplemental calcium use (yes, no), supplemental vitamin D (micrograms per day), and dietary energy (kilocalories per day).
Model 3 was adjusted as in model 2 and by body mass index (calculated as weight in kilograms divided by height in meters squared) (<18.5, 18.5–24.9, 25.0–29.9, or ≥30.0) and fiber intake (<20 g/d, 20 to <30 g/d, 30 to <40 g/d, or ≥40 g/d).
Model 2 was additionally adjusted by the use of hormone therapy among menopausal women (yes, no).
Model 1 was adjusted by age (ie, attained age as time variable) and sex (male, female).
Discussion
Overall, the findings described here demonstrate an association between vegetarian dietary patterns and a reduced risk of colorectal cancers. Significant reductions were also seen for the analysis specific to colon cancer; the analysis specific to rectal cancer was limited by power.
The study has a number of strengths. It was diverse in terms of age, sex, race, geographic location, and socioeconomic status, enhancing the relevance of its findings to the North American population. Homogeneity in certain domains of lifestyle related to the shared religious affiliation of participants, particularly in terms of the low use of tobacco and alcohol, may enhance the internal validity of the study. Vegetarian and nonvegetarian status was determined by precise definitions based on the intake of multiple foods rather than simple self-designation. Associations persisted when controlling for several potential demographic, hereditary, and lifestyle confounders. Many known risk and protective factors (eg, family history, prior endoscopy) demonstrated expected associations with colorectal cancer as covariates in this analysis.
Limitations of the study include the power restrictions of relatively early follow-up, particularly for separate analyses for the 4 vegetarian dietary patterns. Later follow-up will enhance power and allow for additional subgroup analyses. Diet was assessed only at baseline, although dietary change is less likely to be an important factor with early follow-up, and mean self-reported duration of adherence to current dietary patterns in this cohort was long (vegans, 21 years; lacto-ovo vegetarians, 39 years; pescovegetarians, 19 years; semivegetarians, 24 years; and nonvegetarians, 48 years).15 Although analyses controlled for many potential confounders, unknown and unmeasured confounders are always possible. Measurement error may produce bias, although error in the classification of participants into major categories, such as vegetarian and nonvegetarian, seems unlikely to be a frequent occurrence, with this factor being an advantage of analysis by dietary pattern over analysis by a specific food or nutrient.
The results of this study seem consistent with prior evidence that often links the consumption of red meat, especially processed meats, to an increased risk of colorectal cancers.3,5,6 Although reduction in meat intake may be a primary reason for the reduced risk demonstrated in vegetarians, an increase in the consumption of various whole plant foods might also contribute to the reduction. Orlichetal30 described the differences in food consumption for vegetarians compared with nonvegetarians. In addition to reduced consumption of animal products, vegetarians ate less refined grains, added fats, sweets, snacks foods, and caloric beverages than did nonvegetarians and increased amounts of a wide variety of plant foods. Such a pattern might be expected to reduce hyperinsulinemia, which has been proposed as a possible mechanism by which diet may increase colorectal cancer risk.31–38 In a similar manner, some research has suggested that insulin like growth factors and binding proteins may relate to cancer risk,35,39 and Levineet al40 recently linked high levels of protein consumption(particularly animal protein) during middle age to increased levels of insulin like growth factor 1 and to an increased risk of cancer and higher mortality. The association between particular foods and colorectal cancers will be examined later in separate analyses. Adiposity could lie along a causal pathway from dietary pattern to colon cancer. However, the results from models including BMI (ie, mode l3 in Tables 2, 3, and 4) were not greatly attenuated, suggesting that the association may be substantially independent of BMI.
The relatively strong estimate of a protective association in pescovegetarians compared with nonvegetarians (which would remain significant even with a Bonferroni-corrected α value of .0125) is noteworthy and interesting. The strength of this association suggests that future analysis by fish and long-chain ω-3 fatty acid consumption may be beneficial. Such analysis may elucidate whether the reduced risk seen in the pescovegetarian group is attributable to fish consumption or to other aspects of the diet. The existing literature provides some, although inconsistent, support for a possible protective association for fish consumption, particularly for rectal cancer41; evidence for ω-3 fatty acid consumption is limited and inconsistent.42
The estimated overall magnitude of association for all vegetarian dietary patterns compared with the nonvegetarian pattern for colorectal cancer, an approximate 20% reduction in risk, compares favorably with studies of the Mediterranean dietary pattern for this outcome. The European Prospective Investigation into Cancer and Nutrition (EPIC) study43 demonstrated reductions in risk of 8% and 11% for the highest vs lowest Mediterranean pattern score using 2 different scoring approaches. A meta-analysis44 of studies of Mediterranean diet and colorectal cancer showed an overall 10% risk reduction for all cohort studies.
The nonvegetarian group, against which comparisons were made, was already consuming a low-meat diet, with mean intake of only 54.5 g/d of total meat (16.3 g/d of red meat, 20.7 g/d of poultry, and 17.4 g/d of fish) and very little processed meat. For comparison, in the National Institutes of Health (NIH)–AARP (formerly known as the American Association of Retired Persons) study,4 the lowest quintile of red meat consumption for a 2000-kcal/d diet was 17.8 g/d and the highest was133.0g/d. Thus, the AHS-2 nonvegetarian participants consumed slightly less red meat daily compared with the lowest quintile of the NIH-AARP cohort. The AHS-2 nonvegetarian participants were also a low-risk group for colorectal cancer at baseline as evidenced by the SIR value of 0.73 (95% CI, 0.64–0.83). Comparisons of theAHS-2 vegetarians with a more typical American high meat consumption dietary pattern might be expected to demonstrate stronger effects.
The findings of the present study differ from those of the EPIC-Oxford cohort, which is the other major cohort study examining the health effects of vegetarian dietary patterns. The initial results from the EPIC-Oxford study14 found an approximately 50% greater risk of colorectal cancer for vegetarians. Later results from the EPIC-Oxford study45 that were based on approximately twice as many incident cancers no longer demonstrated a significant adverse association for vegetarians but rather a null association. The difference in results between the AHS-2 and EPIC-Oxford studies is in need of explanation. Biological differences between British vegetarians and North American Seventh-Day Adventist vegetarians seem an unlikely explanation. Both studies attempted to control for a variety of important confounders. The approach to ascertaining vegetarian status differed in the 2 studies, but a large measurement error of vegetarian status seems unlikely. Some of the discrepancy may be explained by dietary differences. TheAHS-2 cohort members30 ate substantially more fruits and vegetables compared with the EPIC-Oxford participants.46 The AHS-28 vegans had a substantially greater intake of both dietary fiber and vitamin C than their EPIC-Oxford counterparts.47 Indeed, since foods containing dietary fiber may be protective against colorectal cancer,2,48 such differences in diet between the groups of vegetarians may affect their risk. However, given that the evidence for a link between red meat and processed meat consumption and increased risk of colorectal cancer is considered convincing,2,7 the EPIC-Oxford study results remain surprising. If interpreted causally, the results might suggest either that the potential beneficial effects of the elimination of red and processed meats by British vegetarians are negated by other potentially deleterious aspects of their vegetarian diets or that their meat avoidance is not beneficial. In fact, a UK pooling study49 including EPIC-Oxford cohort members did not demonstrate an association between red meat consumption and colorectal cancer risk. Conversely, red meat consumption was associated with colorectal cancer risk in the entire European EPIC cohort.5 Given the currently available results, such divergent findings seem difficult to fully explain.
Conclusions
We found that vegetarian dietary patterns in a large North American cohort, particularly the pescovegetarian dietary pattern, were associated with lower risk of all colorectal cancer as well as colon cancer separately. The evidence that vegetarian diets similar to those of our study participants may be associated with a reduced risk of colorectal cancer, along with prior evidence of the potential reduced risk of obesity, hypertension, diabetes, and mortality, should be considered carefully in making dietary choices and in giving dietary guidance.
Acknowledgments
Funding/Support: Project support was obtained from National Cancer Institute (NCI) grant 1U01CA152939 (Dr Fraser) and World Cancer Research Fund (WCRF) grant 2009/93 (Dr Fraser).
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Fraser had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Orlich, Singh, Knutsen, Beeson, Jaceldo-Siegl.
Acquisition, analysis, or interpretation of data: Orlich, Sabaté, Fan, Sveen, Bennett, Knutsen, Beeson, Jaceldo-Siegl, Butler, Herring, Fraser.
Drafting of the manuscript: Orlich.
Critical revision of the manuscript for important intellectual content: Singh, Sabaté, Fan, Sveen, Bennett, Knutsen, Beeson, Jaceldo-Siegl, Butler, Herring, Fraser.
Statistical analysis: Orlich, Singh, Fan.
Obtained funding: Knutsen, Fraser.
Administrative, technical, or material support: Sveen, Bennett, Herring, Fraser.
Study supervision: Sabaté, Knutsen, Fraser.
Conflict of Interest Disclosures: Dr Orlich reports receiving a small honorarium from the Northern California Conference of Seventh-Day Adventists to partially defray travel expenses for a speaking engagement at which he gave an overview and update of Adventist Health Studies research and a small honorarium from the Southern California Conference of Seventh-Day Adventists for a speaking engagement at which he lectured on lifestyle approaches for chronic disease prevention. No other conflicts are reported.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the NCI, WCRF, or the participating cancer registries. The ideas and opinions expressed herein are those of the authors and endorsement by the NCI, WCRF, or their contractors or subcontractors is not intended nor should it be inferred.
Previous Presentation: The findings of this study were presented as a poster at the Society for Epidemiologic Research annual meeting; June 26, 2014; Seattle, Washington.
Additional Contributions: Cancer incidence data were provided by the Alabama State Cancer Registry, Alaska Cancer Registry, Arizona Cancer Registry, Arkansas Cancer Registry, California Cancer Registry, Colorado Cancer Registry, Connecticut Tumor Registry, District of Columbia Cancer Registry, Delaware Cancer Registry, Florida Cancer Data System, Georgia Department of Public Health, Hawaii Tumor Registry, Cancer Registry of Idaho, Illinois State Cancer Registry, Indiana State Cancer Registry, Iowa Cancer Registry, Kansas Cancer Registry, Kentucky Cancer Registry, Louisiana Tumor Registry, Maryland Cancer Registry, Massachusetts Cancer Registry, Michigan Cancer Surveillance System, Minnesota Cancer Surveillance System, Mississippi Cancer Registry, Missouri Cancer Registry and Research Center, Montana Central Tumor Registry, Nebraska Cancer Registry, Nevada Central Cancer Registry, New Hampshire State Cancer Registry, New Jersey State Cancer Registry, New Mexico Tumor Registry, New York State Cancer Registry, North Carolina Central Cancer Registry, North Dakota Statewide Cancer Registry, Cancer Data Registry of Ohio, Oklahoma Central Cancer Registry, Oregon State Cancer Registry, Pennsylvania Cancer Registry, Rhode Island Cancer Registry, South Carolina Cancer Registry, South Dakota Cancer Registry, Tennessee Cancer Registry, Texas Cancer Registry, Utah Cancer Registry (NCI contract HHSN261201300071), Vermont Cancer Registry, Virginia Cancer Registry, Washington State Cancer Registry, West Virginia Cancer Registry, and Wyoming Cancer Surveillance Program.
REFERENCES
- 1.American Cancer Society. Colorectal cancer. [Accessed May 19, 2014]; http://www.cancer.org/acs/groups/cid/documents/webcontent/003096-pdf.pdf. [Google Scholar]
- 2.Continuous Update Project Report: Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. Washington, DC: American Institute for Cancer Research; 2011. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 3.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4(12):e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70(6):2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation Into Cancer and Nutrition. J Natl Cancer Inst. 2005;97(12):906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DSM, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 8.Rizzo NS, Jaceldo-Siegl K, Sabaté J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113(12):1610–1619. doi: 10.1016/j.jand.2013.06.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser GE. Vegetarianism and obesity, hypertension, diabetes, and arthritis. In: Fraser GE, editor. Diet, Life Expectancy, and Chronic Disease: Studies of Seventh-Day Adventists and Other Vegetarians. New York, NY: Oxford Univ Press; 2003. pp. 129–148. [Google Scholar]
- 10.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009;32(5):791–796. doi: 10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer EA, Appleby PN, Davey GK, Key TJ. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes Relat Metab Disord. 2003;27(6):728–734. doi: 10.1038/sj.ijo.0802300. [DOI] [PubMed] [Google Scholar]
- 12.Newby PK, Tucker KL, Wolk A. Risk of overweight and obesity among semivegetarian, lactovegetarian, and vegan women. Am J Clin Nutr. 2005;81(6):1267–1274. doi: 10.1093/ajcn/81.6.1267. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Cancer incidence in vegetarians: results from the European Prospective Investigation Into Cancer and Nutrition (EPIC-Oxford) Am J Clin Nutr. 2009;89(5):1620S–1626S. doi: 10.3945/ajcn.2009.26736M. [DOI] [PubMed] [Google Scholar]
- 15.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173(13):1230–1238. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2) Public Health Nutr. 2012;15(10):1909–1916. doi: 10.1017/S1368980011003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser GE, Katuli S, Anousheh R, Knutsen SF, Herring P, Fan J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr. 2015;18(3):537–545. doi: 10.1017/S1368980014000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzo NS, Sabaté J, Jaceldo-Siegl K, Fraser GE. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the Adventist Health Study 2. Diabetes Care. 2011;34(5):1225–1227. doi: 10.2337/dc10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23(4):292–299. doi: 10.1016/j.numecd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2013;22(2):286–294. doi: 10.1158/1055-9965.EPI-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148(8):761–774. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 22.Butler TL, Fraser GE, Beeson WL, et al. Cohort profile: the Adventist Health Study-2 (AHS-2) Int J Epidemiol. 2008;37(2):260–265. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 23.Jaceldo-Siegl K, Knutsen SF, Sabaté J, et al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2) Public Health Nutr. 2010;13(6):812–819. doi: 10.1017/S1368980009992072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaceldo-Siegl K, Fan J, Sabaté J, et al. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr. 2011;14(11):1988–1997. doi: 10.1017/S1368980011000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2011. Bethesda, MD: National Cancer Institute; [Accessed July 9, 2014]. http://seer.cancer.gov/csr/1975_2011/. [Google Scholar]
- 26.Fraser G, Yan R. Guided multiple imputation of missing data: using a subsample to strengthen the missing-at-random assumption. Epidemiology. 2007;18(2):246–252. doi: 10.1097/01.ede.0000254708.40228.8b. [DOI] [PubMed] [Google Scholar]
- 27.Fraser GE, Yan R, Butler TL, Jaceldo-Siegl K, Beeson WL, Chan J. Missing data in a long food frequency questionnaire: are imputed zeroes correct? Epidemiology. 2009;20(2):289–294. doi: 10.1097/EDE.0b013e31819642c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Team R Development Core Team. A language and environment for statistical computing. [Accessed July 15, 2011]; http://www.R-project.org/. [Google Scholar]
- 29.Harrell FE., Jr Hmisc: Harrell miscellaneous. [Accessed March 1, 2014]; http://CRAN.R-project.org/package=Hmisc. [Google Scholar]
- 30.Orlich MJ, Jaceldo-Siegl K, Sabaté J, Fan J, Singh PN, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112(10):1644–1653. doi: 10.1017/S000711451400261X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;3(8):687–695. [PubMed] [Google Scholar]
- 32.Giovannucci E. Diet, body weight, and colorectal cancer: a summary of the epidemiologic evidence. J Womens Health (Larchmt) 2003;12(2):173–182. doi: 10.1089/154099903321576574. [DOI] [PubMed] [Google Scholar]
- 33.Tran TT, Naigamwalla D, Oprescu AI, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147(4):1830–1837. doi: 10.1210/en.2005-1012. [DOI] [PubMed] [Google Scholar]
- 34.Choi Y, Giovannucci E, Lee JE. Glycaemic index and glycaemic load in relation to risk of diabetes-related cancers: a meta-analysis. Br J Nutr. 2012;108(11):1934–1947. doi: 10.1017/S0007114512003984. [DOI] [PubMed] [Google Scholar]
- 35.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14(4):850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 36.Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, Giovannucci E. Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):138–147. [PubMed] [Google Scholar]
- 37.Tsai C-J, Giovannucci EL. Hyperinsulinemia, insulin resistance, vitamin D, and colorectal cancer among whites and African Americans. Dig Dis Sci. 2012;57(10):2497–2503. doi: 10.1007/s10620-012-2198-0. [DOI] [PubMed] [Google Scholar]
- 38.Bao Y, Nimptsch K, Meyerhardt JA, et al. Dietary insulin load, dietary insulin index, and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3020–3026. doi: 10.1158/1055-9965.EPI-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giovannucci E, Pollak M, Liu Y, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12(2):84–89. [PubMed] [Google Scholar]
- 40.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Feng B, Li K, et al. Fish consumption and colorectal cancer risk in humans: a systematic review and meta-analysis. Am J Med. 2012;125(6):551–559. doi: 10.1016/j.amjmed.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012;107(S2) suppl 2:S228–S239. doi: 10.1017/S0007114512001614. [DOI] [PubMed] [Google Scholar]
- 43.Bamia C, Lagiou P, Buckland G, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28(4):317–328. doi: 10.1007/s10654-013-9795-x. [DOI] [PubMed] [Google Scholar]
- 44.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer. 2014;135(8):1884–1897. doi: 10.1002/ijc.28824. [DOI] [PubMed] [Google Scholar]
- 45.Key TJ, Appleby PN, Spencer EA, et al. Cancer incidence in British vegetarians. Br J Cancer. 2009;101(1):192–197. doi: 10.1038/sj.bjc.6605098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agudo A, Slimani N, Ocké MC, et al. Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr. 2002;5(6B):1179–1196. doi: 10.1079/PHN2002398. [DOI] [PubMed] [Google Scholar]
- 47.Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6(3):259–269. doi: 10.1079/PHN2002430. [DOI] [PubMed] [Google Scholar]
- 48.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer EA, Key TJ, Appleby PN, et al. Meat, poultry and fish and risk of colorectal cancer: pooled analysis of data from the UK Dietary Cohort Consortium. Cancer Causes Control. 2010;21(9):1417–1425. doi: 10.1007/s10552-010-9569-7. [DOI] [PubMed] [Google Scholar]